Albumin Submicron Particles with Entrapped Riboflavin—Fabrication and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
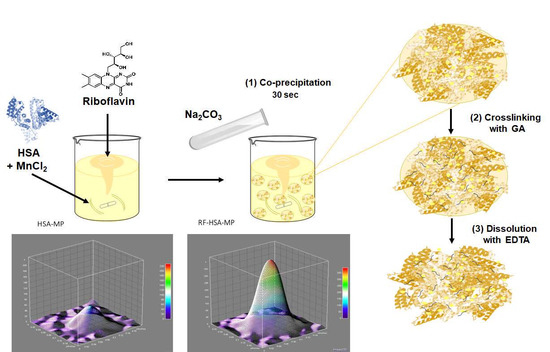
2.2. Fabrication and Characterization of RF-HSA-MPs
2.2.1. Fabrication of RF-HSA-MPs Particles
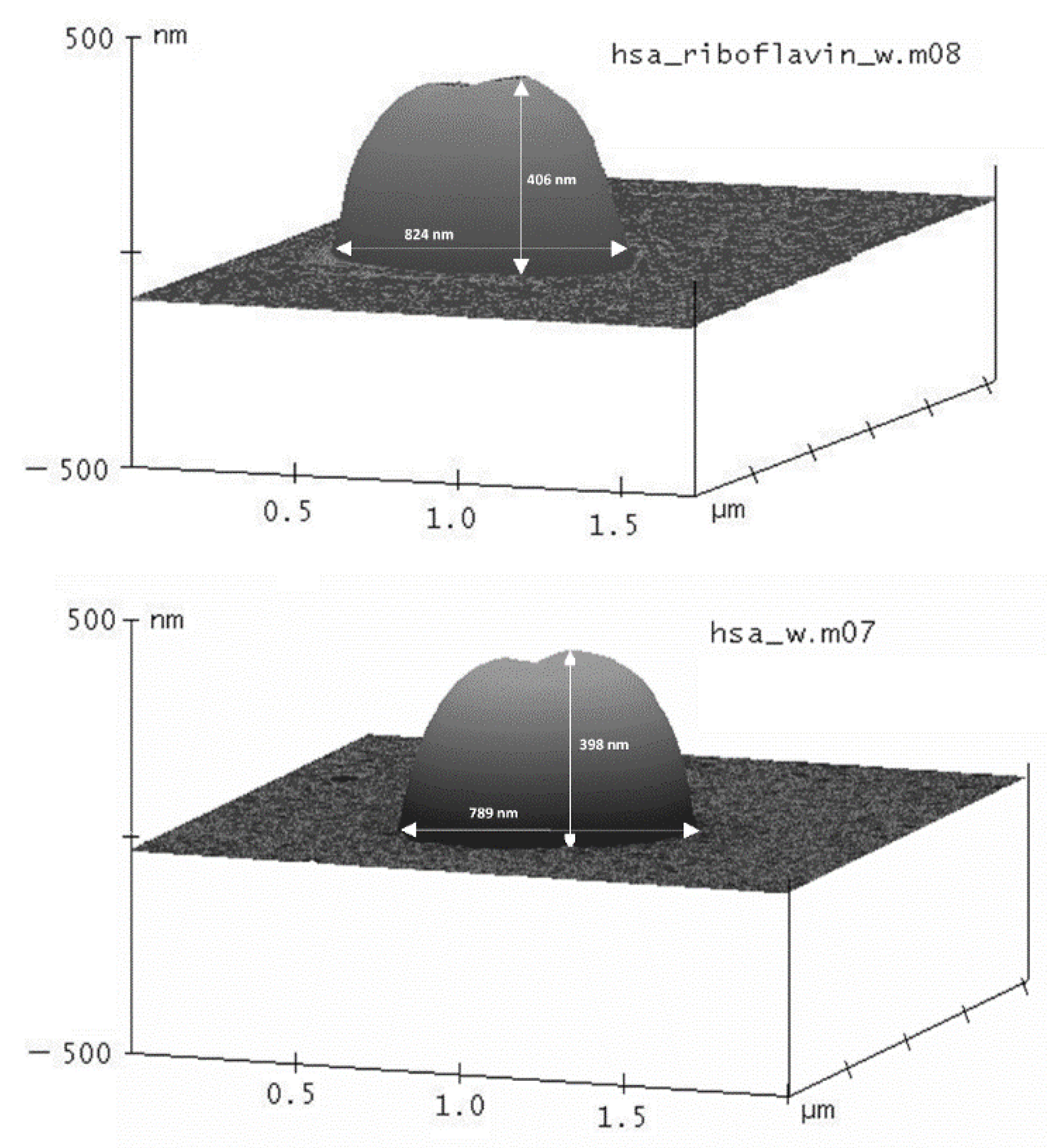
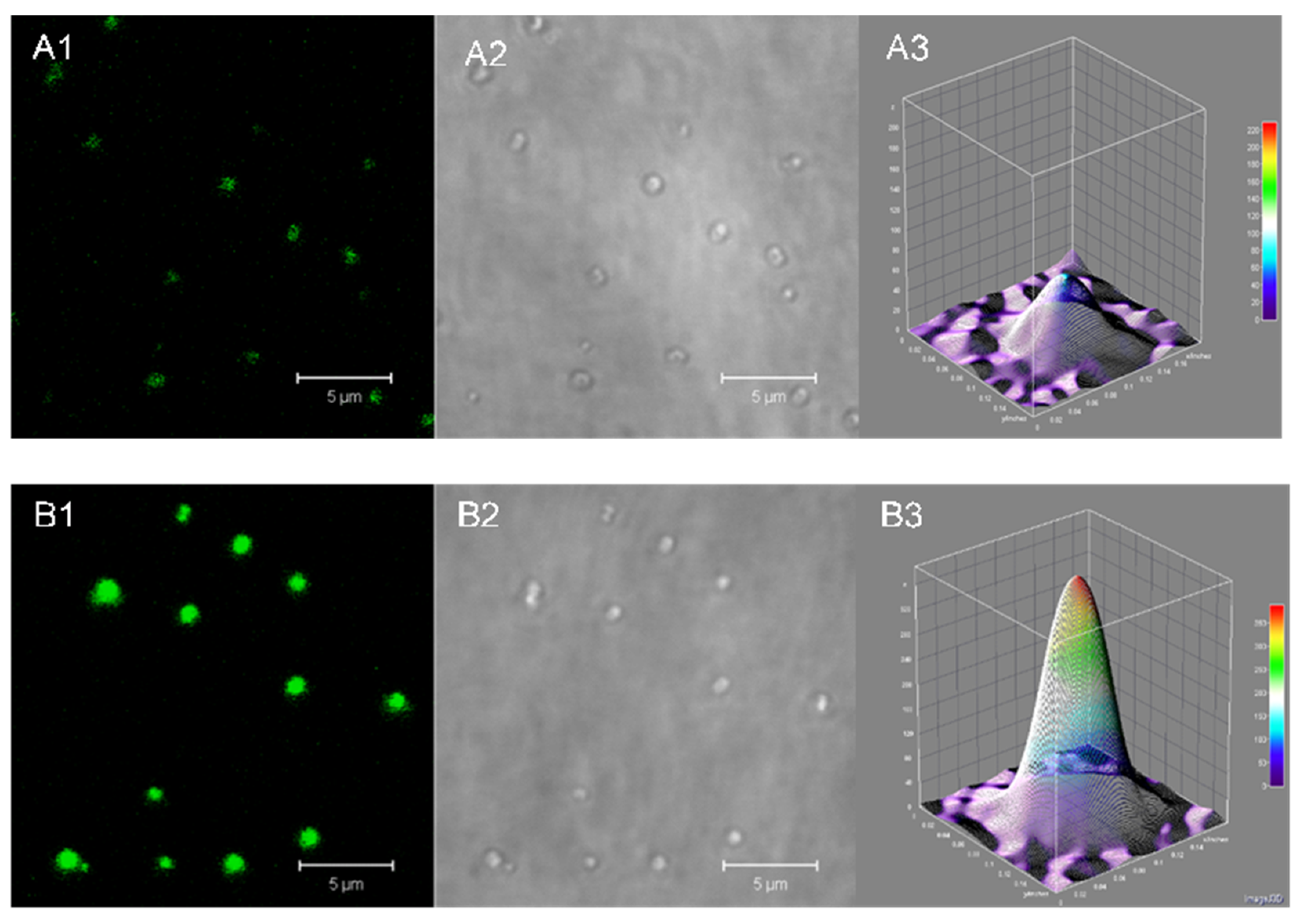
2.2.2. Size, Zeta-Potential and Morphology of the HSA-MPs and RF-HSA-MPs
2.2.3. Intrinsic Fluorescence of the HSA-MPs and RF-HSA-MPs
2.3. In Vitro Release of RF from the RF-HSA-MPs
2.4. Hemocompatibility of HSA-MPs and RF-HSA MPs
2.4.1. Hemolysis Test
2.4.2. Phagocytosis Test
2.4.3. Platelet Activation Test
3. Results and Discussion
3.1. Fabrication and Characterization of RF-HSA-MPs
3.1.1. HSA and RF Content, Size, Zeta-Potential and Morphology
3.1.2. Intrinsic Fluorescence of the HSA-MPs and RF-HSA-MPs
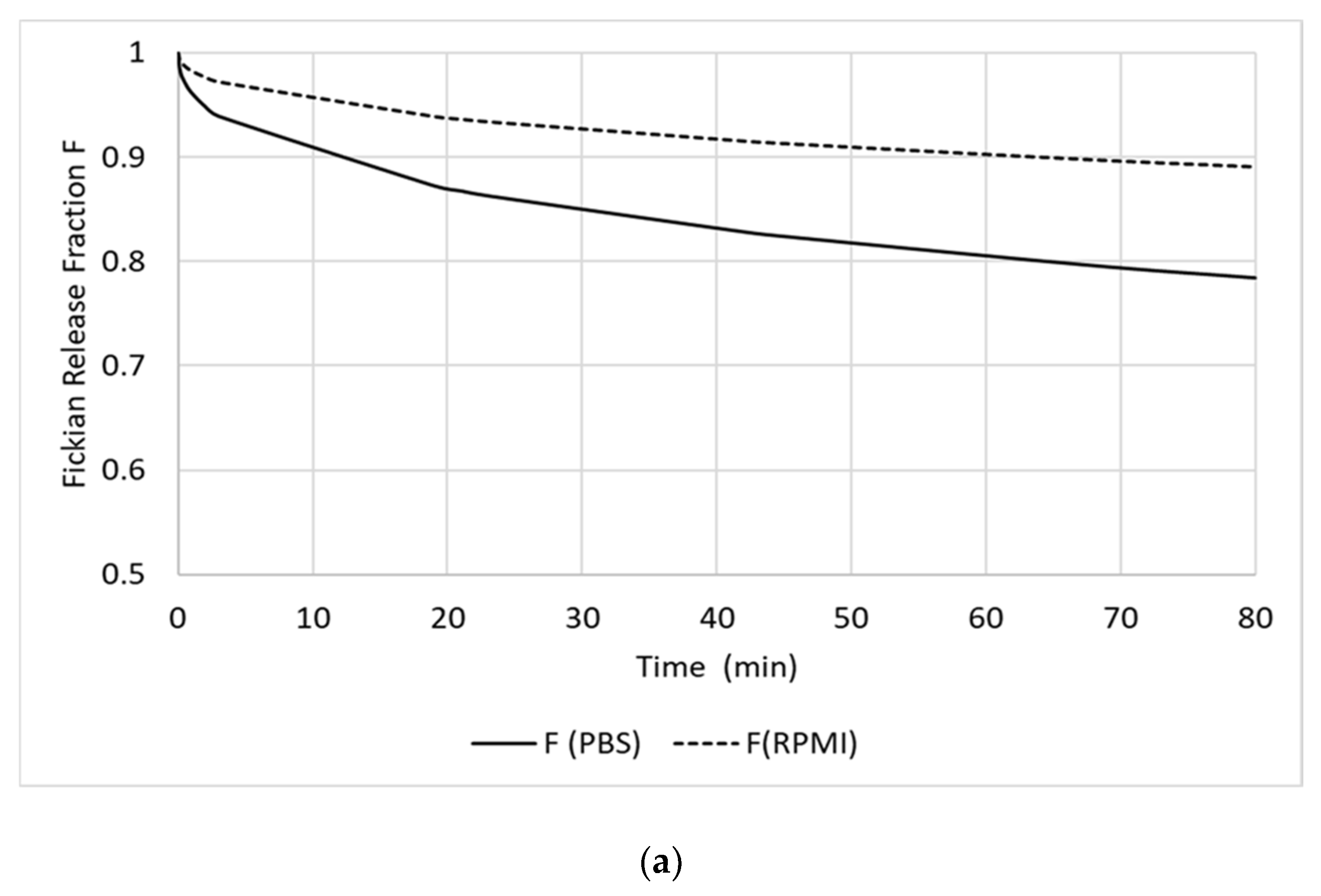
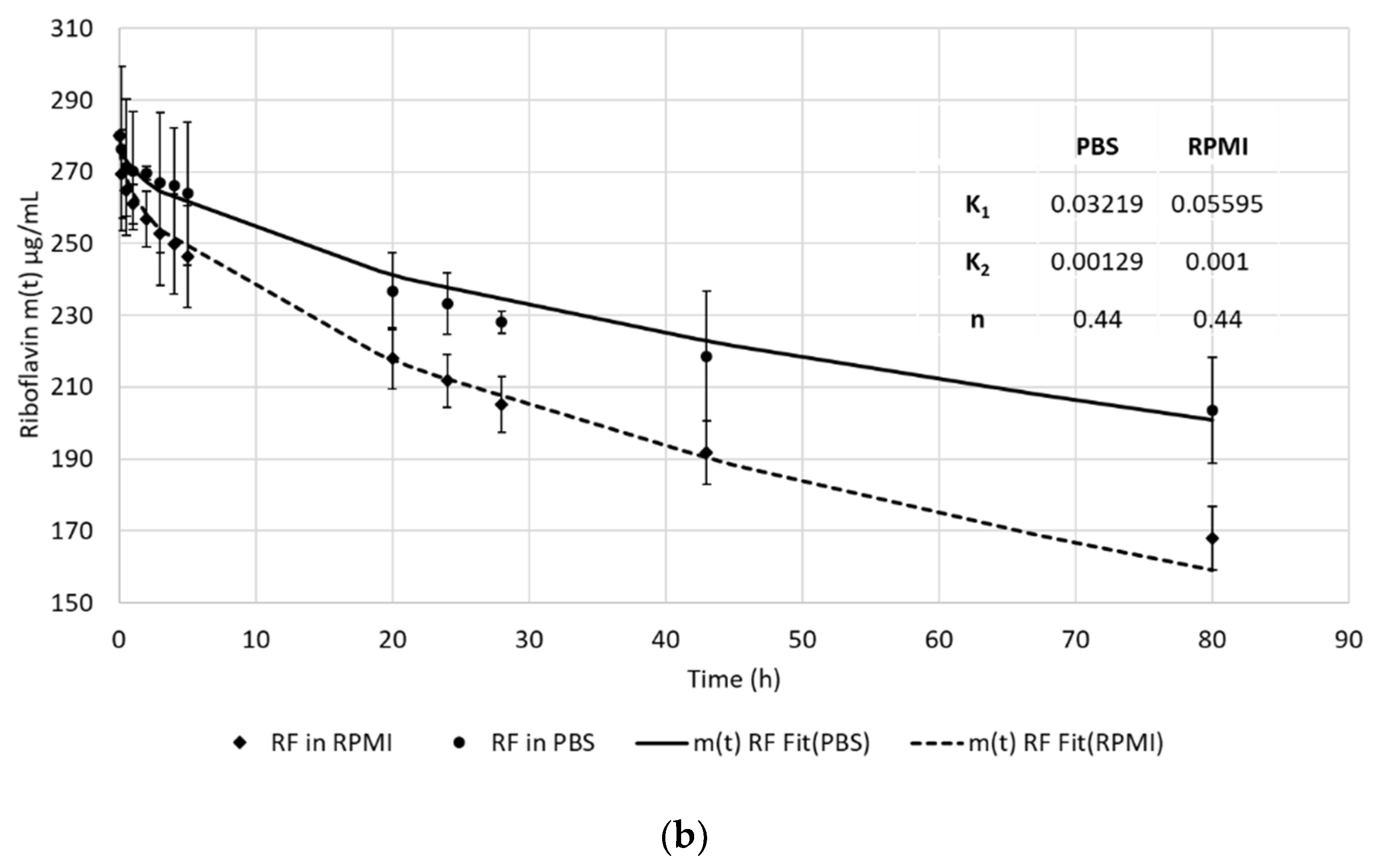
3.2. In Vitro Release of RF from the RF-HSA-MPs
3.3. Hemocompatibility of RF-HSA MPs
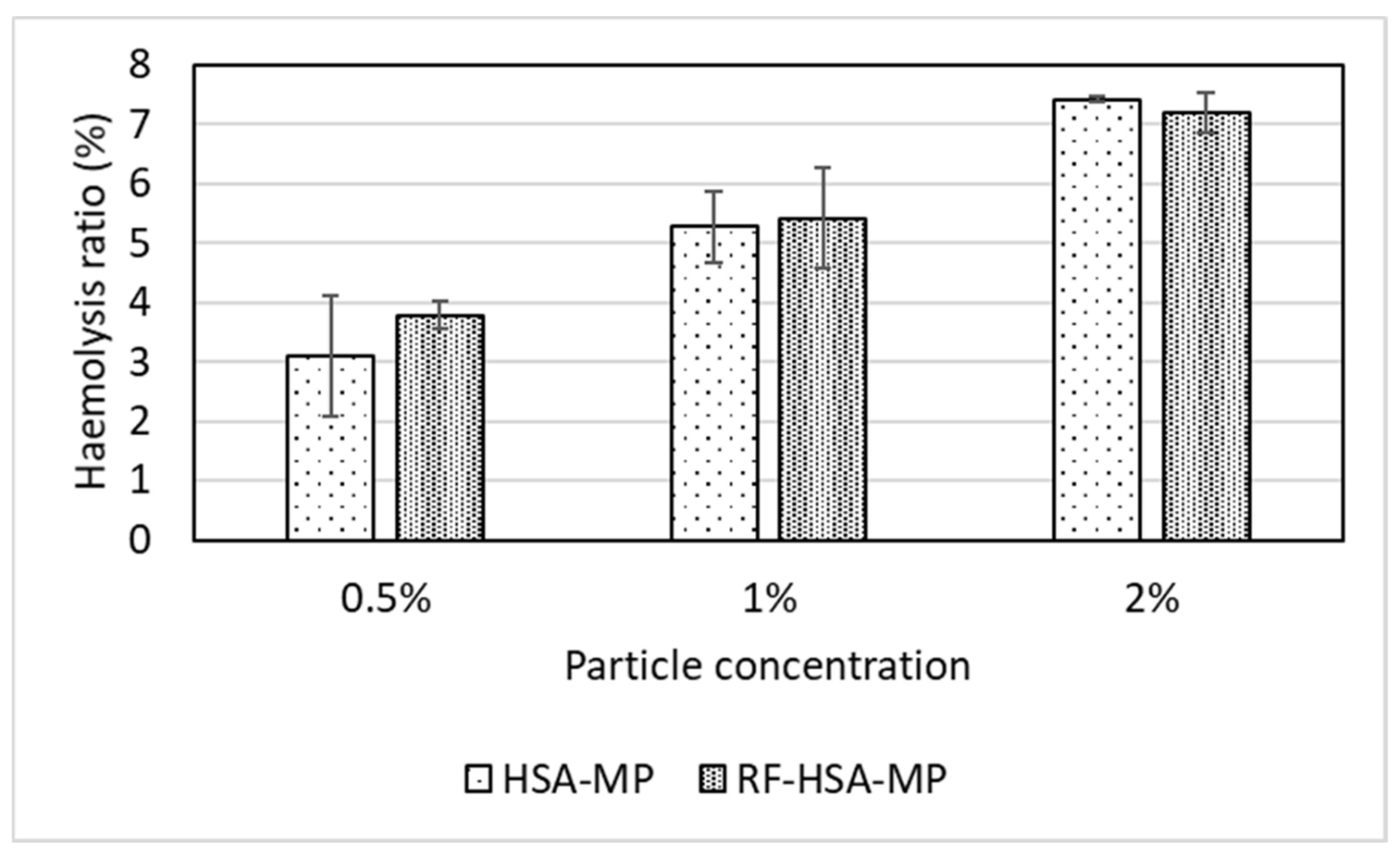
3.3.1. Hemolysis Test
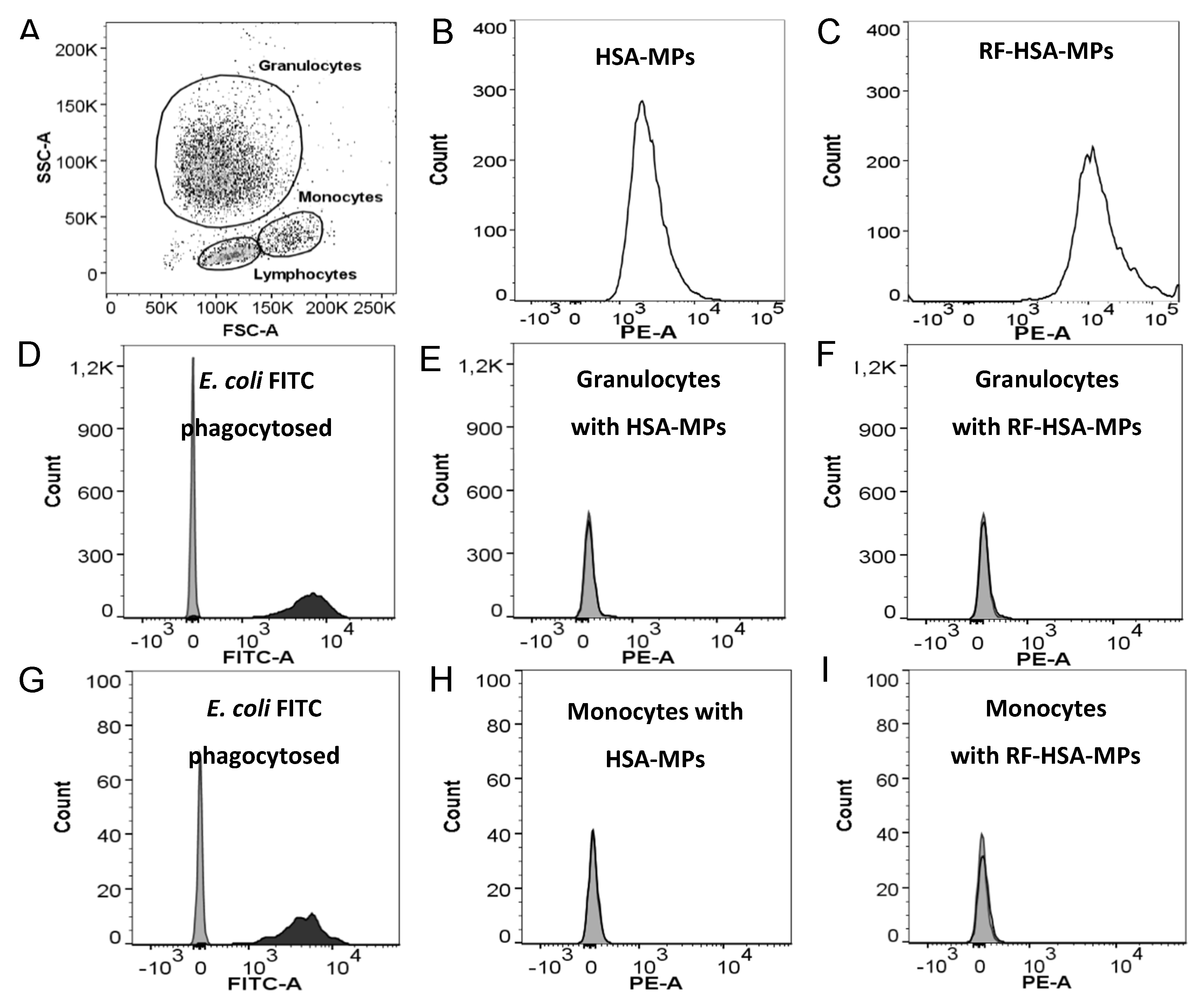
3.3.2. Phagocytosis Test
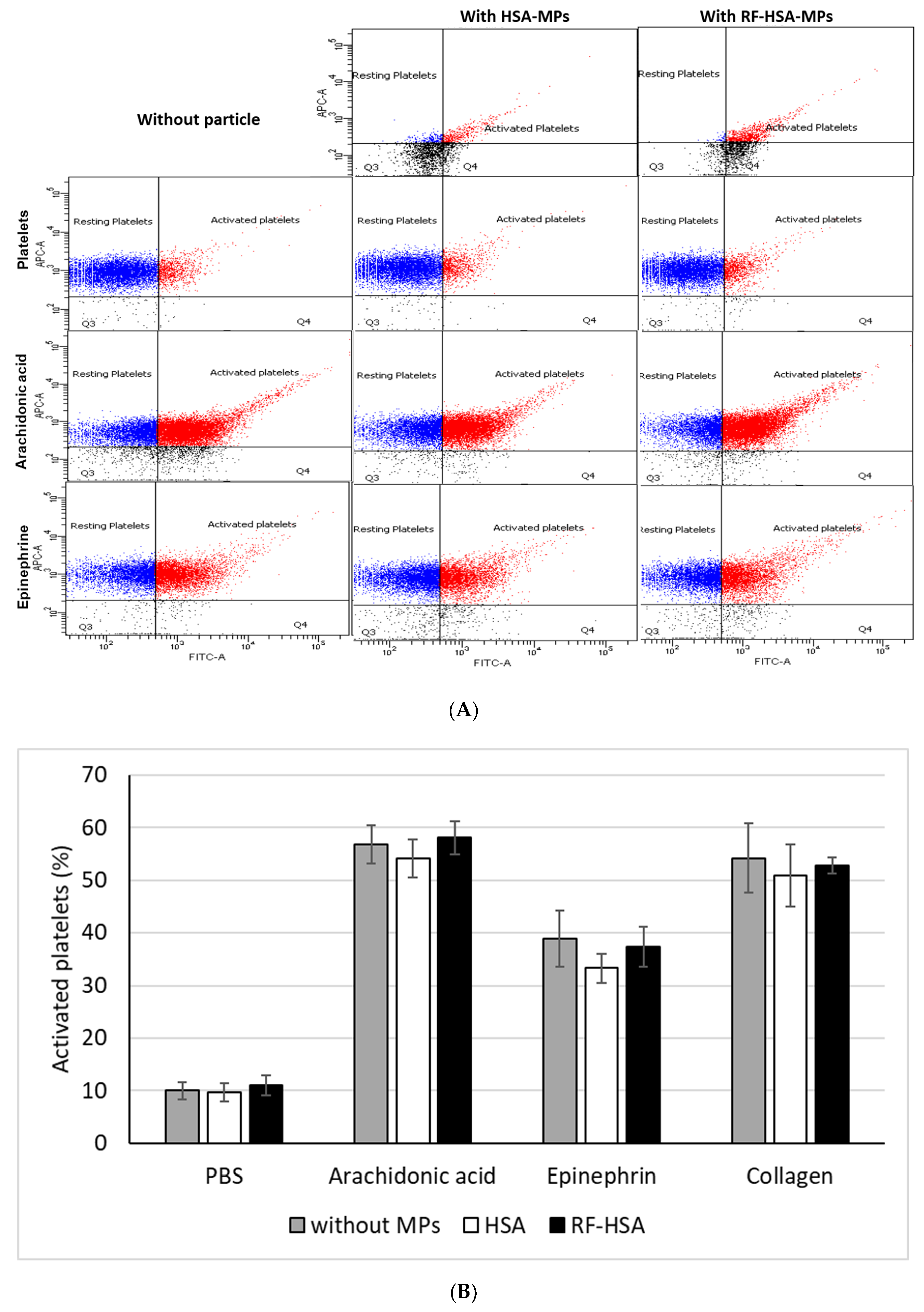
3.3.3. Platelet Activation Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, G.J.; Egan, T.; Jacquier, J.C.; O’Sullivan, M.; O’Riordan, E.D. Kinetics of immobilisation and release of tryptophan, riboflavin and peptides from whey protein microbeads. Food Chem. 2015, 180, 150–155. [Google Scholar] [CrossRef]
