An Up-To-Date Review on Biomedical Applications of Palladium Nanoparticles
Abstract
1. Introduction
2. Synthesis and Characterization of Palladium Nanoparticles for Biomedical Applications
2.1. Synthesis
2.2. Functionalization Procedures
2.3. Stabilization Procedures
2.4. Physicochemical Properties
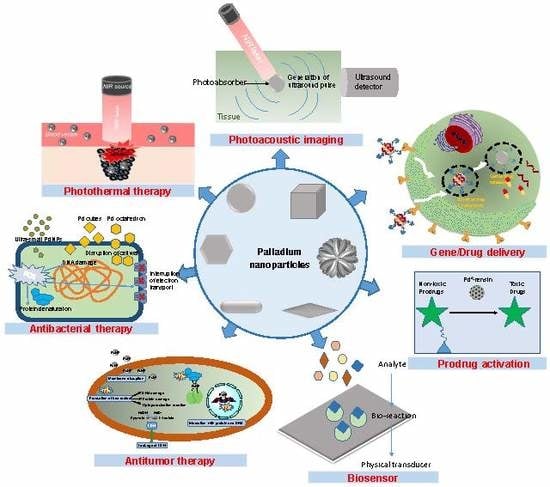
3. Palladium Nanoparticles for Cancer/Infection Photothermal Therapy
4. Palladium Nanoparticles for Photoacoustic Imaging
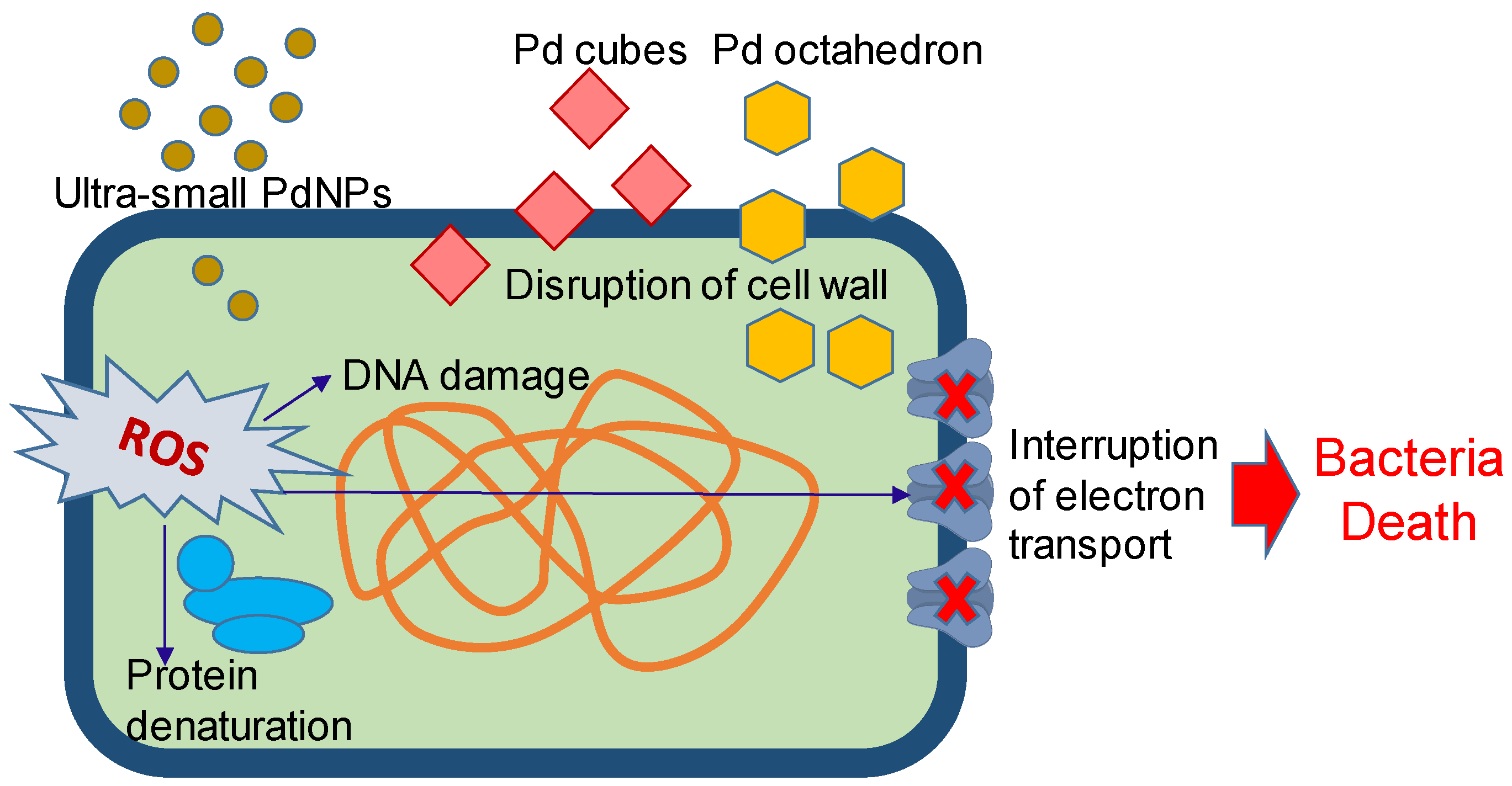
5. Palladium Nanoparticles for Antibacterial Therapy
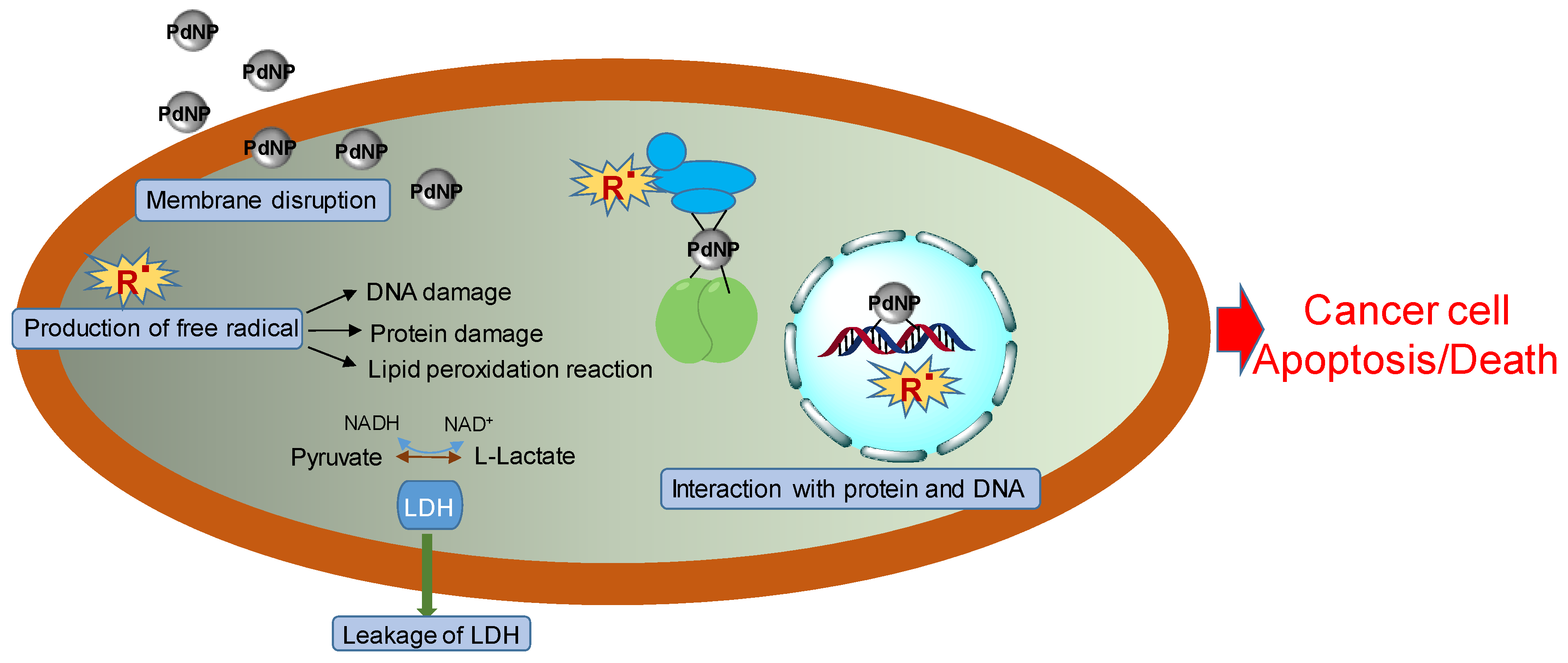
6. Palladium Nanoparticles for Antitumor Therapy
7. Palladium Nanoparticles for Gene and Drug Delivery
8. Palladium Nanoparticles for Prodrug Activation and Transformation Processes
9. Palladium Nanoparticles for Biosensor
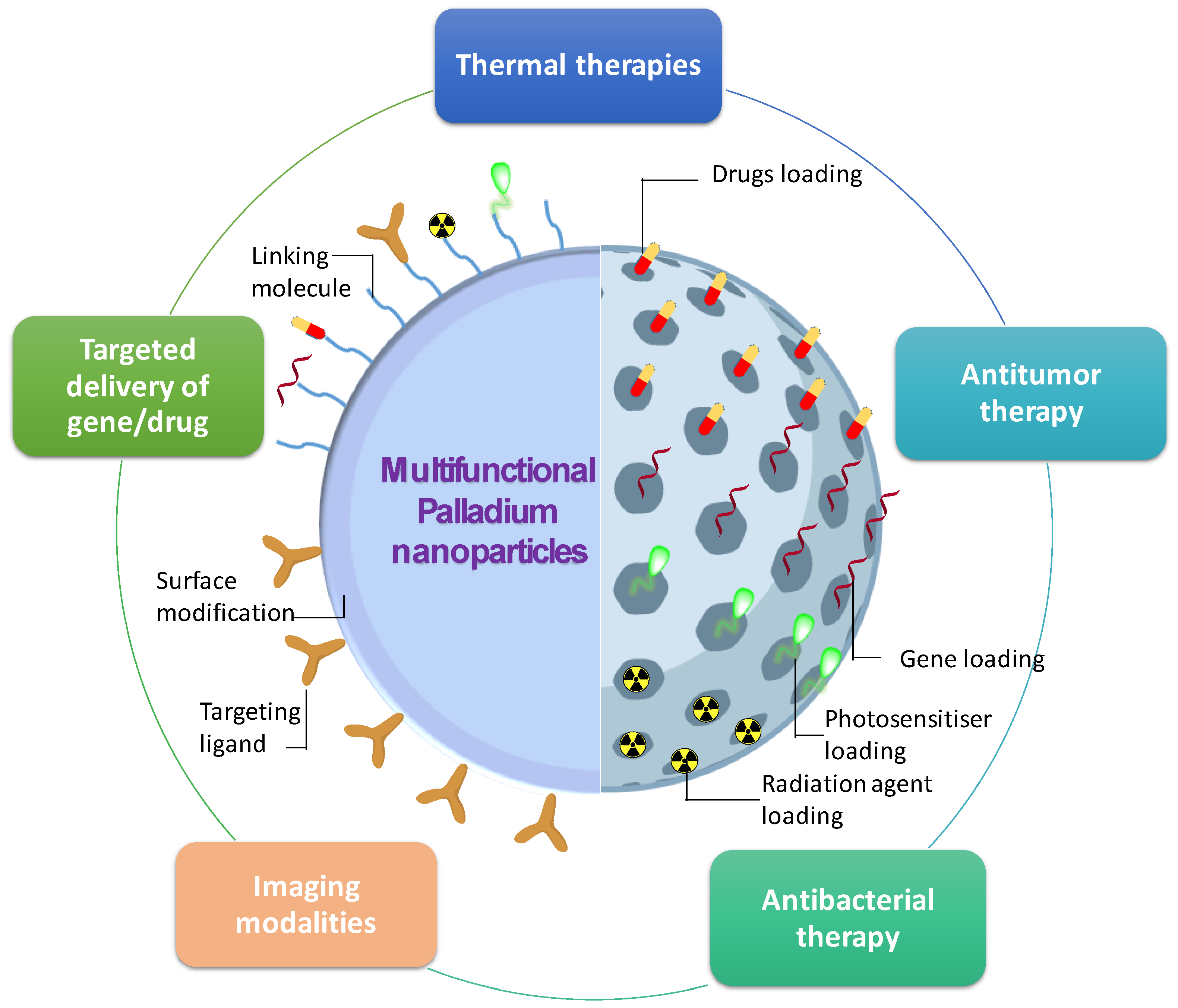
10. Multifunctional Palladium Nanoparticles
11. Toxicity, Pharmacokinetics, and Biodistribution of Palladium Nanoparticles
11.1. Toxicity
11.2. Pharmacokinetics
11.3. Biodistribution
12. Concluding Remarks and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Hyeon, T. Applications of inorganic nanoparticles as therapeutic agents. Nanotechnology 2014, 25, 012001. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic Functionalized Nanoparticles for Biomedical, Drug Delivery and Imaging Applications. Nanoscale Res. Lett. 2019, 14, 188. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.; Birla, S.; Yadav, A.; Santos, C. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2015, 19, 1–24. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.D.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Chen, A.; Ostrom, C. Palladium-Based Nanomaterials: Synthesis and Electrochemical Applications. Chem. Rev. 2015, 115, 11999–12044. [Google Scholar] [CrossRef]
- Saldan, I.; Semenyuk, Y.; Marchuk, I.; Reshetnyak, O. Chemical synthesis and application of palladium nanoparticles. J. Mater. Sci. 2015, 50, 2337–2354. [Google Scholar] [CrossRef]
- Cheong, S.; Watt, J.D.; Tilley, R.D. Shape control of platinum and palladium nanoparticles for catalysis. Nanoscale 2010, 2, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, T.; Miyake, M. Size Control of Palladium Nanoparticles and Their Crystal Structures. Chem. Mater. 1998, 10, 594–600. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Bui, N.Q.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Seo, H.; Phuoc, N.T.; Vy Phan, T.T.; Kim, H.; Lee, K.D.; et al. Multimodal tumor-homing chitosan oligosaccharide-coated biocompatible palladium nanoparticles for photo-based imaging and therapy. Sci. Rep. 2018, 8, 500. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Jiang, H.; Zhou, Y.; Liu, P.; Jia, Y.; Ye, C. Peptide-coated palladium nanoparticle for highly sensitive bioanalysis of trypsin in human urine samples. Nanomater. Nanotechnol. 2018, 8, 1847980418820391. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.-D.; Zhao, L.; Lin, M.; Sun, H.-Z.; Sun, H.-C.; Yang, B. Polypyrrole-coated flower-like Pd nanoparticles (Pd NPs@PPy) with enhanced stability and heat conversion efficiency for cancer photothermal therapy. RSC Adv. 2016, 6, 15854–15860. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef]
