Oriented Cell Alignment Induced by a Nanostructured Titanium Surface Enhances Expression of Cell Differentiation Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Nanostructuration
2.3. Surface Characterization
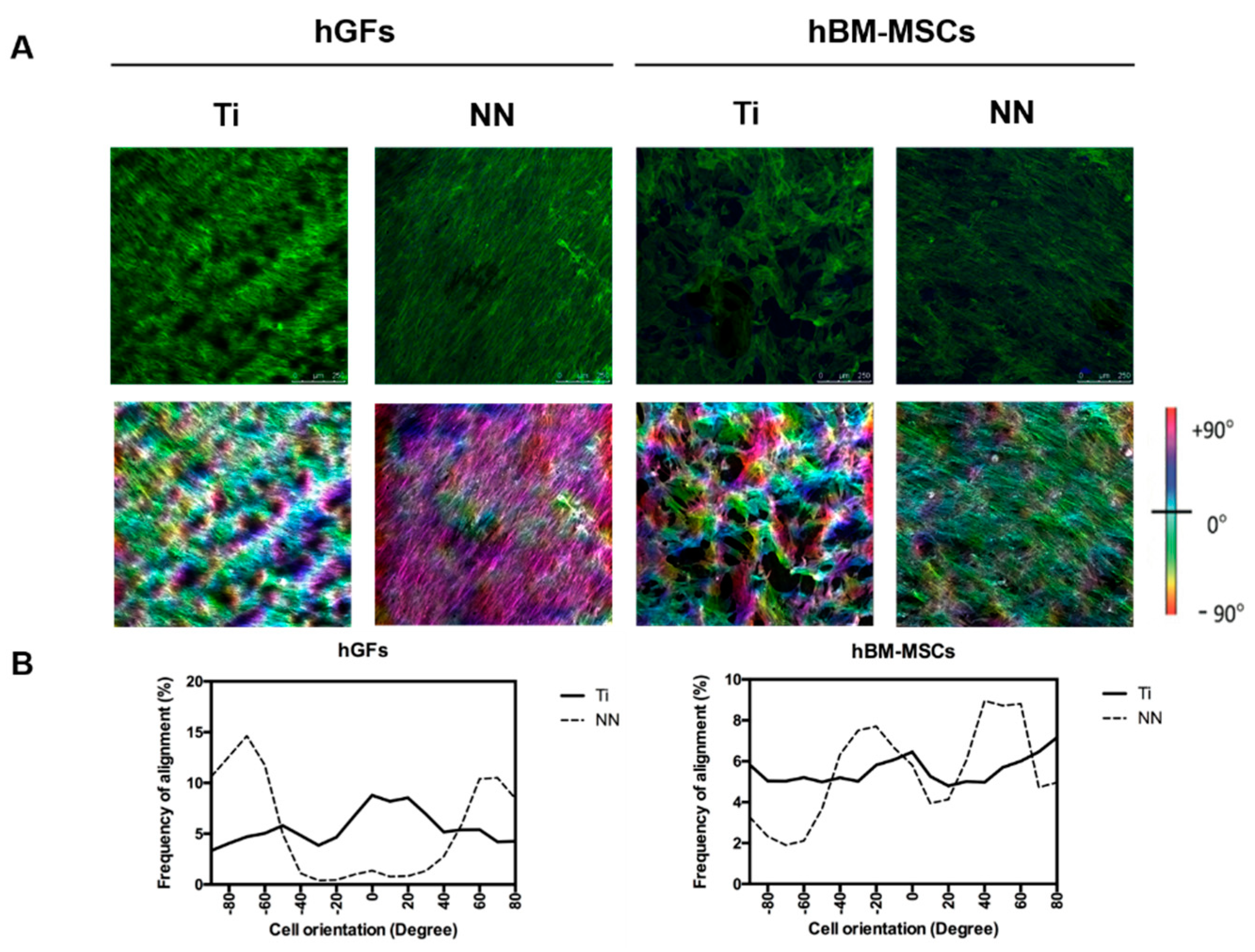
2.4. Cell Culture
2.5. Analysis of Particle Release from the Different Surfaces and Its Effect on Biocompatibility
2.6. Bioactivity of Nanostructured Surfaces
2.7. Cell Adhesion
2.8. Cytotoxicity Assay
2.9. Metabolic Activity
2.10. Collagen Quantification
2.11. Alkaline Phosphatase (ALP) Activity
2.12. Phalloidin-Fluorescein Isothiocyanate (FITC)
2.13. Statistical Analysis
3. Results
3.1. Characterization of Surface Topography and Wettability
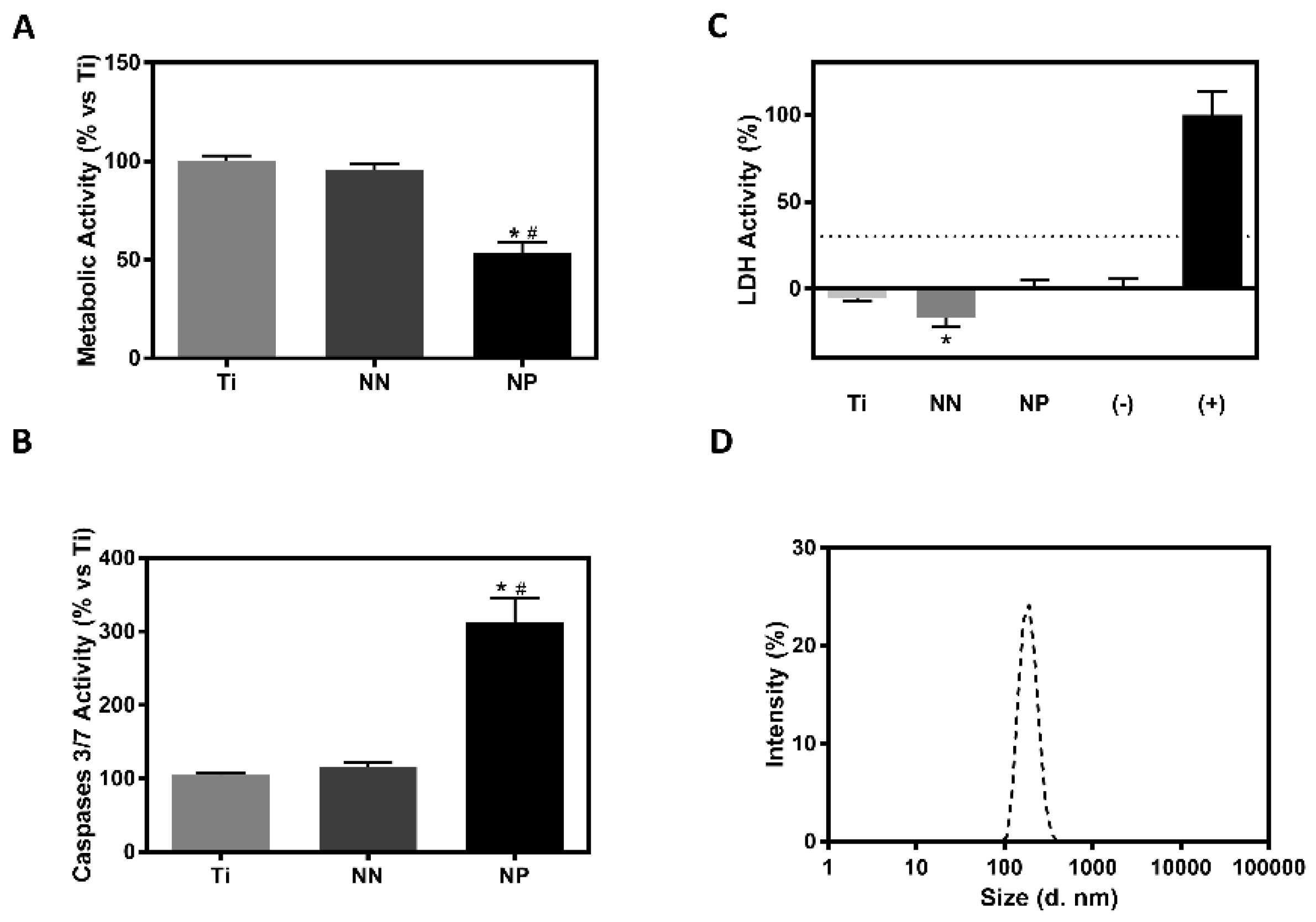
3.2. Analysis of Particle Release from the Different Surfaces and Its Effect on Biocompatibility
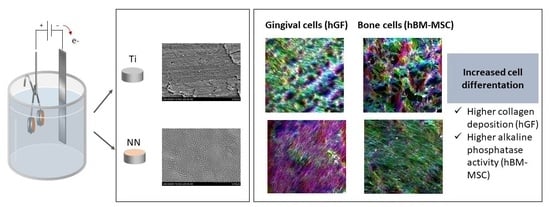
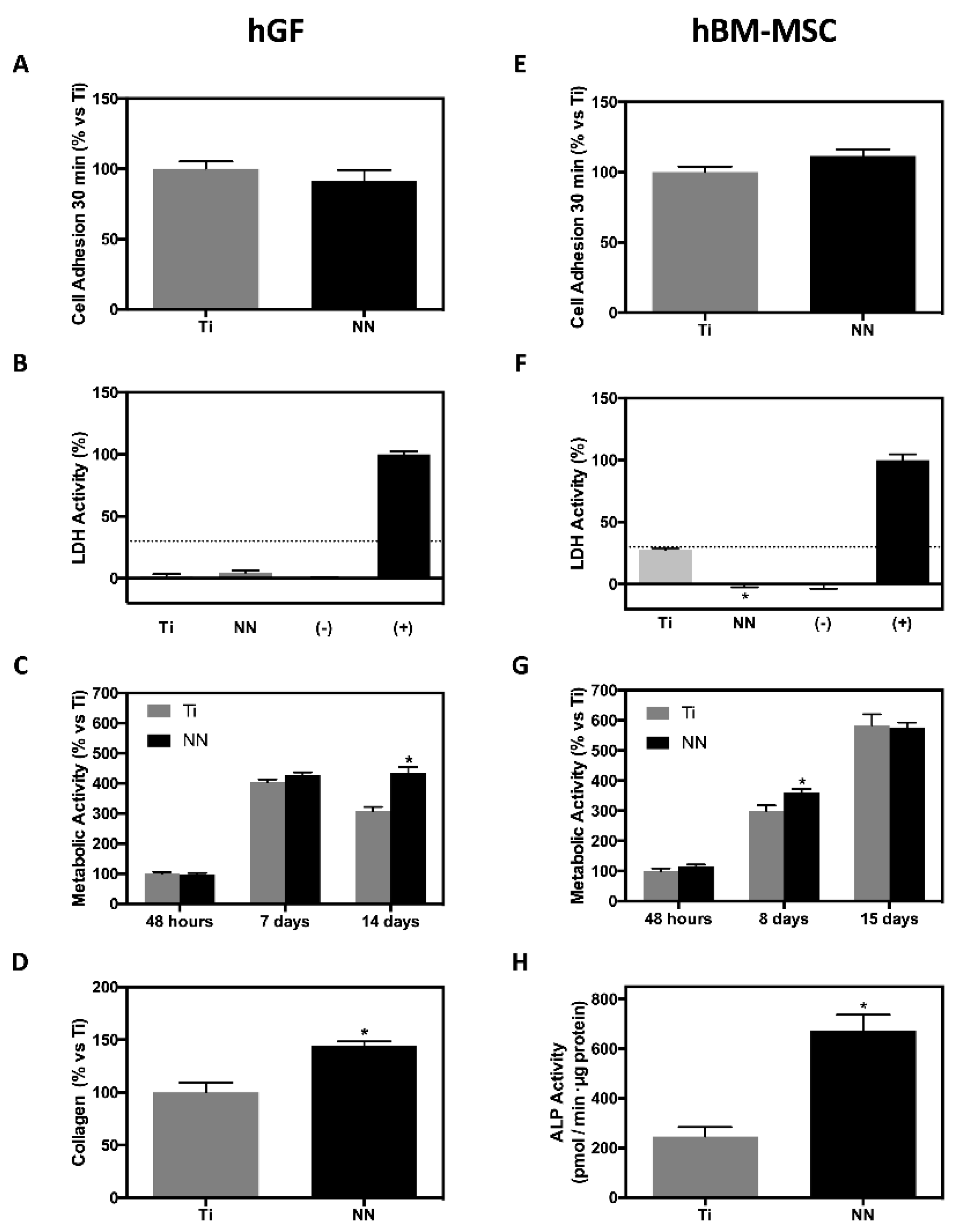
3.3. Bioactivity of NN Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Córdoba, A.; Satué, M.; Gómez-Florit, M.; Hierro-Oliva, M.; Petzold, C.; Lyngstadaas, S.P.; González-Martín, M.L.; Monjo, M.; Ramis, J.M. Flavonoid-Modified Surfaces: Multifunctional Bioactive Biomaterials with Osteopromotive, Anti-Inflammatory, and Anti-Fibrotic Potential. Adv. Healthc. Mater. 2015, 4, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin. Oral Implant. Res. 2008, 19, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Dobbenga, S.