- Machado, D.; Shishido, S.M.; Queiroz, K.C.S.; Oliveira, D.N.; Faria, A.L.C.; Catharino, R.R.; Spek, C.A.; Ferreira, C.V. Irradiated riboflavin diminishes the aggressiveness of melanoma in vitro and in vivo. PLoS ONE 2013, 8, e54269. [Google Scholar] [CrossRef]
- Chaves Neto, A.H.; Pelizzaro-Rocha, K.J.; Fernandes, M.N.; Ferreira-Halder, C.V. Antitumor activity of irradiated riboflavin on human renal carcinoma cell line 786-O. Tumor Biol. 2015, 36, 595–604. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Kolaczkowska, E.; Plytycz, B. Modulation of zymosan-induced peritonitis by riboflavin co-injection, pre-injection or post-injection in male Swiss mice. Life Sci. 2012, 91, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Powers, H.J. Current knowledge concerning optimum nutritional status of riboflavin, niacin and pyridoxine. Proc. Nutr. Soc. 1999, 58, 435–440. [Google Scholar] [CrossRef]
- De Souza, A.C.S.; Kodach, L.; Gadelha, F.R.; Bos, C.L.; Cavagis, A.D.M.; Aoyama, H.; Peppelenbosch, M.P.; Ferreira, C.V. A promising action of riboflavin as a mediator of leukaemia cell death. Apoptosis 2006, 11, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- De Souza Queiroz, K.C.; Zambuzzi, W.F.; Santos de Souza, A.C.; da Silva, R.A.; Machado, D.; Justo, G.Z.; Carvalho, H.F.; Peppelenbosch, M.P.; Ferreira, C.V. A possible anti-proliferative and anti-metastatic effect of irradiated riboflavin in solid tumours. Cancer Lett. 2007, 258, 126–134. [Google Scholar] [CrossRef]
