Two-Step Solvothermal Synthesis of (Zn0.5Co0.5Fe2O4/Mn0.5Ni0.5Fe2O4)@C-MWCNTs Hybrid with Enhanced Low Frequency Microwave Absorbing Performance
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of Zn0.5Co0.5Fe2O4 NPs, Zn0.5Co0.5Fe2O4/Mn0.5Ni0.5Fe2O4 and (Zn0.5Co0.5Fe2O4/Mn0.5Ni0.5Fe2O4)@C-MWCNTs hybrids
2.3. Characterization
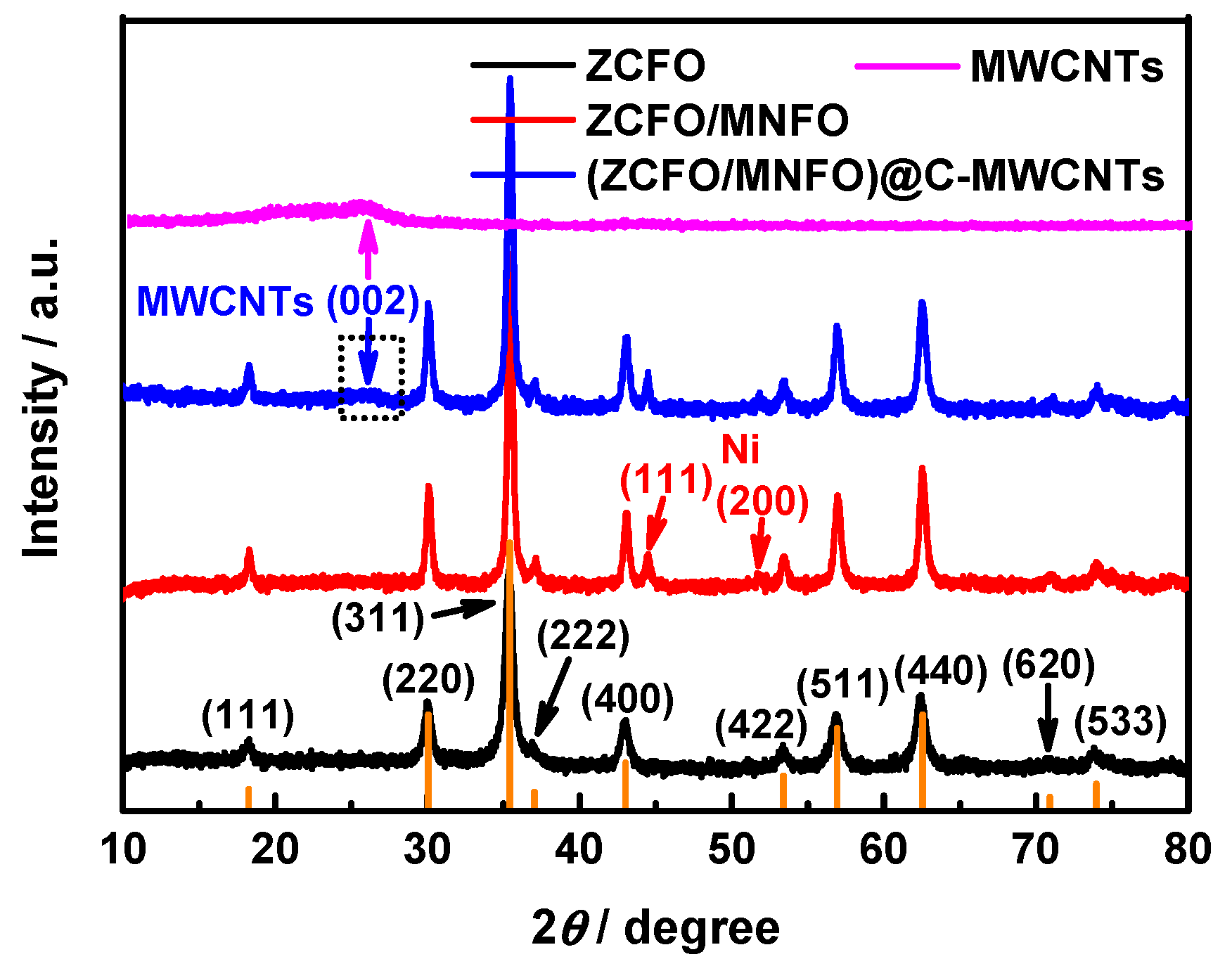
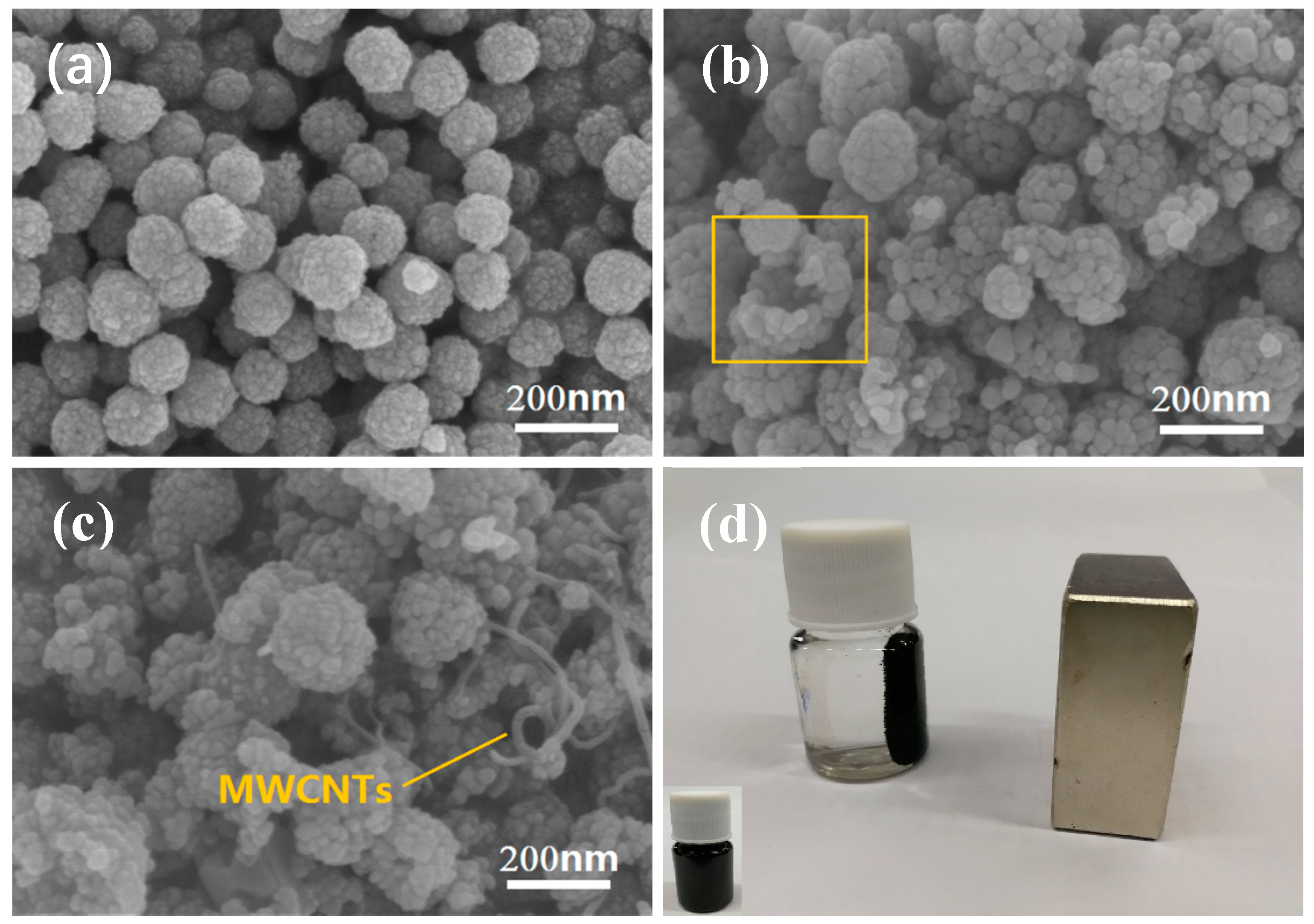
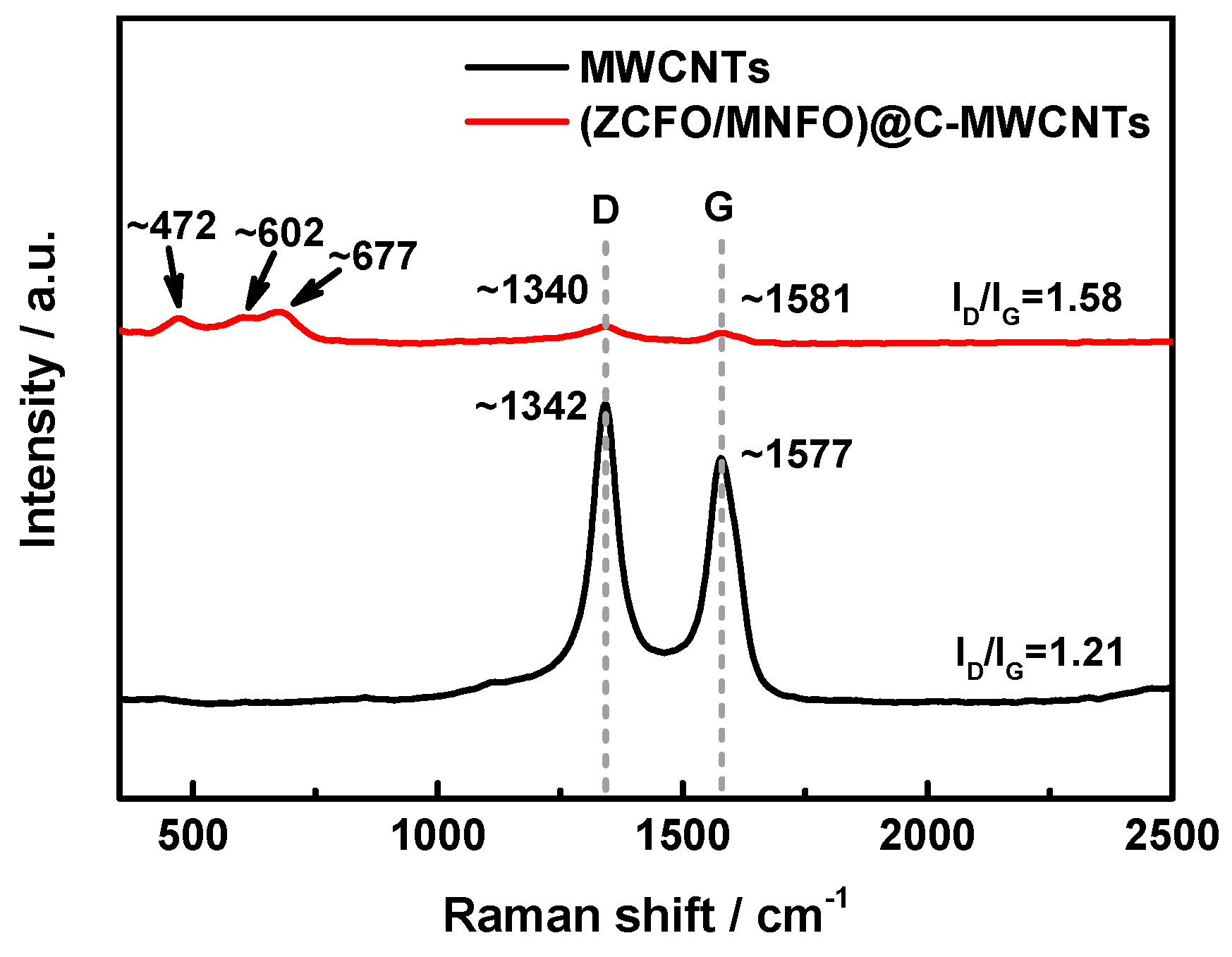
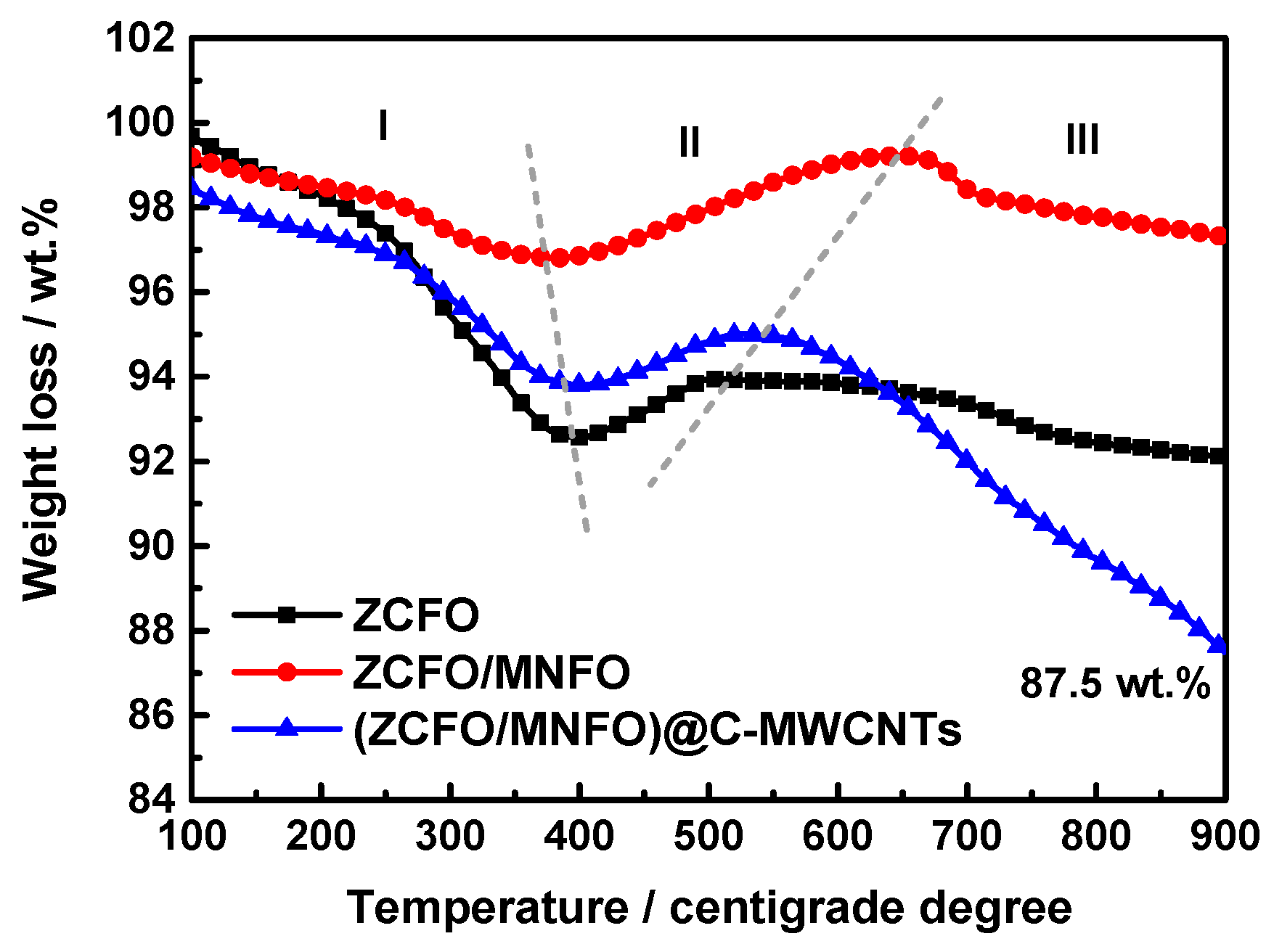
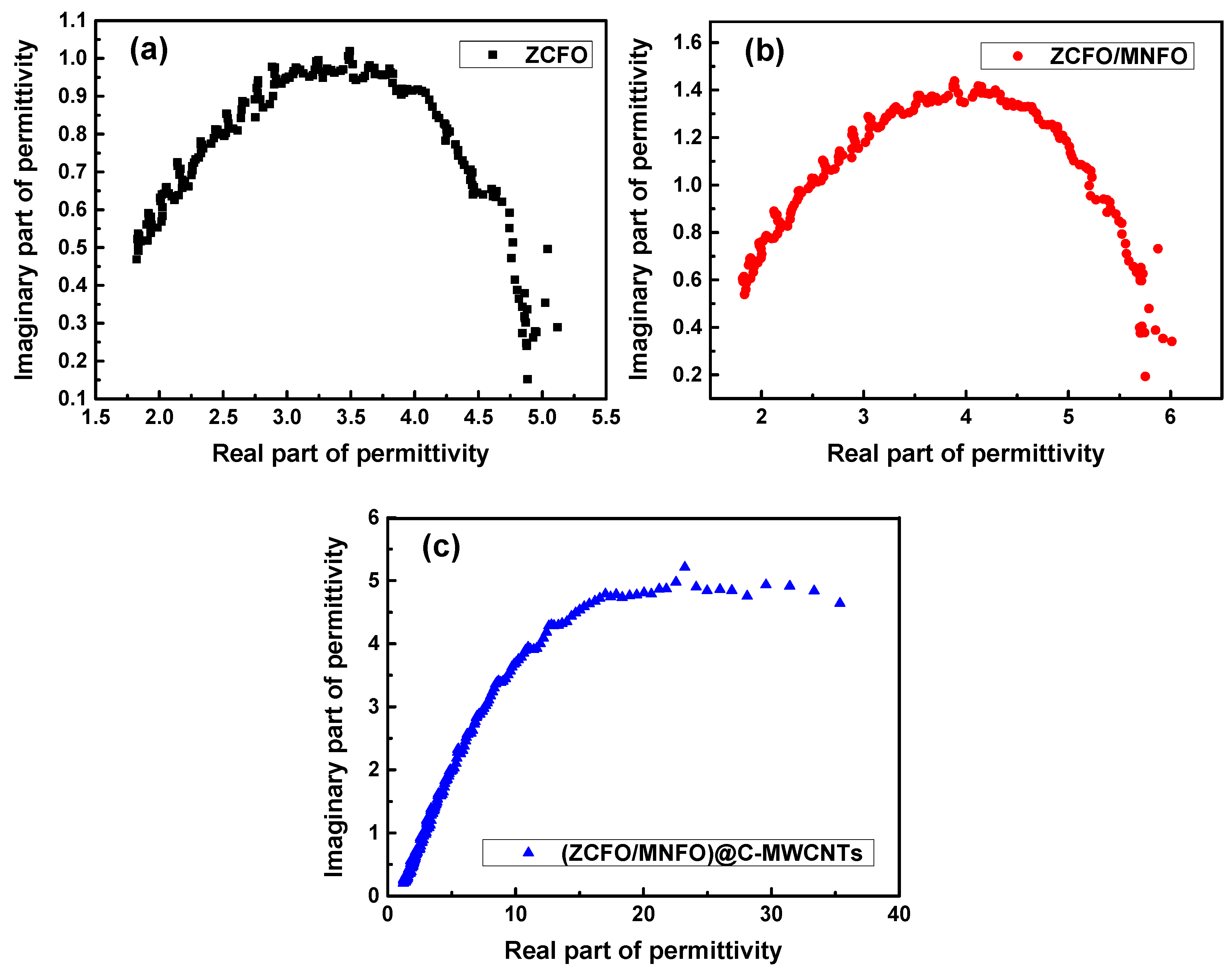
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ling, J.; Zhai, W.; Feng, W.; Shen, B.; Zhang, J.; Zheng, W. Facile preparation of lightweight microcellular polyetherimide/graphene composite foams for electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2013, 5, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Meng, F.; Zhou, Z.; Tian, X.; Shan, L.; Zhu, S.; Xu, X.; Jiang, M.; Wang, L.; Hui, D.; et al. One-step synthesis of graphene/polyaniline hybrids by in situ intercalation polymerization and their electromagnetic properties. Nanoscale 2014, 6, 8140–8148. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Yin, X.; Yuan, X.; Yuan, Y.; Zhang, Y.; Liu, X.; Cheng, L.; Zhang, L. Electromagnetic wave absorption properties of graphene modified with carbon nanotube/poly (dimethyl siloxane) composites. Carbon 2014, 73, 185–193. [Google Scholar] [CrossRef]
- Lan, D.; Qin, M.; Yang, R.; Chen, S.; Wu, H.; Fan, Y.; Fu, Q.; Zhang, F. Facile synthesis of hierarchical chrysanthemum-like copper cobaltate-copper oxide composites for enhanced microwave absorption performance. J. Colloid Interface Sci. 2019, 533, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Zhang, W.; Huang, S. One-pot synthesis of MnFe2O4 nanoparticles decorated reduced graphene oxide for enhanced microwave absorption properties. Mater. Technol. 2017, 32, 32–37. [Google Scholar] [CrossRef]
- Wu, H.; Qu, S.; Lin, K.; Qing, Y.; Wang, L.; Fan, Y.; Fu, Q.; Zhang, F. Enhanced low-frequency microwave absorbing property of SCFs@TiO2 composite. Powder Technol. 2018, 333, 153–159. [Google Scholar] [CrossRef]
- Luo, H.; Chen, W.; Zhou, W.; Long, L.; Deng, L.; Xiao, P.; Li, Y. Carbon fiber/Si3N4 composites with SiC nanofiber interphase for enhanced microwave absorption properties. Ceram. Int. 2017, 43, 12328–12332. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Yu, D. Three-phase heterostructures f-NiFe2O4/PANI/PI EMI shielding fabric with high microwave absorption performance. Appl. Surf. Sci. 2017, 425, 518–525. [Google Scholar] [CrossRef]
