1. Introduction
Owing to the environmental issues with the state-of-the-art piezoelectric materials based on lead zirconate titanate and other lead containing compounds, potassium sodium niobate (K
0.5Na
0.5NbO
3, KNN) has become an extensively investigated system [
1] due to its elevated Currie temperature (
TC), up to 420 °C, and high longitudinal piezoelectric coefficient (
d33) reported by Saito et al. on KNN-based ceramics in 2004 [
2]. Since that time, an increasing interest has been focused on KNN-based bulk materials [
1,
2,
3,
4] as possible alternatives in various applications, such as sensors, actuators, energy harvesting and microelectromechanical systems (MEMS) [
5]. Considering that, numerous efforts have been made by researchers to fabricate KNN and KNN-based thin films due to their potential applications in sensor and actuator micro-devices, as well as miniaturized MEMS [
6]. However, the reported piezoelectric performance for KNN-based thin films is significantly inferior compared to that of bulk KNN-based materials mainly due to the volatility of alkaline elements, composition deviation from stoichiometry, issues related to the formation of secondary phases, and high leakage current density [
6].
Certain advances in the electrical properties of KNN-based thin films have been observed upon the addition of K and/or Na excess in the precursors of the sol–gel derived KNN thin films [
6,
7,
8]. In particular, Ahn et al. reported that when the solution contained 20% alkali-excess, the preferentially (100) oriented films revealed the best electrical properties [
7]. The 250 nm thick films were prepared from solutions with 0.1 M concentration, performing final heating by insertion into furnace at 700 °C for 30 min. Further, Kupec et al. have shown that the optimal electrical response can be observed at only 5% excess of potassium, when the 250 nm thick films are prepared from a solution with 0.4 M concentration and annealed at 750 °C for 5 min using rapid thermal annealing (RTA) [
8].
However, there has been no systematic study presenting solely the solution concentration effect on the properties of KNN thin films. Moreover, although such studies have been reported for 1 µm thick Pb(Zr
0.52Ti
0.48)O
3 (PZT) films on Pt(111)/Ti/SiO
2/Si(100) [
9] and 400 nm thick 0.755Bi
0.5Na
0.5TiO
3–0.065BaTiO
3–0.18SrTiO
3 (BNT–BT–ST) films on LaNiO
3(100) buffered Pt(111)/Ti/SiO
2/Si substrates [
10], their results are rather contradictive. The PZT films were found to be more (111) textured with increasing solution concentration that resulted in an enhanced piezoresponse [
9]. In contrast, higher piezoresponse was reported for (100) oriented BNT–BT–ST films obtained from 0.1 M solution compared to that for randomly oriented films prepared from the solution with a higher concentration of 0.2 M [
10]. Therefore, each material can have its own solution concentration—texturing—piezoresponse relationship that has to be established.
Furthermore, in our recent work we have shown that substrates, used for the film deposition, also have a determining role on the final properties of the films [
11]. Thus, platinized Si, mainly reported as substrate for KNN [
6,
7,
8] and other piezoelectric films [
9,
10], has been shown to induce large tensile residual stress in sol–gel derived undoped KNN films due to thermal expansion mismatch, thus diminishing out-of-plane dielectric, ferroelectric, and piezoelectric response of the films [
11]. On the other hand, platinized SrTiO
3 substrates, possessing much larger thermal expansion coefficient (TEC), have been shown to induce residual compressive stress in KNN films, significantly enhancing out-of-plane permittivity, polarization, and piezoresponse [
11]. Therefore, to study only the influence of the solution conditions on the properties of polycrystalline KNN thin films, minimizing the effect of the substrate, one should choose a substrate with similar TEC. In the case of KNN, sapphire (Al
2O
3) has very similar thermal expansion properties. Nevertheless, sol–gel derived KNN thin films deposited on platinized sapphire have not yet been studied. To the best of our knowledge, there was just a report on over 10 µm thick films of KNN with complex perovskite materials, aerosol deposited on platinized sapphire [
12], and a recent article on 510 nm thick KNN films, pulsed laser deposited on sapphire without a Pt layer [
13].
In this work and within this context, crack-free KNN thin films are prepared by chemical solution deposition from 0.2 and 0.4 M precursor solution with 5% solely potassium excess and 20% alkali (potassium and sodium) excess on platinized sapphire substrates, being then annealed using RTA. Performing atomic force microscopy (AFM), scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) analyses in combination with X-ray diffraction, dielectric spectroscopy and piezo-force microscopy (PFM) characterization, a correlation between the electrical properties in the parallel plate capacitor geometry, piezoresponse and the solution conditions for KNN films deposited on platinized Al2O3 was found.