- Reddy, H.L.; Dayan, A.D.; Cavagnaro, J.; Gad, S.; Li, J.; Goodrich, R.P. Toxicity testing of a novel riboflavin-based technology for pathogen reduction and white blood cell inactivation. Transfus. Med. Rev. 2008, 22, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Pron, G.; Ieraci, L.; Kaulback, K. Collagen cross-linking using riboflavin and ultraviolet-a for corneal thinning disorders: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2011, 11, 1–89. [Google Scholar] [PubMed]
- Filipowicz, A.; Wołowiec, S. Solubility and in vitro transdermal diffusion of riboflavin assisted by PAMAM dendrimers. Int. J. Pharm. 2011, 408, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Memarpoor-Yazdi, M.; Mahaki, H. Probing the interaction of human serum albumin with vitamin B2 (riboflavin) and L-Arginine (L-Arg) using multi-spectroscopic, molecular modeling and zeta potential techniques. J. Lumin. 2013, 136, 150–159. [Google Scholar] [CrossRef]
- Zhao, H.; Ge, M.; Zhang, Z.; Wang, W.; Wu, G. Spectroscopic studies on the interaction between riboflavin and albumins. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Georgieva, R.; Steffen, A.; Smuda, K.; Bäumler, H. Structure and properties of hybrid biopolymer particles fabricated by co-precipitation cross-linking dissolution procedure. J. Colloid Interface Sci. 2018, 514, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, H.; Xiong, Y.; Liu, Z.Z.; Patzak, A.; Georgieva, R. Novel