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Nanopattern-induced osteogenic differentiation of stem cells—A systematic review. Acta Biomater. 2016, 46, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Metavarayuth, K.; Sitasuwan, P.; Zhao, X.; Lin, Y.; Wang, Q. Influence of Surface Topographical Cues on the Differentiation of Mesenchymal Stem Cells in Vitro. ACS Biomater. Sci. Eng. 2016, 2, 142–151. [Google Scholar] [CrossRef]
- Ferrà-Cañellas, M.d.M.; Llopis-Grimalt, M.A.; Monjo, M.; Ramis, J.M. Tuning Nanopore Diameter of Titanium Surfaces to Improve Human Gingival Fibroblast Response. Int. J. Mol. Sci. 2018, 19, 2881. [Google Scholar] [CrossRef]
- Necula, M.G.; Mazare, A.; Ion, R.N.; Ozkan, S.; Park, J.; Schmuki, P.; Cimpean, A. Lateral Spacing of TiO2 Nanotubes Modulates Osteoblast Behavior. Material 2019, 12, 2956. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. Engl. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Narendrakumar, K.; Kulkarni, M.; Addison, O.; Mazare, A.; Junkar, I.; Schmuki, P.; Sammons, R.; Iglič, A. Adherence of oral streptococci to nanostructured titanium surfaces. Dent. Mater. 2015, 1–9. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Von Der Mark, K.; Schmuki, P. Nanosize and Vitality: TiO2 Nanotube Diameter Directs Cell Fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Schlegel, K.A.; Neukam, F.W.; von der Mark, K.; Schmuki, P. TiO2 nanotube surfaces: 15 nm—An optimal length scale of surface topography for cell adhesion and differentiation. Small 2009, 5, 666–671. [Google Scholar] [CrossRef]