3. Results and Discussion
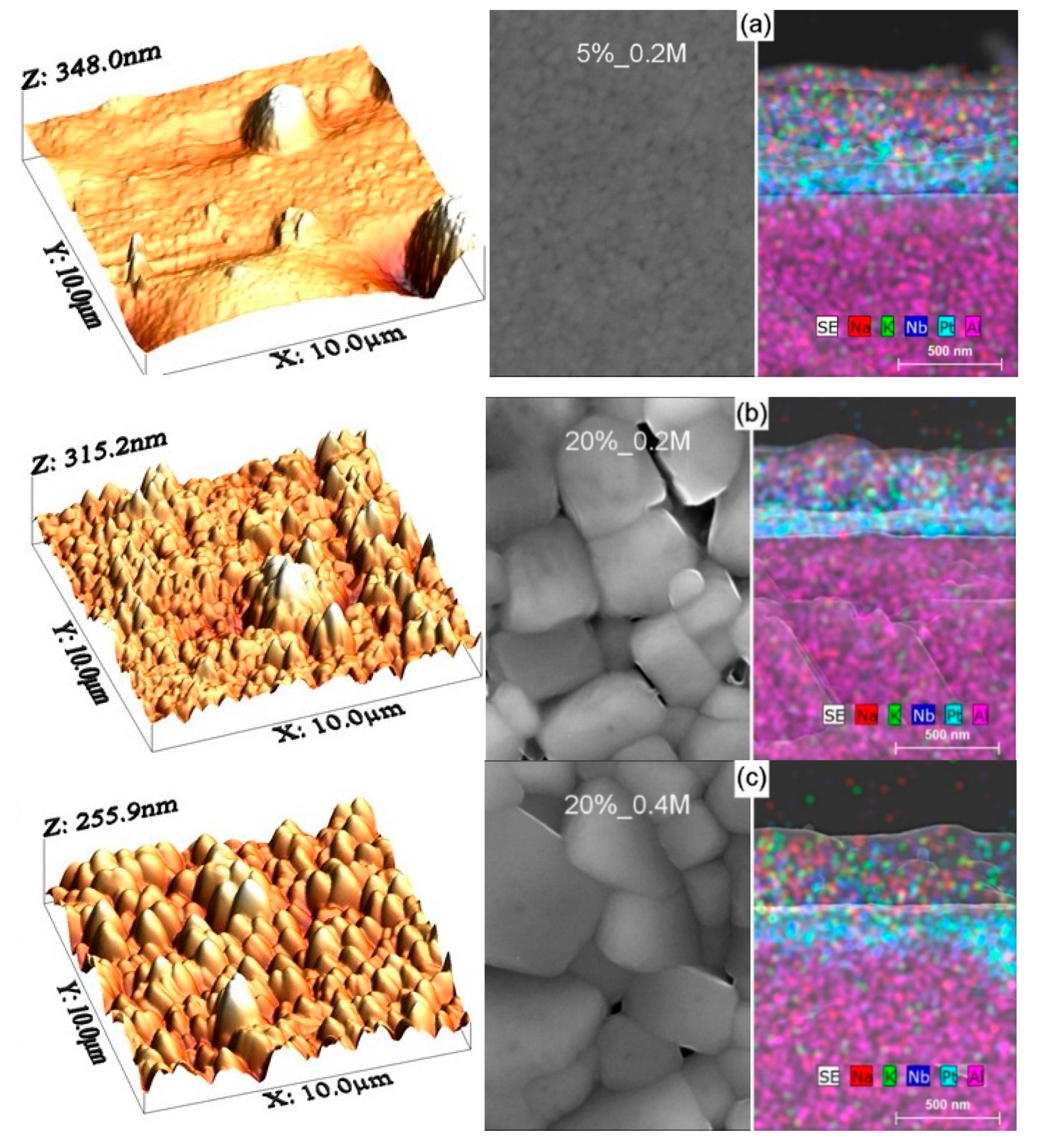
Figure 1a–c shows the typical AFM surface morphology as well as SEM top-view and cross-sectional microstructures of KNN thin films deposited on platinized Al
2O
3 substrates from three solutions under study. The surface roughness, obtained from the AFM data, as well as the average lateral grain size and the film thickness, deduced from the field emission SEM analysis, are presented in
Table 1. In particular, the
Table 1 shows that root mean square (RMS) values of surface roughness in a 10 µm × 10 µm area of 37 ± 2 nm for all three kinds of the KNN films under study. In the case of 5%_0.2 M films, however, the roughness in a 2 µm × 2 µm area is as low as 3 nm, but some holes and hills of µm size result in the average roughness of 39 nm for the 10 µm × 10 µm area.