hemoglobin particles—Promising new-generation hemoglobin-based oxygen carriers. Artif. Organs 2014, 38, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, H.; Georgieva, R. Micro-Particles, Blood-Substitute and Method for Forming Same. Patent EP07112474.7A, 13 July 2007. [Google Scholar]
- Bäumler, H.; Georgieva, R. Coupled enzyme reactions in multicompartment microparticles. Biomacromolecules 2010, 11, 1480–1487. [Google Scholar] [CrossRef]
- Xiong, Y.; Steffen, A.; Andreas, K.; Müller, S.; Sternberg, N.; Georgieva, R.; Bäumler, H. Hemoglobin-based oxygen carrier microparticles: Synthesis, properties, and in vitro and in vivo investigations. Biomacromolecules 2012, 13, 3292–3300. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Z.Z.; Georgieva, R.; Smuda, K.; Steffen, A.; Sendeski, M.; Voigt, A.; Patzak, A.; Bäumler, H. Nonvasoconstrictive hemoglobin particles as oxygen carriers. ACS Nano 2013, 7, 7454–7461. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Wu, L.; Li, W.; Chen, J.; Cheng, H.; Pan, J.; Cai, B. Hyaluronic acid-coated bovine serum albumin nanoparticles loaded with brucine as selective nanovectors for intra-articular injection. Int. J. Nanomed. 2013, 8, 3843–3853. [Google Scholar] [CrossRef]
- Lee, J.Y.; Bae, K.H.; Kim, J.S.; Nam, Y.S.; Park, T.G. Intracellular delivery of paclitaxel using oil-free, shell cross-linked HSA-multi-armed PEG nanocapsules. Biomaterials 2011, 32, 8635–8644. [Google Scholar] [CrossRef]