- Yun, S.; Kirakosyan, A.; Surabhi, S.; Jeong, J.R.; Choi, J. Controlled morphology of MWCNTs driven by polymer-grafted nanoparticles for enhanced microwave absorption. J. Mater. Chem. C 2017, 5, 8436–8443. [Google Scholar] [CrossRef]
- Ali, N.N.; Atassi, Y.; Salloum, A.; Charba, A.; Malki, A.; Jafarian, M. Comparative study of microwave absorption characteristics of (Polyaniline/NiZn ferrite) nanocomposites with different ferrite percentages. Mater. Chem. Phys. 2018, 211, 79–87. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Gong, H.; Zhang, Y.; Lin, X.; Xie, B.; Mao, J. Electromagnetic wave absorption properties of nickel-containing polymerderived SiCN ceramics. Ceram. Int. 2018, 44, 10945–10950. [Google Scholar] [CrossRef]
- Reddy, M.P.; Mohamed, A.M.A.; Ramana, M.V.; Zhou, X.B.; Huang, Q. Spark plasma sintering and microwave electromagnetic properties of MnFe2O4 ceramics. J. Magn. Magn. Mater. 2015, 395, 185–189. [Google Scholar] [CrossRef]
- Wei, H.; Yin, X.; Hou, Z.; Jiang, F.; Xu, H.; Li, M.; Zhang, L.; Cheng, L. A novel SiC-based microwave absorption ceramic with Sc2Si2O7 as transparent matrix. J. Eur. Ceram. Soc. 2018, 38, 4189–4197. [Google Scholar] [CrossRef]
- Wei, C.; Shen, X.; Song, F.; Zhu, Y.; Wang, Y. Double-layer microwave absorber based on nanocrystalline Zn0.5Ni0.5Fe2O4/α-Fe microfibers. Mater. Des. 2012, 35, 363–368. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, L.; Liang, Z.; Wang, X.; Li, X. A wide frequency absorbing material added CIPs using the fuse deposition modeling. J. Alloys Compd. 2017, 704, 593–598. [Google Scholar] [CrossRef]
- Khani, O.; Shoushtari, M.Z.; Ackland, K.; Stamenov, P. The structural, magnetic and microwave properties of spherical and flake shaped carbonyl iron particles as thin multilayer microwave absorbers. J. Magn. Magn. Mater. 2017, 428, 28–35. [Google Scholar] [CrossRef]
- Wu, H.; Wu, G.; Ren, Y.; Yang, L.; Wang, L.; Li, X. Co2+/Co3+ ratio dependence of electromagnetic wave absorption in hierarchical NiCo2O4-CoNiO2 hybrids. J Mater. Chem. C 2015, 3, 7677–7690. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Zhang, W.; Huang, S. Synthesis and electromagnetic absorption properties of Ag-coated reduced graphene oxide with MnFe2O4 particles. J. Magn. Magn. Mater. 2016, 404, 58–63. [Google Scholar] [CrossRef]