No peelings or cracks were found at the dense film surfaces both at AFM (left part of
Figure 1) and SEM top-view images (central part of
Figure 1). At the same time, there is an evident lateral grain size variation from ~110 nm in average for 5%_0.2 M films to ~480 and ~600 nm for 20%_0.2 M and 20%_0.4 M films, respectively, as also indicated in
Table 1. Thus, besides a strong increase of the grain size with the alkali excess amount observed previously [
7,
8] there is also slight grain size increase with the solution concentration.
The right part of
Figure 1 shows simultaneously the SEM cross-sectional microstructure and EDS elemental maps of the KNN thin films grown on platinized Al
2O
3 substrates using three different alkali excess and/or concentration precursor solutions (for separated elemental maps and SEM cross-sectional microstructure see
Figures S1–S3 in Supplementary Materials). As expected, the average film thickness of 340 ± 10 nm is similar for all three kinds of the films, whereas elemental maps give a possibility for clear determination of the KNN films consisting of Na, K, and Nb oxides, the Pt electrode layer and the Al oxide substrate. Moreover, for 5%_0.2 M films, there are several grains across the film thickness, whereas for 20%_0.2 M and 20%_0.4 M films, some regions with single grains across the film thickness are seen in agreement with the lateral grain size variation.
Further, the EDS analysis was performed on KNN thin films to estimate their chemical composition. Typical energy dispersive spectra are depicted in
Figure 2, clearly presenting Na, Nb and K peaks. In the case of 20%_0.4 M films, there is also a separated peak of Al, possibly from the sample holder. The relative elemental content of K, Na, and Nb in the 4 representative spots and in average for the investigated KNN films are plotted in inset of
Figure 2, and the derived (K + Na)/Nb ratio is presented in
Table 1.
The results of the EDS analysis of all the KNN films reveal that the contents of K, Na, and Nb deviate for a few atomic percent from the respective stoichiometric values, which in relation to Nb content are 50 at % of K, 50 at % of Na, and 100 at % of Nb. In average, the films demonstrate some excess of alkali content in relation to that of Nb (shown also in
Table 1) and a slightly higher content of Na compared to K with regard to the stoichiometric KNN composition values. The average values of K and Na fraction in relation to that of Nb in 5%_0.2M films are 42 ± 4 and 59 ± 3 at %, respectively. These values are well comparable to those of 45 ± 6 and 56 ± 6 at %, obtained for K and Na, respectively, in KNN films deposited on platinized Si substrates from solutions with 5% excess of potassium and 0.4 M concentration by Kupec et al. [
8]. However, since in both our and cited cases, the films were prepared from K-rich solutions, while Na content cannot be higher than 50 at %, the observed Na fraction > 50 at % and thereby Na/K ratio > 1 cannot be explained other than by some overestimation of Na and/or underestimation of K content during the EDS fitting treatment. Despite that, we can still do some comparison between the films under study.
Thus, the films with 20% of alkali excess contain in average a larger fraction of K but pretty much the same fraction of Na, compared to 5%_0.2M films, that results in an increase of the (Na + K)/Nb ratio from 1.01 to 1.08 in the case of 20%_0.2M and to 1.07 in the case of 20%_0.4M films. Namely, 20%_0.2 M films possess 50 ± 4 at % of K and 58 ± 3 at % of Na, while the average values for K and Na in 20%_0.4 M films are equal to 51 ± 4 at % and 56 ± 3 at %, respectively. Therefore, from the absolute values, one could conclude that in both these films the most volatile element is K, of which content in the films is lower. However, taking into account that compared to the EDS analysis of the 5%_0.2 M films the excess of Na gives no increment in the Na content within the 20%_0.2M and 20%_0.4M films in contrast to K excess, the most volatile element is evidently Na. It is interesting to note that the (K + Na)/Nb ratio of 1.07 was also obtained by Kupec et al. [
8] in KNN films with 10 mol % K- or 10 mol % Na-initial-excess, although such excess should not give the ratio value above 1.05. Overall, KNN films prepared by us from the solutions with 5% K excess revealed a decrease of the (K + Na)/Nb ratio from an initial 1.025 in the solution to 1.01 in the films. An alkali excess as high as 20 mol % of K and Na exhibited larger alkali losses, diminishing the ratio from 1.2 in the solution to 1.07–1.08 in the films.