- Von Storp, B.; Engel, A.; Boeker, A.; Ploeger, M.; Langer, K. Albumin nanoparticles with predictable size by desolvation procedure. J. Microencapsul. 2012, 29, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Mahor, S.; Rawat, A.; Gupta, P.N.; Dubey, P.; Khatri, K.; Vyas, S.P. Targeted brain delivery of AZT via transferrin anchored pegylated albumin nanoparticles. J. Drug Target. 2006, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yao, P.; He, F.; Yu, C.; Huang, C. Nanoparticles with dextran/chitosan shell and BSA/chitosan core-doxorubicin loading and delivery. Int. J. Pharm. 2010, 393, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Heng, D.; Ng, W.K.; Chan, H.K.; Tan, R.B.H. Nano spray drying: A novel method for preparing protein nanoparticles for protein therapy. Int. J. Pharm. 2011, 403, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Fisher, M.; Juliano, R.L. Targeted albumin-based nanoparticles for delivery of amphipathic drugs. Bioconjug. Chem. 2011, 22, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Kaewprayoon, W.; Zhou, H.; Georgieva, R.; Bäumler, H. RBC aggregation in dextran solutions can be measured by flow cytometry. Clin. Hemorheol. Microcirc. 2017, 65, 93–101. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Rahimi-Nasrabadi, M.; Davoudi-Dehaghani, A.A.; Javidan, A.; Zahedi, M.M.; Hajimirsadeghi, S.S. Statistical optimization of experimental parameters for synthesis of manganese carbonate and manganese oxide nanoparticles. Mater. Res. Bull. 2012, 47, 1045–1050. [Google Scholar] [CrossRef]