- Lamolle, S.F.; Monjo, M.; Lyngstadaas, S.P.; Ellingsen, J.E.; Haugen, H.J. Titanium implant surface modification by cathodic reduction in hydrofluoric acid: Surface characterization and in vivo performance. J. Biomed. Mater. Res. A 2009, 88, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Florit, M.; Ramis, J.M.; Xing, R.; Taxt-Lamolle, S.; Haugen, H.J.; Lyngstadaas, S.P.; Monjo, M. Differential response of human gingival fibroblasts to titanium- and titanium-zirconium-modified surfaces. J. Periodontal Res. 2014, 49, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Satué, M.; Petzold, C.; Córdoba, A.; Ramis, J.M.; Monjo, M. UV photoactivation of 7-dehydrocholesterol on titanium implants enhances osteoblast differentiation and decreases Rankl gene expression. Acta Biomater. 2013, 9, 5759–5770. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Berger, S.; Hauser, C.; Meyer, K.; Yang, M.; Schmuki, P. Transition of TiO2 nanotubes to nanopores for electrolytes with very low water contents. Electrochem. Commun. 2010, 12, 1184–1186. [Google Scholar] [CrossRef]
- Garcia-contreras, R.; Scougall-vilchis, R.J.; Contreras-bulnes, R.; Kanda, Y.; Nakajima, H.; Sakagami, H. Effects of TiO2 Nano Glass Ionomer Cements Against Normal and Cancer Oral Cells. In Vivo 2014, 28, 895–907. [Google Scholar]