- Adams, B.D.; Chen, A. The role of palladium in a hydrogen economy. Mater. Today 2011, 14, 282–289. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Silva, S.F.; Coelho, L.; Frazao, O.; Santos, J.L.; Malcata, F.X. A Review of Palladium-Based Fiber-Optic Sensors for Molecular Hydrogen Detection. IEEE Sens. J. 2012, 12, 93–102. [Google Scholar] [CrossRef]
- Moreno-Mañas, M.; Pleixats, R. Formation of Carbon−Carbon Bonds under Catalysis by Transition-Metal Nanoparticles. Acc. Chem. Res. 2003, 36, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Mpungose, P.P.; Vundla, Z.P.; Maguire, G.E.M.; Friedrich, H.B. The Current Status of Heterogeneous Palladium Catalysed Heck and Suzuki Cross-Coupling Reactions. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Dumas, A.; Couvreur, P. Palladium: A future key player in the nanomedical field? Chem. Sci. 2015, 6, 2153–2157. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.-W.; Fan, S.-X.; Wang, F.; Sun, L.-D.; Zheng, X.-Y.; Yan, C.-H. Porous Pd nanoparticles with high photothermal conversion efficiency for efficient ablation of cancer cells. Nanoscale 2014, 6, 4345–4351. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.V.; Hoang, G.; Nguyen, V.T.; Nguyen, T.P.; Kim, H.H.; Mondal, S.; Manivasagan, P.; Moorthy, M.S.; Lee, K.D.; Junghwan, O. Chitosan as a stabilizer and size-control agent for synthesis of porous flower-shaped palladium nanoparticles and their applications on photo-based therapies. Carbohydr. Polym. 2019, 205, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Chen, M.; Sun, X.; Rong, P.; Zheng, N.; Chen, X. Palladium nanosheets as highly stable and effective contrast agents for in vivo photoacoustic molecular imaging. Nanoscale 2014, 6, 1271–1276. [Google Scholar] [CrossRef]
- Gil, Y.-G.; Kang, S.; Chae, A.; Kim, Y.-K.; Min, D.-H.; Jang, H. Synthesis of porous Pd nanoparticles by therapeutic chaga extract for highly efficient tri-modal cancer treatment. Nanoscale 2018, 10, 19810–19817. [Google Scholar] [CrossRef]
- Kang, S.; Shin, W.; Kang, K.; Choi, M.-H.; Kim, Y.-J.; Kim, Y.-K.; Min, D.-H.; Jang, H. Revisiting of Pd Nanoparticles in Cancer Treatment: All-Round Excellence of Porous Pd Nanoplates in Gene-Thermo Combinational Therapy. ACS Appl. Mater. Interfaces 2018, 10, 13819–13828. [Google Scholar] [CrossRef]
- Weiss, J.T.; Dawson, J.C.; Fraser, C.; Rybski, W.; Torres-Sánchez, C.; Bradley, M.; Patton, E.E.; Carragher, N.O.; Unciti-Broceta, A. Development and Bioorthogonal Activation of Palladium-Labile Prodrugs of Gemcitabine. J. Med. Chem. 2014, 57, 5395–5404. [Google Scholar] [CrossRef]
- Weiss, J.T.; Dawson, J.C.; Macleod, K.G.; Rybski, W.; Fraser, C.; Torres-Sánchez, C.; Patton, E.E.; Bradley, M.; Carragher, N.O.; Unciti-Broceta, A. Extracellular palladium-catalysed dealkylation of 5-fluoro-1-propargyl-uracil as a bioorthogonally activated prodrug approach. Nat. Commun. 2014, 5, 3277. [Google Scholar] [CrossRef]