- Wu, H.; Wu, G.; Wang, L. Peculiar porous α-Fe2O3, γ-Fe2O3 and Fe3O4 nanospheres: Facile synthesis and electromagnetic properties. Powder Technol. 2015, 269, 443–451. [Google Scholar] [CrossRef]
- Lan, Y.; Li, X.; Zong, Y.; Li, Z.; Sun, Y.; Tan, G.; Feng, J.; Ren, Z.; Zheng, X. In-situsynthesis of carbon nanotubes decorated by magnetite nanoclusters and their applications as highly efficient and enhanced microwave absorber. Ceram. Int. 2016, 42, 19110–19118. [Google Scholar] [CrossRef]
- Xiang, J.; Hou, Z.; Zhang, X.; Gong, L.; Wu, Z.; Mi, J. Facile synthesis and enhanced microwave absorption properties of multiferroic Ni0.4Co0.2Zn0.4Fe2O4/BaTiO3 composite fibers. J. Alloys Compd. 2018, 737, 412–420. [Google Scholar] [CrossRef]
- Ismail, M.M.; Rafeeq, S.N.; Sulaiman, J.M.A.; Mandal, A. Electromagnetic interference shielding and microwave absorption properties of cobalt ferrite CoFe2O4/polyaniline composite. Appl. Phys. A 2018, 124, 380. [Google Scholar] [CrossRef]
- Chen, Q.; Li, L.; Wang, Z.; Ge, Y.; Zhou, C.; Yi, J. Synthesis and enhanced microwave absorption performance of CIP@SiO2@Mn0.6Zn0.4Fe2O4 ferrite composites. J. Alloys Compd. 2019, 779, 720–727. [Google Scholar] [CrossRef]
- Acharya, S.; Ray, J.; Patro, T.U.; Alegaonkar, P.; Datar, S. Microwave absorption properties of reduced graphene oxide strontium hexaferrite/poly (methyl methacrylate) composites. Nanotechnology 2018, 29, 115605. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dai, Y.; Gong, W.; Geng, D.; Ma, S.; Li, D.; Liu, W.; Zhang, Z. Broadband microwave absorption of CoNi@C nanocapsules enhanced by dual dielectric relaxation and multiple magnetic resonances. Appl. Phys. Lett. 2013, 102, 223113. [Google Scholar] [CrossRef]
- Han, M.; Liang, D.; Deng, L. Fabrication and electromagnetic wave absorption properties of amorphous Fe79Si16B5 microwires. Appl. Phys. Lett. 2011, 99, 082503. [Google Scholar]
- Zhu, L.; Zeng, X.; Chen, M.; Yu, R. Controllable permittivity in 3D Fe3O4/CNTs network for remarkable microwave absorption performances. RSC Adv. 2017, 7, 26801–26808. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, X.; Zhang, Q.; Chen, H.; Chen, X. Fe3O4 nanoparticles decorated MWCNTs@C ferrite nanocomposites and their enhanced microwave absorption properties. J. Magn. Magn. Mater. 2018, 452, 55–63. [Google Scholar] [CrossRef]