- Tay, C.Y.; Fang, W.; Setyawati, M.I.; Chia, S.L.; Tan, K.S.; Hong, C.H.L.; Leong, D.T. Nano-hydroxyapatite and nano-titanium dioxide exhibit different subcellular distribution and apoptotic profile in human oral epithelium. ACS Appl. Mater. Interfaces 2014, 6, 6248–6256. [Google Scholar] [CrossRef]
- Palaiologou, A.A.; Yukna, R.A.; Moses, R.; Lallier, T.E. Gingival, dermal, and periodontal ligament fibroblasts express different extracellular matrix receptors. J. Periodontol. 2001, 72, 798–807. [Google Scholar] [CrossRef]
- Bartold, P.M.; Walsh, L.J.; Narayanan, A.S. Molecular and cell biology of the gingiva. Periodontology 2000, 24, 28–55. [Google Scholar] [CrossRef]
- Abiko, Y.; Hiratsuka, K.; Kiyama-Kishikawa, M.; Tsushima, K.; Ohta, M.; Sasahara, H. Profiling of differentially expressed genes in human gingival epithelial cells and fibroblasts by DNA microarray. J. Oral Sci. 2004, 46, 19–24. [Google Scholar] [CrossRef][Green Version]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, 96–101. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Hirachi, A.; Hasegawa, N.; Iwata, T.; Hamaguchi, H.; Shiba, H.; Takata, T.; Kato, Y.; Kurihara, H. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J. Periodontol. 2004, 75, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, X.; Li, S.; Lai, R.; Zhou, Z.; Zhang, Y.; Zhou, L. Effects of titania nanotubes with or without bovine serum albumin loaded on human gingival fibroblasts. Int. J. Nanomed. 2014, 9, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Kung, K.-C.; Lee, T.-M.; Wang, C.-C. The use of factorial design for proliferation assay of hFOB cells on different TiO2 nanotube arrays. Sci. Res. Essays 2011, 6, 5750–5756. [Google Scholar] [CrossRef]