- Azizi, S.; Mahdavi Shahri, M.; Rahman, H.S.; Rahim, R.A.; Rasedee, A.; Mohamad, R. Green synthesis palladium nanoparticles mediated by white tea (Camellia sinensis) extract with antioxidant, antibacterial, and antiproliferative activities toward the human leukemia (MOLT-4) cell line. Int. J. Nanomed. 2017, 12, 8841–8853. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Tiloke, C.; Phulukdaree, A.; Ranjan, B.; Chuturgoon, A.; Singh, S.; Gengan, R.M. Biosynthesis of palladium nanoparticles by using Moringa oleifera flower extract and their catalytic and biological properties. J. Photochem. Photobiol. B Biol. 2016, 165, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.P.; Walker, K.A.; Obare, S.O.; Docherty, K.M. Size-Dependent Antimicrobial Effects of Novel Palladium Nanoparticles. PLoS ONE 2014, 9, e85981. [Google Scholar] [CrossRef]
- Fang, G.; Li, W.; Shen, X.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.; Chai, Z.; Chen, C.; Ge, C.; Zhou, R. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat. Commun. 2018, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, D.P.; Hirsch, L.R.; Halas, N.J.; Payne, J.D.; West, J.L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic clinical imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Wu, D.; Huang, L.; Jiang, M.S.; Jiang, H. Contrast agents for photoacoustic and thermoacoustic imaging: A review. Int. J. Mol. Sci. 2014, 15, 23616–23639. [Google Scholar] [CrossRef]
- Huang, X.; Tang, S.; Mu, X.; Dai, Y.; Chen, G.; Zhou, Z.; Ruan, F.; Yang, Z.; Zheng, N. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotechnol. 2011, 6, 28–32. [Google Scholar] [CrossRef]
- Tang, S.; Chen, M.; Zheng, N. Sub-10-nm Pd nanosheets with renal clearance for efficient near-infrared photothermal cancer therapy. Small 2014, 10, 3139–3144. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Huynh, T.-C.; Oh, J. Photothermal Responsive Porous Membrane for Treatment of Infected Wound. Polymers 2019, 11, 1679. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tang, S.; Guo, Z.; Wang, X.; Mo, S.; Huang, X.; Liu, G.; Zheng, N. Core–Shell Pd@Au Nanoplates as Theranostic Agents for In-Vivo Photoacoustic Imaging, CT Imaging and Photothermal Therapy. Adv. Mater. 2014, 26, 8210–8216. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Tang, S.; Liu, P.; Fang, X.; Gong, J.; Zheng, N. Pd nanosheet-covered hollow mesoporous silica nanoparticles as a platform for the chemo-photothermal treatment of cancer cells. Small 2012, 8, 3816–3822. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, N.; He, L.; Liu, G.; Ling, D.; Su, X.; Sun, X. Porous hollow palladium nanoplatform for imaging-guided trimodal chemo-, photothermal-, and radiotherapy. Nano Res. 2017, 11. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.S.; Jin, Z.; Zhao, P.; Zhang, H.; Wen, Y.; He, Q. Porphyrin–palladium hydride MOF nanoparticles for tumor-targeting photoacoustic imaging-guided hydrogenothermal cancer therapy. Nanoscale Horiz. 2019, 4, 1185–1193. [Google Scholar] [CrossRef]
- Attar, A.; Altikatoglu Yapaoz, M. Biosynthesis of palladium nanoparticles using Diospyros kaki leaf extract and determination of antibacterial efficacy. Prep. Biochem. Biotechnol. 2018, 48, 629–634. [Google Scholar] [CrossRef]