- Fanali, G.; Cao, Y.; Ascenzi, P.; Fasano, M. Mn(II) binding to human serum albumin: A 1H-NMR relaxometric study. J. Inorg. Biochem. 2012, 117, 198–203. [Google Scholar] [CrossRef]
- Petrov, A.I.; Volodkin, D.V.; Sukhorukov, G.B. Protein calcium-carbonate coprecipitation: A tool for protein encapsulation. Biotechnol. Prog. 2005, 21, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Vantsyan, M.A.; Kochetkov, A.A.; Marchenko, I.V.; Kiryukhin, Y.I.; Nabatov, B.V.; Artemov, V.V.; Bukreeva, T.V. Nanostructured calcium carbonate particles as fluorophore carriers. Crystallogr. Rep. 2015, 60, 951–958. [Google Scholar] [CrossRef]
- Lauth, V.; Maas, M.; Rezwan, K. Coacervate-directed synthesis of CaCO3 microcarriers for pH-responsive delivery of biomolecules. J. Mater. Chem. B 2014, 2, 7725–7731. [Google Scholar] [CrossRef]
- Simioni, A.R.; de Jesus, P.C.C.; Tedesco, A.C. Layer-by-layer hollow photosensitizer microcapsule design via a manganese carbonate hard template for photodynamic therapy in cells. Photodiagn. Photodyn. Ther. 2018, 22, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Gao, C. Selective removal of particle cores to fabricate manganese carbonate hollow spheres and composite microcapsules. Colloids Surf. A Physicochem. Eng. Asp. 2007, 295, 233–238. [Google Scholar] [CrossRef]