- Yu, Y.; Ran, Q.; Shen, X.; Zheng, H.; Cai, K. Enzyme responsive titanium substrates with antibacterial property and osteo/angio-genic differentiation potentials. Colloids Surf. B Biointerfaces 2020, 185, 110592. [Google Scholar] [CrossRef] [PubMed]
- Gongadze, E.; Kabaso, D.; Bauer, S.; Slivnik, T.; Schmuki, P.; van Rienen, U.; Iglič, A. Adhesion of osteoblasts to a nanorough titanium implant surface. Int. J. Nanomed. 2011, 6, 1801–1816. [Google Scholar] [CrossRef]
- Hughes, F.J. Stem Cell Biology and Tissue Engineering in Dental Sciences; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780123971579. [Google Scholar]
- Gulati, K.; Maher, S.; Findlay, D.M.; Losic, D. Titania nanotubes for orchestrating osteogenesis at the bone-implant interface. Nanomedicine 2016, 11, 1847–1864. [Google Scholar] [CrossRef]
- Lai, M.; Cai, K.; Hu, Y.; Yang, X.; Liu, Q. Regulation of the behaviors of mesenchymal stem cells by surface nanostructured titanium. Colloids Surf. B Biointerfaces 2012, 97, 211–220. [Google Scholar] [CrossRef]
- Reich, U.; Fadeeva, E.; Warnecke, A.; Paasche, G.; Müller, P.; Chichkov, B.; Stöjver, T.; Lenarz, T.; Reuter, G. Directing neuronal cell growth on implant material surfaces by microstructuring. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100 B, 940–947. [Google Scholar] [CrossRef]
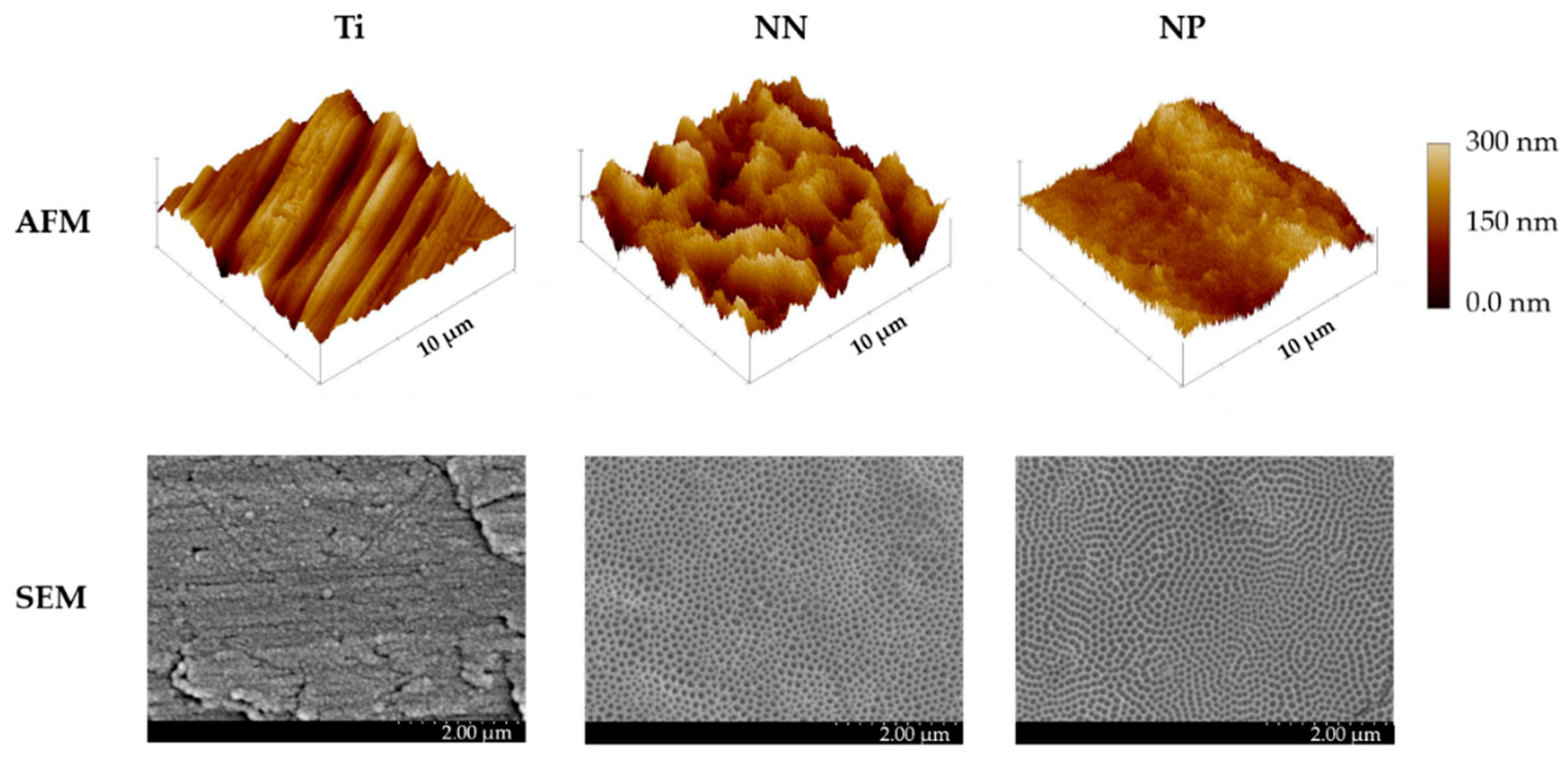