- Shao, Y.; Lu, W.; Chen, H.; Xiao, J.Q.; Qiu, Y.; Chou, T.W. Flexible ultra-thin Fe3O4/MnO2 core-shell decorated CNT composite with enhanced electromagnetic wave absorption performance. Compos. Part B Eng. 2018, 144, 111–117. [Google Scholar] [CrossRef]
- Yu, L.; Lan, X.; Wei, C.; Li, X.; Qi, X.; Xu, T.; Li, C.; Li, C.; Wang, Z. MWCNT/NiO-Fe3O4 hybrid nanotubes for efficient electromagnetic wave absorption. J. Alloys Compd. 2018, 748, 111–116. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.L.; Peng, Q.; Wang, X.; Chen, J.P.; Li, Y.D. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. 2005, 117, 2842–2845. [Google Scholar] [CrossRef]
- Yin, P.; Deng, Y.; Zhang, L.; Wu, W.; Wang, J.; Feng, X.; Sun, X.; Li, H.; Tao, Y. One-step hydrothermal synthesis and enhanced microwave absorption properties of Ni0.5Co0.5Fe2O4/graphene composites in low frequency band. Ceram. Int. 2018, 44, 20896–20905. [Google Scholar] [CrossRef]
- Nicolson, A.M.; Ross, G.F. Measurement of the intrinsic properties of materials by time-domain techniques. IEEE Trans. Instrum. Meas. 1970, 19, 377–382. [Google Scholar] [CrossRef]
- Lemdek, E.M.; Benkhouja, K.; Jaafari, K.; Bettach, M.; Tahiri, M.; Masaif, N.; Jennane; Lotfi, E.M. Structural and magnetic properties of nanometric Zn0.5Co0.5Fe2O4 prepared by hydrothermal method. Mol. Cryst. Liq. Cryst. 2016, 627, 125–132. [Google Scholar] [CrossRef]
- Chicinas, I.; Marinca, T.F.; Neamtu, B.V.; Popa, F.; Isnard, O.; Pop, V. Synthesis, structural, and magnetic properties of nanocrystalline/nanosized manganese-nickel ferrite-Mn0.5Ni0.5Fe2O4. IEEE Trans. Magn. 2014, 50, 2800704. [Google Scholar] [CrossRef]
- Tong, X.; Zeng, M.; Xu, H.; Li, J. Synthesis and lithium storage performance of graphene/Co3O4 microrods hybrids. J. Mater. Sci. Mater. Electron. 2016, 27, 7657–7664. [Google Scholar] [CrossRef]
- Shen, Q.; Yang, J.; Chen, K.L.; Wang, H.; Liu, J.B.; Yan, H. Co3O4 nanorods-graphene composites as catalysts for rechargeable zinc-air battery. J. Solid State Electrochem. 2016, 20, 3331–3336. [Google Scholar] [CrossRef]
- Peng, J.; Peng, Z.; Zhu, Z.; Augustine, R.; Mahmoud, M.M.; Tang, H.; Rao, M.; Zhang, Y.; Li, G.; Jiang, T. Achieving ultra-high electromagnetic wave absorption by anchoring Co0.33Ni0.33Mn0.33Fe2O4 nanoparticles on graphene sheets using microwave-assisted polyol method. Ceram. Int. 2018, 44, 21015–21026. [Google Scholar] [CrossRef]