- Zhu, H.; Stein, E.W.; Lu, Z.; Lvov, Y.M.; McShane, M.J. Synthesis of size-controlled monodisperse manganese carbonate microparticles as templates for uniform polyelectrolyte microcapsule formation. Chem. Mater. 2005, 17, 2323–2328. [Google Scholar] [CrossRef]
- Antipov, A.A.; Shchukin, D.; Fedutik, Y.; Petrov, A.I.; Sukhorukov, G.B.; Möhwald, H. Carbonate microparticles for hollow polyelectrolyte capsules fabrication. Colloids Surf. A Physicochem. Eng. Asp. 2003, 224, 175–183. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, F.; Allen, C. Influence of serum protein on polycarbonate-based copolymer micelles as a delivery system for a hydrophobic anti-cancer agent. J. Control. Release 2005, 103, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Reipa, V.; Hitchins, V.M.; Goering, P.L.; Malinauskas, R.A. Physicochemical characterization and in vitro hemolysis evaluation of silver nanoparticles. Toxicol. Sci. 2011, 123, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Zook, J.M.; MacCuspie, R.I.; Locascio, L.E.; Halter, M.D.; Elliott, J.T. Stable nanoparticle aggregates/agglomerates of different sizes and the effect of their size on hemolytic cytotoxicity. Nanotoxicology 2011, 5, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Vadon, M.; Rinner, B.; Novak, A.; Wintersteiger, R.; Fröhlich, E. The role of nanoparticle size in hemocompatibility. Toxicology 2009, 258, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Evora, C.; Soriano, I.; Rogers, R.A.; Shakesheff, K.M.; Hanes, J.; Langer, R. Relating