| Parameter | Ti | NN | NP |
|---|---|---|---|
| Porous size (nm) | - | 77.7 ± 0.7 × 47.4 ± 0.5 | 52.9 ± 0.9 |
| Contact Angle (°) | 71.7 ± 8.7 | 84.3 ± 3.8 | 17.7 ± 1.3 |
| Ra (nm) | 28.9 ± 0.7 | 55.8 ± 1.6 * | 31.3 ± 1.9 # |
| Sku | 6.78 ± 2.96 | 2.81 ± 0.13 | 3.74 ± 0.39 |
| Ssk | 0.34 ± 0.24 | 0.07 ± 0.04 | 0.20 ± 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llopis-Grimalt, M.A.; Amengual-Tugores, A.M.; Monjo, M.; Ramis, J.M. Oriented Cell Alignment Induced by a Nanostructured Titanium Surface Enhances Expression of Cell Differentiation Markers. Nanomaterials 2019, 9, 1661. https://doi.org/10.3390/nano9121661
Llopis-Grimalt MA, Amengual-Tugores AM, Monjo M, Ramis JM. Oriented Cell Alignment Induced by a Nanostructured Titanium Surface Enhances Expression of Cell Differentiation Markers. Nanomaterials. 2019; 9(12):1661. https://doi.org/10.3390/nano9121661
Chicago/Turabian StyleLlopis-Grimalt, Maria Antonia, Andreu Miquel Amengual-Tugores, Marta Monjo, and Joana Maria Ramis. 2019. "Oriented Cell Alignment Induced by a Nanostructured Titanium Surface Enhances Expression of Cell Differentiation Markers" Nanomaterials 9, no. 12: 1661. https://doi.org/10.3390/nano9121661
APA StyleLlopis-Grimalt, M. A., Amengual-Tugores, A. M., Monjo, M., & Ramis, J. M. (2019). Oriented Cell Alignment Induced by a Nanostructured Titanium Surface Enhances Expression of Cell Differentiation Markers. Nanomaterials, 9(12), 1661. https://doi.org/10.3390/nano9121661