The increased alkali to niobium ratio could not, however, be identified by XRD, as seen from
Figure 3, showing X-ray diffraction patterns of KNN thin films, prepared from the solutions with different amounts of alkali excess and/or concentrations. Besides the peak of the substrate Pt layer, only the perovskite phase peaks could be observed. Therefore, the alkali excess incorporates into the films either in the form of the amorphous alkali-rich phase or structural defects like the Ruddlesden–Popper (RP) structure in Sr-rich SrTiO
3 [
15].
The degree of the preferred orientation in the thin film samples was quantitated by the Lotgering factor (F), which ranges from 0 for a randomly oriented phase to 1 for a perfectly oriented phase [
16]. The Lotgering factor for (100) preferential orientation
F(100) was calculated as
where
and
with
and
being the intensities of (
hkl) peaks for the textured and randomly oriented KNN thin films, respectively.
Table 1 shows that there is no clear preferred orientation in the 5%_0.2 M films with an
F(100) value of just 0.13. (100) preferential orientation appears, however, with the increasing amount of alkali excess in the solution, when
F(100) value increases to 0.39 for 20%_0.2 M films. Moreover, when the solution concentration is doubled there is even higher growth of
F(100) to 0.72 for 20%_0.4 M films. Such high value indicates a significant texturing of these films along (100) crystallographic direction. The observed (100) preferential orientation for the films with high alkali excess is in agreement with other reports on alkali niobate thin films [
6,
7,
8], although its increase with the solution concentration was not reported so far.
To have more complete characterization of the texture quality of KNN films deposited on platinized Al
2O
3 substrates, X-ray pole figure measurements, which reflect the preferred orientation, of the crystal lattice in the material, were performed at the fixed 2θ angle of (100) peak, as shown in the inserted illustrations of
Figure 3. For the KNN thin film with 20% alkali excess, the projected intensities are all grouped in a central circle near a tilt angle of 0°. It is an obvious indication that these films are oriented along (100), as XRD patterns suggested. In contrast, KNN thin films with 5% potassium excess and 0.2 M concentration, pole figures show the most diffuse distribution of the lines indicating absence of the preferred crystallographic orientation along (100).
The dielectric properties of the 5%_0.2 M, 20%_0.2 M, and 20%_0.4 M films grown on platinized Al
2O
3 and measured at room temperature as a function of frequency (10
2–10
6 Hz) are shown in
Figure 4. All the films show similar behavior of the relative dielectric permittivity ε
r (
Figure 4a), that is, a decrease when the frequency increases. However, for 5%_0.2 M films, the decrease is much stronger than for 20 mol % alkali excess films. Such high permittivity variation looks to be supported by two dielectric relaxations, which can be determined in the dissipation factor tanδ variation with frequency (
Figure 4b).
The relative permittivity and dissipation factor values of the films at 10 kHz are listed in
Table 1. These results show the highest ε
r and lowest tanδ for 20%_0.2 M films. Both decrease of the alkali excess content and increase of the solution concentration suppress the permittivity and enhance the dissipation factor. Films prepared from solution with just 5% excess of potassium show lower relative permittivity values at 10 kHz and stronger dielectric relaxation, comparing to that of 20%_0.2 M, due to significantly smaller grain size and possible Maxwell–Wagner-type polarization mechanism observed in the low frequency range at room temperature, respectively. In the case of the solution concentration variation, the observed variation of the dielectric response can be explained in terms of a higher (100) orientation degree of 20%_0.4 M thin films with respect to 20%_0.2 M. Indeed, some decrease of the dielectric permittivity and increase of the dissipation factor was reported for (100)-oriented KNN-based films in comparison to (110)- and (111)-oriented ones by Chen et al. [
17]. Therefore, these results, although not optimized so far, indicate that there is a competition between the grain size and (100) orientation effects on the dielectric response of KNN thin films. In addition, the permittivity values of our films at 10 kHz are lower than those of 300–600 observed by Kupec et al. [
8], perhaps due to higher porosity of our films. However, the reduced permittivity is not a drawback but an advantage of the materials for piezoelectric applications, if their piezoelectric properties are not suppressed [
18].