- Shu, R.; Li, W.; Wu, Y.; Zhang, J.; Zhang, G. Nitrogen-doped Co-C/MWCNTs nanocomposites derived from bimetallic metal-organic frameworks for electromagnetic wave absorption in the X-band. Chem. Eng. J. 2019, 362, 513–524. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Y.; Zhang, X. Cubic NiFe2O4 particles on graphene-polyaniline and their enhanced microwave absorption properties. Compos. Sci. Technol. 2015, 107, 54–60. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, G.S.; Cao, W.Q.; Wei, Y.Z.; Liang, J.F.; Guo, L.; Cao, M.S. Enhanced microwave absorption property of reduced graphene oxide (RGO)-MnFe2O4 nanocomposites and polyvinylidene fluoride. ACS Appl. Mater. Interfaces 2014, 6, 7471–7478. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xie, A.M.; Sun, M.X.; Wang, Y.; Wang, M.Y. Reduced graphene oxide (RGO) modified sponge-like polypyrrole (PPy) aerogel for excellent electromagnetic absorption. J. Mater. Chem. A 2015, 3, 14358–14369. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.N.; Jiang, K.D.; Tong, G.X.; Lv, T.X.; Wu, W.H. Controllable synthesis of elliptical Fe3O4@C and Fe3O4/Fe@C nanorings for plasmon resonance-enhanced microwave absorption. J. Mater. Chem. C 2016, 4, 7316–7323. [Google Scholar] [CrossRef]
- Wu, M.; Yang, E.; Qi, X.; Xie, R.; Bai, Z.; Qin, S.; Zhong, W.; Du, Y. Constructing different categories of heterostructured magnetic nanoparticles@carbon nanotubes-reduced graphene oxide, and their tunable excellent microwave absorption capabilities. J. Alloys Compd. 2019, 785, 1126–1136. [Google Scholar] [CrossRef]
- Song, W.L.; Cao, M.S.; Lu, M.M.; Liu, J.; Yuan, J.; Fan, L.Z. Improved dielectric properties and highly efficient and broadened bandwidth electromagnetic attenuation of thickness-decreased carbon nanosheet/wax composites. J. Mater. Chem. C 2013, 1, 1846–1854. [Google Scholar] [CrossRef]
- Liu, X.F.; Cui, X.R.; Chen, Y.X.; Zhang, X.J.; Yu, R.H.; Wang, G.S.; Ma, H. Modulation of electromagnetic wave absorption by carbon shell thickness in carbon encapsulated magnetite nanospindles-poly (vinylidene fluoride) composites. Carbon 2015, 95, 870–878. [Google Scholar] [CrossRef]