- Bhakyaraj, K.; Kumaraguru, S.; Gopinath, K.; Sabitha, V.; Periyannan, K.; Karthika, V.; Arumugam, D.S.; Muthukumaran, U.; Jayakumar, K.; Mohan, S.; et al. Eco-Friendly Synthesis of Palladium Nanoparticles Using Melia azedarach Leaf Extract and Their Evaluation for Antimicrobial and Larvicidal Activities. J. Clust. Sci. 2016, 28. [Google Scholar] [CrossRef]
- Balbín, A.; Gaballo, F.; Ceballos-Torres, J.; Prashar, S.; Fajardo, M.; Kaluđerović, G.N.; Gómez-Ruiz, S. Dual application of Pd nanoparticles supported on mesoporous silica SBA-15 and MSU-2: Supported catalysts for C–C coupling reactions and cytotoxic agents against human cancer cell lines. RSC Adv. 2014, 4, 54775–54787. [Google Scholar] [CrossRef]
- Hiral, V.; Rahul, S.; Amanullakhan, P. Palladium Nanoparticles Mediated Through Bauhinia variegata: Potent In vitro Anticancer Activity Against MCF-7 Cell Lines and Antimicrobial Assay. Curr. Nanomater. 2018, 3, 168–177. [Google Scholar] [CrossRef]
- Shanthi, K.; Vimala, K.; Gopi, D.; Kannan, S. Fabrication of a pH responsive DOX conjugated PEGylated palladium nanoparticle mediated drug delivery system: An in vitro and in vivo evaluation. RSC Adv. 2015, 5, 44998–45014. [Google Scholar] [CrossRef]
- Yi, X.; Wu, Y.; Tan, G.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Wang, Z.; Pang, J.; Ning, C. Palladium nanoparticles entrapped in a self-supporting nanoporous gold wire as sensitive dopamine biosensor. Sci. Rep. 2017, 7, 7941. [Google Scholar] [CrossRef] [PubMed]
- Nikam, A.V.; Prasad, B.L.V.; Kulkarni, A.A. Wet chemical synthesis of metal oxide nanoparticles: A review. Cryst. Eng. Comm. 2018, 20, 5091–5107. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Sun, L.-D.; Xu, C.-H.; Wang, J.; Yu, J.C.; Yan, C.-H. Porous Single-Crystalline Palladium Nanoparticles with High Catalytic Activities. Angew. Chem. Int. Ed. 2012, 51, 4872–4876. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, L.-D.; Feng, W.; Chen, H.; Yeung, M.H.; Wang, J.; Yan, C.-H. Heteroepitaxial Growth of Core–Shell and Core–Multishell Nanocrystals Composed of Palladium and Gold. Small 2010, 6, 2566–2575. [Google Scholar] [CrossRef]
- Di, L.; Li, Z.; Zhang, X.; Wang, H.; Fan, Z. Reduction of supported metal ions by a safe atmospheric pressure alcohol cold plasma method. Catal. Today 2019, 337, 55–62. [Google Scholar] [CrossRef]
- Sauvageau, J.-F.; Turgeon, S.; Chevallier, P.; Fortin, M.-A. Colloidal Suspensions of Platinum Group Metal Nanoparticles (Pt, Pd, Rh) Synthesized by Dielectric Barrier Discharge Plasma (DBD). Part. Part. Syst. Charact. 2018, 35, 1700365. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Jiang, Z.; Xie, Z. Synthesis of size-controlled monodisperse Pd nanoparticles via a non-aqueous seed-mediated growth. Nanoscale Res. Lett. 2012, 7, 312. [Google Scholar] [CrossRef]
- Li, C.; Sato, T.; Yamauchi, Y. Size-controlled synthesis of mesoporous palladium nanoparticles as highly active and stable electrocatalysts. Chem. Commun. 2014, 50, 11753–11756. [Google Scholar] [CrossRef]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539–2544. [Google Scholar] [CrossRef]
- Díaz-Sánchez, M.; Díaz-García, D.; Prashar, S.; Gómez-Ruiz, S. Palladium nanoparticles supported on silica, alumina or titania: Greener alternatives for Suzuki–Miyaura and other C–C coupling reactions. Environ. Chem. Lett. 2019, 17, 1585–1602. [Google Scholar] [CrossRef]