The piezoelectric properties of the KNN films estimated using PFM are presented in
Figure 5, giving clear qualitative comparison for the films with similar thickness and measured at the same conditions. All the curves exhibit typical hysteresis loops, indicating the presence of piezoelectricity at least at local scale. However, the shape of the loops is more diffused in the case of 5%_0.2 M films, whereas for 20%_0.2 M and 20%_0.4 M films it is more quadratic-like. Despite of some offset, the coercive field can be estimated as about 3 times lower in the case of 20%_0.2 M and 20%_0.4 M films comparing to 5%_0.2 M films. Regarding the piezoelectric coefficient, it continuously increases from 5%_0.2 M to 20%_0.2 M and further to 20%_0.4 M films, being thus in correlation with
F(100) variation. An increase of the local piezoresponse with (100) orientation was also reported by Chen et al. [
17].
It is known that the ferroelectric and electromechanical response of a material is directly related to its polar domain structure. In ferroelectric KNN thin films, energy minimization results in multiple domains, separated by domains walls. These domain walls have well-defined orientations that minimize energy by maintaining compatibility of strain and polarization across the wall. The ferroelectric domains could adopt 60°, 90°, 120°, and 180° domain walls, as in the case of KNbO
3 and KNN [
19,
20]. Thus, the domain structures dictate the effective properties of the crystals.
Figure 6 shows topography and out-of-plane PFM signal taken on KNN thin films deposited on platinized Al
2O
3 substrates from solutions with 5% potassium excess and 0.2 M concentration. As the topography (
Figure 6a) presents very small grains, domains with out-of-plane component of polarization are also small but numerous, as seen in
Figure 6b. The dark regions in the piezo-response images represent the domains with polarization oriented towards substrate, and bright regions to domains with polarization oriented to the film surface.
Figure 7 shows topography and the out-of-plane PFM signal taken on KNN thin film deposited on platinized Al
2O
3 substrates using solution with 20% alkali excess and 0.2 M concentration. In this case, the contrast and domain size are higher than in the case of 5%_0.2M films, while domain number is lower.
Figure 8 shows the topography, piezo-response image, and simultaneous cross-sections of topography and PFM, taken on KNN thin films deposited on platinized Al
2O
3 substrate from solutions with 20% alkali excess and 0.4 M concentration. These films exhibit as some well-defined relatively large domains as nanodomains. The latter can be responsible for the enhanced piezoresponse [
17]. These nanodomains are circled in
Figure 8b, while other similar features corresponding to the horizontal lines in the topography (
Figure 8a) should be artefacts of the PFM. The domains are separated by exactly 180° domain walls, as shown in a scheme also presented in
Figure 8, with a wavy shape, characteristic to this type of the domain walls [
19]. The domain length in the PFM image ranges between 500 and 750 nm and the average domain width is about 100 nm, as can be seen from the domain profile not corresponding to that of topography, as shown in
Figure 8c. The observation of a 180° nanodomain structure in these films should be related with their high (100) texture and submicron grain size. Thus, very regular 180° domain structures was observed in (100) KNN single crystals, comparing to that of polycrystalline KNN [
20], although the domain size was much larger than in our thin films likely due the larger crystallite size.
Although we aware that local effective piezoelectric coefficient values measured in different PFM experiments cannot be compared directly as they are highly correlated with the tip-sample contact stiffness [
21] and therefore many authors of the PFM studies on KNN-based films did not even report the d*
33 values [
17,
22,
23], we had to estimate these values from our PFM data at least for rough comparison with those reported for KNN and other piezoelectric materials. For that, we used identical PFM measurements of a PZT thin film with known d
33 of 160 pm/V, as a reference, and obtained values of 21, 95, and 124 pm/V for 5%_0.2 M, 20%_0.2 M, and 20%_0.4 M KNN films, respectively. The former value is just slightly larger than that of 17 pm/V for pristine KNN film [
24], whereas two latter values are lower than that of 160 pm/V (pC/N) for KNN single crystals [
20] but comparable with or even higher than that of 110 pm/V (pC/N) for conventionally sintered KNN ceramics [
25]. Moreover, they are significantly higher than the values of 40–74 pm/V reported for other KNN-based films [
24,
26,
27] and are comparable with the values of 110–140 pm/V reported for BNT–BT–ST films [
10]. However, in contrast to the BNT–BT–ST films [
10], the higher piezoresponse and (100) preferential orientation were observed in our KNN films obtained from solutions with higher concentration. Such relationship is more similar to that of PZT films, reported to possess d
33 increasing from ~100 to ~160 pC/m with increasing solution concentration and texturing [
9]. However, in that case, the texturing was along (111) but not (100) direction.
Thus, we have shown that 20%_0.4 M KNN films with the more promising piezoresponse and reduced εr, compared to the films from solutions with lower concentration and/or alkali excess. These films derived by low-cost sol–gel method and deposited on well thermally matching platinized sapphire substrates, can be considered for piezoelectric applications.