- Bespyatykh, Y.I.; Kazantseva, N.E. Electromagnetic properties of hybrid polymer composites. J. Commun. Technol. Electron. 2008, 53, 143–154. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, W.; Zhao, J.; Xiao, J.; Lu, L.; Fan, H. Synthesis and characterization of TiO2/polyaniline/graphene oxide bouquet-like composites for enhanced microwave absorption performance. J. Alloys Compd. 2017, 710, 717–724. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Bera, P.; Chakradhar, R.P.S.; Choudhury, B.; Pawar, S.P.; Bose, S.; Nair, R.U.; Barshilia, H.C. Enhanced microwave absorption properties of PMMA modified MnFe2O4-polyaniline nanocomposites. Phys. Chem. Chem. Phys. 2019, 21, 5068–5077. [Google Scholar] [CrossRef] [PubMed]
- Michielssen, E.; Sajer, J.M.; Ranjithan, S.; Mittra, R. Design of lightweight, broadband microwave absorbers using genetic algorithms. IEEE Trans. Microw. Theory Tech. 1993, 41, 1024–1031. [Google Scholar] [CrossRef]
- Shen, G.; Xu, Z.; Li, Y. Absorbing properties and structural design of microwave absorbers based on W-type La-doped ferrite and carbon fiber composites. J. Magn. Magn. Mater. 2006, 301, 325–330. [Google Scholar] [CrossRef]
- Yu, K.; Zeng, M.; Yin, Y.; Zeng, X.; Liu, J.; Li, Y.; Tang, W.; Wang, Y.; An, J.; He, J.; et al. MWCNTs as conductive network for monodispersed Fe3O4 nanoparticles to enhance the wave absorption performances. Adv. Eng. Mater. 2018, 20, 1700543. [Google Scholar] [CrossRef]
- Du, Y.; Liu, W.; Qiang, R.; Wang, Y.; Han, X.; Ma, J.; Xu, P. Shell thickness-dependent microwave absorption of core-shell Fe3O4@C composites. ACS Appl. Mater. Interfaces 2014, 6, 12997–13006. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, T.; Tan, G.G.; Zuo, W.L.; Wei, J.Q.; Qiao, L.; Li, F.S. Microwave absorption properties of oriented Pr2Fe17N3-δ particles/paraffin composite with planar anisotropy. J. Alloys Compd. 2014, 586, 239–243. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, Y.; Xiong, J.; Yang, Y.; Lu, H. Microwave-absorbing properties of de-aggregated flake-shaped carbonyl-iron particle composites at 2–18 GHz. IEEE Trans. Magn. 2006, 42, 1778–1781. [Google Scholar] [CrossRef]
- Fang, J.; Liu, T.; Chen, Z.; Wang, Y.; Wei, W.; Yue, X.; Jiang, Z. A wormhole-like porous carbon/magnetic particles composite as an efficient broadband electromagnetic wave absorber. Nanoscale 2016, 8, 8899–8909. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yao, Z.; Lin, H.; Zhou, J.; Haidry, A.A.; Liu, P. The effect of polymerization temperature and reaction time on microwave absorption properties of Co-doped ZnNi ferrite/polyaniline composites. RSC Adv. 2018, 8, 29344–29355. [Google Scholar] [CrossRef]