- Thapa, R.; Poudel, K.; Soe, Z.; Ou, W.; Jeong, J.-H.; Jin, S.; Ku, S.; Choi, H.-G.; Lee, Y.; Yong, C.; et al. Palladium nanoparticle-decorated 2-D graphene oxide for effective photodynamic and photothermal therapy of prostate solid tumors. Coll. Surf. B Biointerfaces 2018, 169. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Zhang, L.; Xu, G. Shape-Controlled Synthesis of Single-Crystalline Palladium Nanocrystals. ACS Nano 2010, 4, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xia, Y. Shape-Controlled Synthesis of Metal Nanostructures: The Case of Palladium. Adv. Mater. 2007, 19, 3385–3391. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, M.; Xiong, Y.; Lim, B.; Xia, Y. Shape-Controlled Synthesis of Pd Nanocrystals and Their Catalytic Applications. Acc. Chem. Res. 2013, 46, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, S.; He, C.; Mo, S.; Wang, X.; Liu, G.; Zheng, N. Safety profile of two-dimensional Pd nanosheets for photothermal therapy and photoacoustic imaging. Nano Res. 2017, 10, 1234–1248. [Google Scholar] [CrossRef]
- Sugawa, K.; Tahara, H.; Yamashita, A.; Otsuki, J.; Sagara, T.; Harumoto, T.; Yanagida, S. Refractive Index Susceptibility of the Plasmonic Palladium Nanoparticle: Potential as the Third Plasmonic Sensing Material. ACS Nano 2015, 9, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Bui, N.Q.; Moorthy, M.S.; Lee, K.D.; Oh, J. Synthesis and In Vitro Performance of Polypyrrole-Coated Iron-Platinum Nanoparticles for Photothermal Therapy and Photoacoustic Imaging. Nanoscale Res. Lett. 2017, 12, 570. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Nguyen, V.T.; Ahn, S.-H.; Oh, J. Chitosan-mediated facile green synthesis of size-controllable gold nanostars for effective photothermal therapy and photoacoustic imaging. Eur. Polym. J. 2019, 118, 492–501. [Google Scholar] [CrossRef]
- Gai, S.; Yang, G.; Yang, P.; He, F.; Lin, J.; Jin, D.; Xing, B. Recent advances in functional nanomaterials for light–triggered cancer therapy. Nano Today 2018, 19, 146–187. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Plasmonic photo-thermal therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-M.; Conde, J.; Lipinski, T.; Bednarkiewicz, A.; Huang, C.-C. Revisiting the classification of NIR absorbing/emitting nanomaterials for in vivo bio-applications. NPG Asia Mater. 2016, 8. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S. Bioimaging: Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yao, J.; Wang, L.V. Photoacoustic tomography: Principles and advances. Electromagn. Waves 2014, 147, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Yeager, D.; Emelianov, S.Y. Chapter 9—Photoacoustic Imaging for Cancer Diagnosis and Therapy Guidance. In Cancer Theranostics; Chen, X., Wong, S., Eds.; Academic Press: Oxford, UK, 2014; pp. 139–158. [Google Scholar] [CrossRef]