the phagocytosis of microparticles by alveolar macrophages to surface chemistry: The effect of 1,2-dipalmitoylphosphatidylcholine. J. Control. Release 1998, 51, 143–152. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwannasom, N.; Smuda, K.; Kloypan, C.; Kaewprayoon, W.; Baisaeng, N.; Prapan, A.; Chaiwaree, S.; Georgieva, R.; Bäumler, H. Albumin Submicron Particles with Entrapped Riboflavin—Fabrication and Characterization. Nanomaterials 2019, 9, 482. https://doi.org/10.3390/nano9030482
Suwannasom N, Smuda K, Kloypan C, Kaewprayoon W, Baisaeng N, Prapan A, Chaiwaree S, Georgieva R, Bäumler H. Albumin Submicron Particles with Entrapped Riboflavin—Fabrication and Characterization. Nanomaterials. 2019; 9(3):482. https://doi.org/10.3390/nano9030482
Chicago/Turabian StyleSuwannasom, Nittiya, Kathrin Smuda, Chiraphat Kloypan, Waraporn Kaewprayoon, Nuttakorn Baisaeng, Ausanai Prapan, Saranya Chaiwaree, Radostina Georgieva, and Hans Bäumler. 2019. "Albumin Submicron Particles with Entrapped Riboflavin—Fabrication and Characterization" Nanomaterials 9, no. 3: 482. https://doi.org/10.3390/nano9030482
APA StyleSuwannasom, N., Smuda, K., Kloypan, C., Kaewprayoon, W., Baisaeng, N., Prapan, A., Chaiwaree, S., Georgieva, R., & Bäumler, H. (2019). Albumin Submicron Particles with Entrapped Riboflavin—Fabrication and Characterization. Nanomaterials, 9(3), 482. https://doi.org/10.3390/nano9030482