- Long, Q.; Xu, Z.; Xiao, H.; Xie, K. A facile synthesis of a cobalt nanoparticle-graphene nanocomposite with high-performance and triple-band electromagnetic wave absorption properties. RSC Adv. 2018, 8, 1210–1217. [Google Scholar] [CrossRef]
- Lu, B.; Huang, H.; Dong, X.L.; Zhang, X.F.; Lei, J.P.; Sun, J.P.; Dong, C. Influence of alloy components on electromagnetic characteristics of core/shell-type Fe-Ni nanoparticles. J. Appl. Phys. 2008, 104, 114313. [Google Scholar] [CrossRef]
- Liu, X.G.; Ou, Z.Q.; Geng, D.Y.; Han, Z.; Xie, Z.G.; Zhang, Z.D. Enhanced natural resonance and attenuation properties in superparamagnetic graphite-coated FeNi3 nanocapsules. J. Phys. D 2009, 42, 155004. [Google Scholar] [CrossRef]
- Guan, Z.J.; Jiang, J.T.; Chen, N.; Gong, Y.X.; Zhen, L. Carbon-coated CoFe-CoFe2O4 composite particles with high and dual-band electromagnetic wave absorbing properties. Nanotechnology 2018, 29, 305604. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, Q.; Lin, Y.; Yang, H.; Wang, L. Flower-like Bi0.9La0.1FeO3 microspheres modified by reduced graphene oxide as a thin and strong electromagnetic wave absorber. J. Alloys Compd. 2019, 781, 723–733. [Google Scholar] [CrossRef]
- Li, N.; Huang, G.; Xiao, H.; Feng, Q.; Fu, S. Investigations on structure-dependent microwave absorption performance of nano-Fe3O4 coated carbon-based absorbers. Carbon 2019, 144, 216–227. [Google Scholar] [CrossRef]
- Chen, Y.J.; Xiao, G.; Wang, T.S.; Ouyang, Q.Y.; Qi, L.H.; Ma, Y.; Gao, P.; Zhu, C.L.; Cao, M.S.; Jin, H.B. Porous Fe3O4/carbon core/shell nanorods: Synthesis and electromagnetic properties. J. Phys. Chem. C 2011, 115, 13603–13608. [Google Scholar] [CrossRef]
- Moitra, D.; Hazra, S.; Ghosh, B.K.; Jani, R.K.; Patra, M.K.; Vadera, S.R.; Ghosh, N.N. A facile low temperature method for the synthesis of CoFe2O4 nanoparticles possessing excellent microwave absorption properties. RSC Adv. 2015, 5, 51130–51134. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Chen, Y.; Wu, X.; Zhang, W.; Luo, C.; Li, J. Design of hollow ZnFe2O4 microspheres@graphene decorated with TiO2 nanosheets as a high-performance low frequency absorber. Mater. Chem. Phys. 2017, 202, 184–189. [Google Scholar] [CrossRef]