- Akchurin, G.; Khlebtsov, B.; Akchurin, G.; Tuchin, V.; Zharov, V.; Khlebtsov, N. Gold nanoshell photomodification under a single-nanosecond laser pulse accompanied by color-shifting and bubble formation phenomena. Nanotechnology 2007, 19, 015701. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, A.F.; Hassan, H.H. Antimicrobial and antitumor activity of platinum and palladium complexes of novel spherical aramides nanoparticles containing flexibilizing linkages: Structure-property relationship. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 103, 232–245. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, A.; Khan, A.; Zhang, J.; Raza, M.; Rahman, A.U.; Tariq, M.; Ali Khan, U.; Zada, S.; Yuan, Q. Palladium nanoparticles synthesis, characterization using glucosamine as the reductant and stabilizing agent to explore their antibacterial & catalytic applications. Microb. Pathog. 2018, 125, 150–157. [Google Scholar] [CrossRef]
- Cheng, H.; Xie, Y.; Villalobos, L.F.; Song, L.; Peinemann, K.-V.; Nunes, S.; Hong, P.-Y. Antibiofilm effect enhanced by modification of 1,2,3-triazole and palladium nanoparticles on polysulfone membranes. Sci. Rep. 2016, 6, 24289. Available online: https://www.nature.com/articles/srep24289#supplementary-information (accessed on 16 November 2019). [CrossRef]
- Ali, G.W.; El-Hotaby, W.; Hemdan, B.; Abdel-Fattah, W.I. Thermosensitive chitosan/phosphate hydrogel-composites fortified with Ag versus Ag@Pd for biomedical applications. Life Sci. 2018, 194, 185–195. [Google Scholar] [CrossRef]
- Petrarca, C.; Clemente, E.; Di Giampaolo, L.; Mariani-Costantini, R.; Leopold, K.; Schindl, R.; Lotti, L.V.; Mangifesta, R.; Sabbioni, E.; Niu, Q.; et al. Palladium Nanoparticles Induce Disturbances in Cell Cycle Entry and Progression of Peripheral Blood Mononuclear Cells: Paramount Role of Ions. J. Immunol. Res. 2014, 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kim, E.; Han, J.W.; Park, J.H.; Kim, J.H. Green Chemistry Approach for Synthesis of Effective Anticancer Palladium Nanoparticles. Molecules 2015, 20, 22476–22498. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Mao, K.; Ye, X.; Yan, W.; Huang, Y.; Wang, J.; Fu, Y.; Wang, X.; Wu, X.; Xie, Y.; et al. Surface Facet of Palladium Nanocrystals: A Key Parameter to the Activation of Molecular Oxygen for Organic Catalysis and Cancer Treatment. J. Am. Chem. Soc. 2013, 135, 3200–3207. [Google Scholar] [CrossRef] [PubMed]
- Yusop, R.M.; Unciti-Broceta, A.; Johansson, E.M.V.; Sánchez-Martín, R.M.; Bradley, M. Palladium-mediated intracellular chemistry. Nat. Chem. 2011, 3, 239–243. [Google Scholar] [CrossRef]
- Miao, P.; Sheng, S.; Sun, X.; Liu, J.; Huang, G. Lactate dehydrogenase a in cancer: A promising target for diagnosis and therapy: LDHA in Cancer. IUBMB Life 2013, 65, 904–910. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Yun, Y.H.; Lee, B.K.; Park, K. Controlled Drug Delivery: Historical perspective for the next generation. J. Control. Release 2015, 219, 2–7. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef]
- Muhale, F.A.; Wetmore, B.A.; Thomas, R.S.; McLeod, H.L. Systems pharmacology assessment of the 5-fluorouracil pathway. Pharmacogenomics 2011, 12, 341–350. [Google Scholar] [CrossRef]
- Clavadetscher, J.; Indrigo, E.; Chankeshwara, S.V.; Lilienkampf, A.; Bradley, M. In-Cell Dual Drug Synthesis by Cancer-Targeting Palladium Catalysts. Angew. Chem. 2017, 56, 6864–6868. [Google Scholar] [CrossRef]
- Destito, P.; Sousa-Castillo, A.; Couceiro, J.R.; López, F.; Correa-Duarte, M.A.; Mascareñas, J.L. Hollow nanoreactors for Pd-catalyzed Suzuki–Miyaura coupling and O-propargyl cleavage reactions in bio-relevant aqueous media. Chem. Sci. 2019, 10, 2598–2603. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Du, Z.; Ren, J.; Qu, X. Designed heterogeneous palladium catalysts for reversible light-controlled bioorthogonal catalysis in living cells. Nat. Commun. 2018, 9, 1209. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Gade, A.; Gaikwad, S.; Marcatob, P.; Duran, N. Biomedical Applications of Nanobiosensors: The State-of-the-Art. J. Braz. Chem. Soc. 2012, 23, 14–24. [Google Scholar] [CrossRef]
- Soleymani, L.; Fang, Z.; Sargent, E.H.; Kelley, S.O. Programming the detection limits of biosensors through controlled nanostructuring. Nat. Nanotechnol. 2009, 4, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.C.; Gladwin, M.T. The hydrogen highway to reperfusion therapy. Nat. Med. 2007, 13, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Nakashima-Kamimura, N.; Mikami, T.; Ohsawa, I.; Ohta, S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology 2009, 34, 501–508. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, C.; Zhang, J.; Gao, F.; Li, B.; Chuai, Y.; Liu, C.; Cai, J. Hydrogen protects mice from radiation induced thymic lymphoma in BALB/c mice. Int. J. Biol. Sci. 2011, 7, 297–300. [Google Scholar] [CrossRef]
- Griessen, R.; Strohfeldt, N.; Giessen, H. Thermodynamics of the hybrid interaction of hydrogen with palladium nanoparticles. Nat. Mater. 2016, 15, 311–317. [Google Scholar] [CrossRef]
- Wilkinson, K.E.; Palmberg, L.; Witasp, E.; Kupczyk, M.; Feliu, N.; Gerde, P.; Seisenbaeva, G.A.; Fadeel, B.; Dahlén, S.-E.; Kessler, V.G. Solution-Engineered Palladium Nanoparticles: Model for Health Effect Studies of Automotive Particulate Pollution. ACS Nano 2011, 5, 5312–5324. [Google Scholar] [CrossRef]
- Leso, V.; Iavicoli, I. Palladium Nanoparticles: Toxicological Effects and Potential Implications for Occupational Risk Assessment. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Iavicoli, I.; Fontana, L.; Leso, V.; Corbi, M.; Marinaccio, A.; Leopold, K.; Schindl, R.; Lucchetti, D.; Calapà, F.; Sgambato, A. Subchronic exposure to palladium nanoparticles affects serum levels of cytokines in female Wistar rats. Hum. Exp. Toxicol. 2017, 37, 309–320. [Google Scholar] [CrossRef] [PubMed]
| Pd-Based Materials | Surface Modification | Size | Shape | Potential Biomedical Applications | Ref. |
|---|---|---|---|---|---|
| Pd nanosheets | 28, 46 and 60 nm | Ultrathin hexagon | Photothermal therapy | [39] | |
| Pd nanosheets | Reduced glutathione | <10 nm | Hexagon | Photothermal therapy | [40] |
| PdNPs | Methoxy-terminated PEG–thiol | ≤30 nm | Porous architecture | Photothermal therapy | [24] |
| PdNPs | Cancer cell targeting RGD peptide | 22.26 ± 0.97 nm | Porous architecture | Photothermal therapy | [13] |
| Photoacoustic imaging | |||||
| PdNPs embedded in chitosan/polyvinyl alcohol membrane | 30.2 ± 17.2 nm | Flower-like shape | Photothermal therapy | [41] | |
| Wound healing | |||||
| Pd nanosheets | 16 nm | Hexagon | Photoacoustic imaging | [26] | |
| Pd@gold nanoplates | Thiol-polyethylene glycol | Diameter: 30 nm | Hexagon | Photoacoustic imaging | [42] |
| Thickness: 4 nm | Computed tomography | ||||
| Pd nanosheets covered hollow mesoporous silica nanoparticles | 3-aminopropyltrimethoxysilane | 170 nm | sphere | Photothermal therapy | [43] |
| Drug delivery | |||||
| PdNPs (carrying cancer drug and radiation agent) | 58 ± 4 nm | Porous hollow nanoplatforms | Photothermal therapy | [44] | |
| Drug delivery | |||||
| Radiotherapy | |||||
| Hydrogenated porphyrin-Pd-organic framework nanoparticles | 93 nm | Framework architecture | Photoacoustic imaging | [45] | |
| Hydrogenothermal therapy | |||||
| PdNPs | 2.0 ± 0.1 nm, 2.5 ± 0.2 nm, and 3.1 ± 0.2 nm. | Sphere | Antibacterial therapy | [33] | |
| Pd nanocrystals | ~10 nm | Cube Octahedron | Antibacterial therapy | [34] | |
| PdNPs | 98 ± 36 nm | Sphere | Antibacterial therapy | [46] | |
| Pd@White tea NPs (synthesized by Camellia sinensis leaf extract) | 6 nm to 18 nm | Sphere | Antibacterial therapy | [31] | |
| Anticancer therapy | |||||
| PdNPs (synthesized by Melia azedarach leaf extract) | 10 nm to 20 nm | Sphere | Antibacterial therapy | [47] | |
| Larvicidal activities | |||||
| Pd NPs supported on mesoporous silica (SBA-15–Pd and MSU-2–Pd) | 29 ± 9 nm for SBA-15–Pd | Sphere | Anticancer therapy | [48] | |
| 28 ± 5 nm for MSU-2–Pd | |||||
| PdNPs (synthesized by Bauhinia variegata bark extract) | 2.87 nm | Cube | Anticancer therapy | [49] | |
| PdNPs (synthesized by biomass waste petal of Moringa oleifera) | 10 nm to 50 nm | Sphere | Anticancer therapy | [32] | |
| PdNPs | 80 nm to 100 nm | Porous architecture | Gene delivery | [28] | |
| PdNPs (synthesized by chaga mushroom (Inonotus obliquus) extract) | ~100 nm | Porous architecture | Photothermal therapy | [27] | |
| Anticancer therapy | |||||
| Drug delivery | |||||
| PdNPs | PEG-hydrazide | 17 ± 2 nm | Sphere | Drug delivery | [50] |
| Heterogeneous Pd0 resin | ~150 µm | Sphere | Prodrug activation (5-fluorouracil and gemcitabine) | [29,30] | |
| PdNPs into self-supporting nanoporous gold wire | 10 nm | Nanowires | Biosensors (Dopamine detection) | [51] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, T.T.V.; Huynh, T.-C.; Manivasagan, P.; Mondal, S.; Oh, J. An Up-To-Date Review on Biomedical Applications of Palladium Nanoparticles. Nanomaterials 2020, 10, 66. https://doi.org/10.3390/nano10010066
Phan TTV, Huynh T-C, Manivasagan P, Mondal S, Oh J. An Up-To-Date Review on Biomedical Applications of Palladium Nanoparticles. Nanomaterials. 2020; 10(1):66. https://doi.org/10.3390/nano10010066
Chicago/Turabian StylePhan, Thi Tuong Vy, Thanh-Canh Huynh, Panchanathan Manivasagan, Sudip Mondal, and Junghwan Oh. 2020. "An Up-To-Date Review on Biomedical Applications of Palladium Nanoparticles" Nanomaterials 10, no. 1: 66. https://doi.org/10.3390/nano10010066
APA StylePhan, T. T. V., Huynh, T.-C., Manivasagan, P., Mondal, S., & Oh, J. (2020). An Up-To-Date Review on Biomedical Applications of Palladium Nanoparticles. Nanomaterials, 10(1), 66. https://doi.org/10.3390/nano10010066