- Javid, M.; Zhou, Y.; Zhou, T.; Wang, D.; Zhou, L.; Shah, A.; Duan, Y.; Dong, X.; Zhang, Z. In-situ fabrication of Fe@ZrO2 nanochains for the heat-resistant electromagnetic wave absorber. Mater. Lett. 2019, 242, 199–202. [Google Scholar] [CrossRef]
- Li, G.; Sheng, L.; Yu, L.; An, K.; Ren, W.; Zhao, X. Electromagnetic and microwave absorption properties of single-walled carbon nanotubes and CoFe2O4 nanocomposites. Mater. Sci. Eng. B 2015, 193, 153–159. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Or, S.W.; Ho, S.L. Exchange coupling and microwave absorption in core/shell-structured hard/soft ferrite-based CoFe2O4/NiFe2O4 nanocapsules. AIP Adv. 2017, 7, 056403. [Google Scholar] [CrossRef]
- Cheng, Y.; Tan, M.; Hu, P.; Zhang, X.; Sun, B.; Yan, L.; Zhou, S.; Han, W. Strong and thermostable SiC nanowires/graphene aerogel with enhanced hydrophobicity and electromagnetic wave absorption property. Appl. Surf. Sci. 2018, 448, 138–144. [Google Scholar] [CrossRef]
- Zhu, T.; Chang, S.; Song, Y.F.; Lahoubi, M.; Wang, W. PVP-encapsulated CoFe2O4/rGO composites with controllable electromagnetic wave absorption performance. Chem. Eng. J. 2019, 373, 755–766. [Google Scholar] [CrossRef]
- Genc, F.; Turhan, E.; Kavas, H.; Topal, U.; Baykal, A.; Sozeri, H. Magnetic and microwave absorption properties of NixZn0.9-xMn0.1Fe2O4 prepared by boron addition. J. Supercond. Nov. Magn. 2015, 28, 1047–1050. [Google Scholar] [CrossRef]
- Lu, S.W.; Yuan, C.J.; Jia, C.X.; Ma, K.M.; Wang, X.Q. Preparation and low-frequency microwave-absorbing properties of MWCNTs/Co-Ni/Fe3O4 hybrid material. Funct. Mater. Lett. 2016, 9, 1650035. [Google Scholar] [CrossRef]
- Lv, H.; Yang, Z.; Wang, P.L.; Ji, G.; Song, J.; Zheng, L.; Zeng, H.; Xu, Z.J. A voltage-boosting strategy enabling a low-frequency, flexible electromagnetic wave absorption device. Adv. Mater. 2018, 30, 1706343. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; He, Z.; Dong, W.; Wu, W.; Tong, G. Broadband and strong microwave absorption of Fe/Fe3C/C coreeshell spherical chains enhanced by dual dielectric relaxation and dual magnetic resonances. J. Alloys Compd. 2019, 782, 193–202. [Google Scholar] [CrossRef]




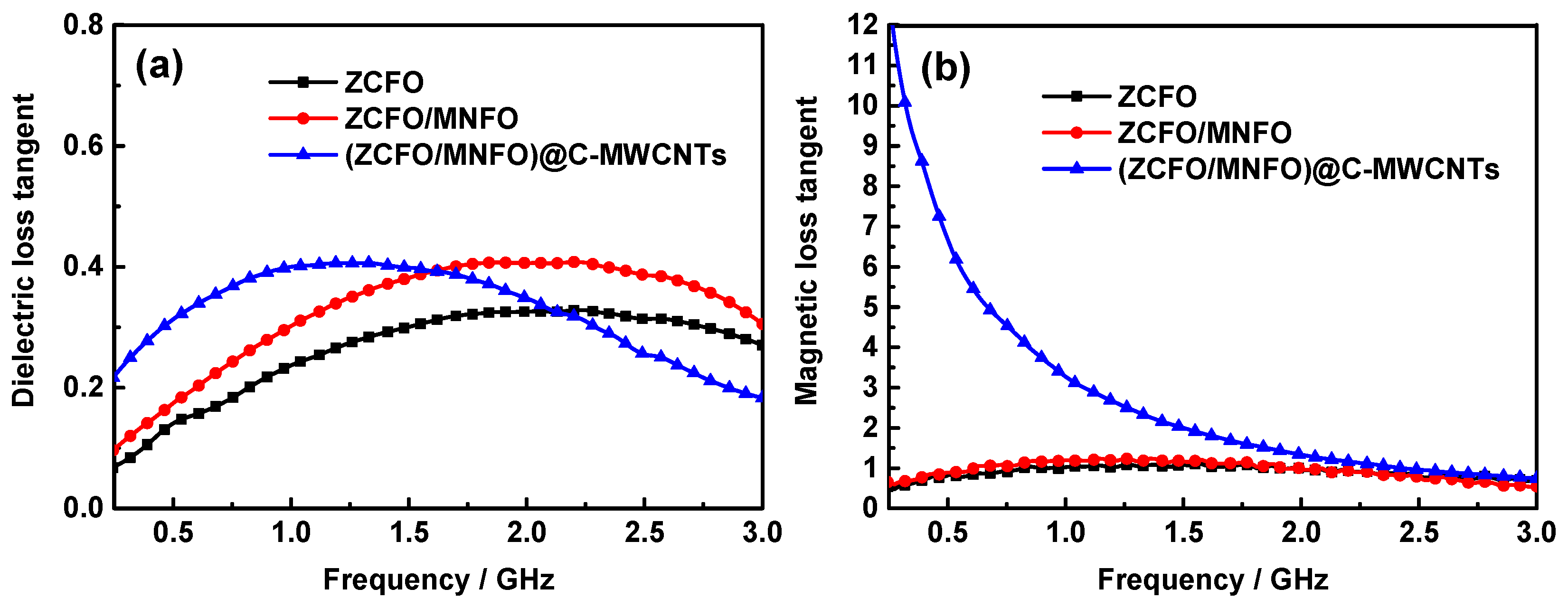
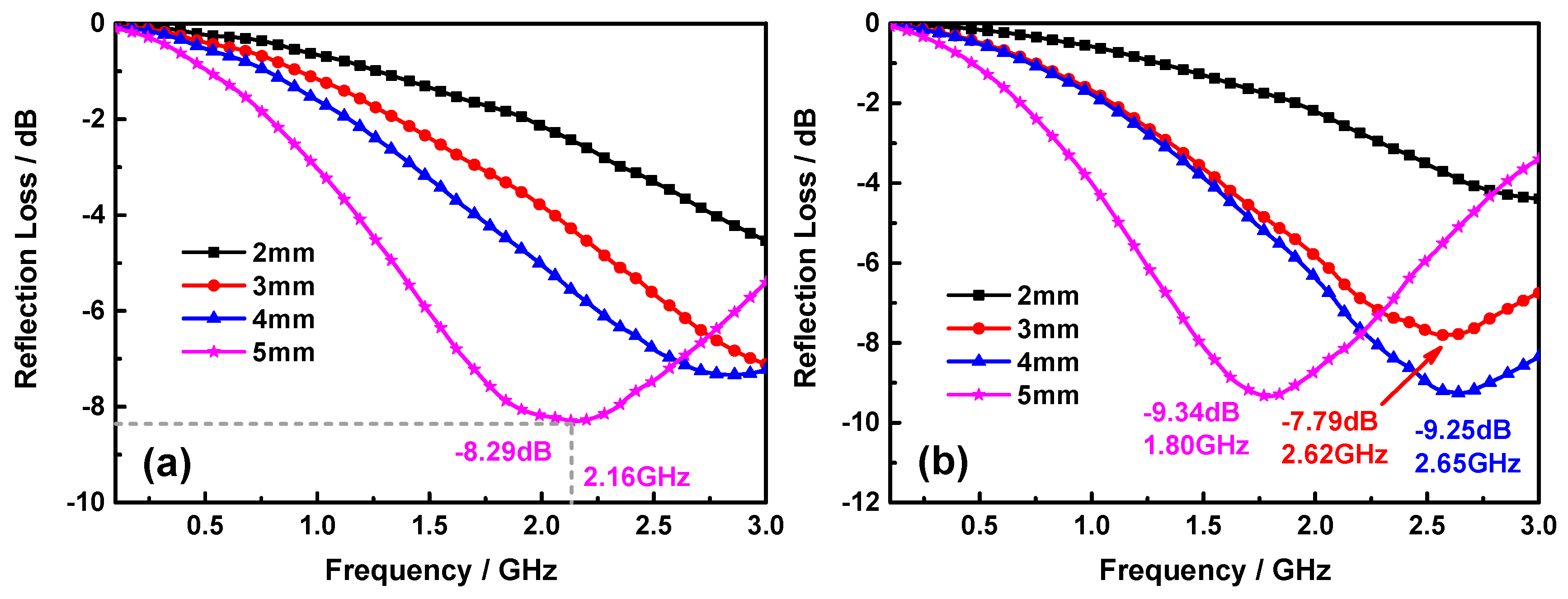


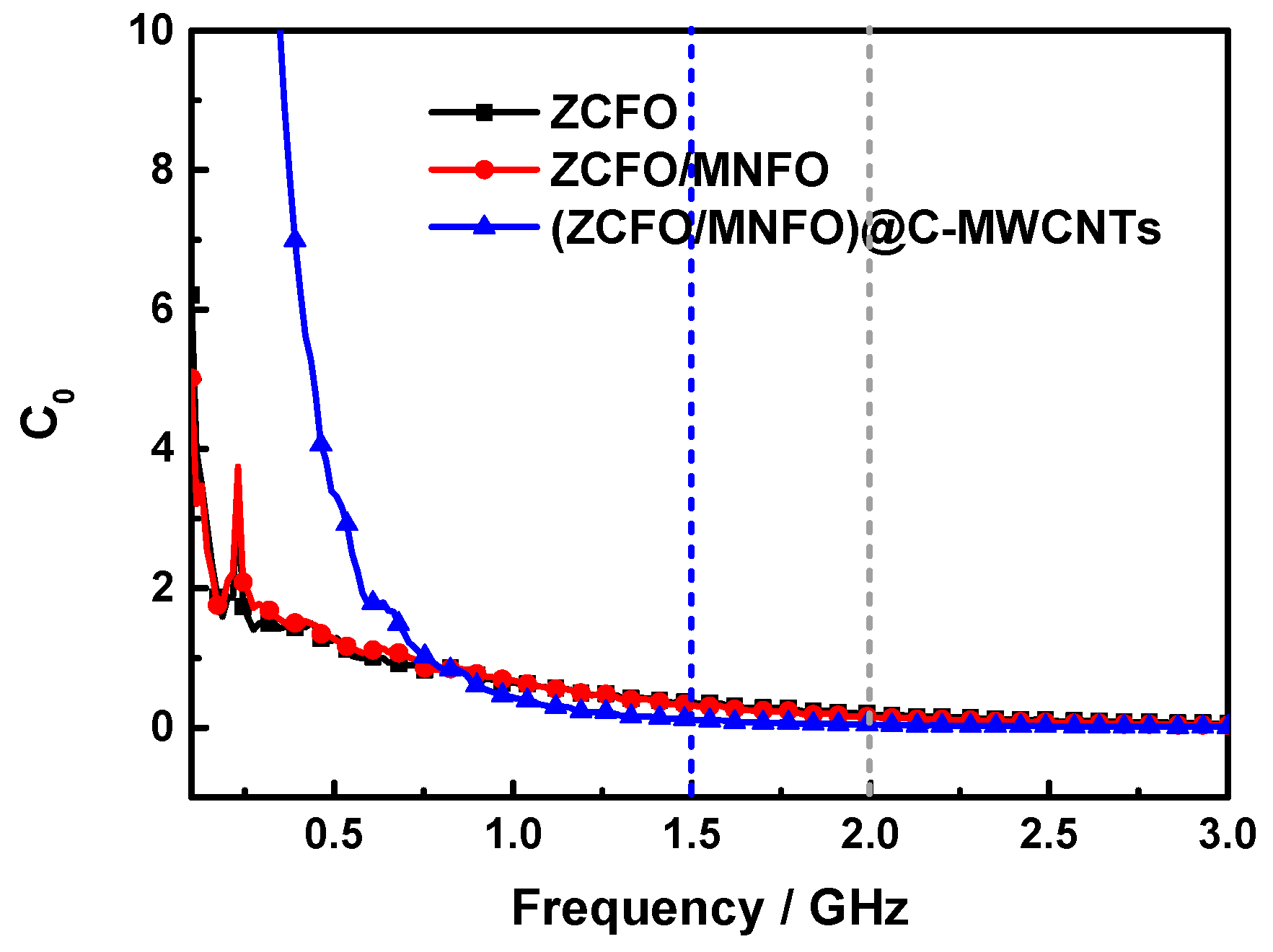
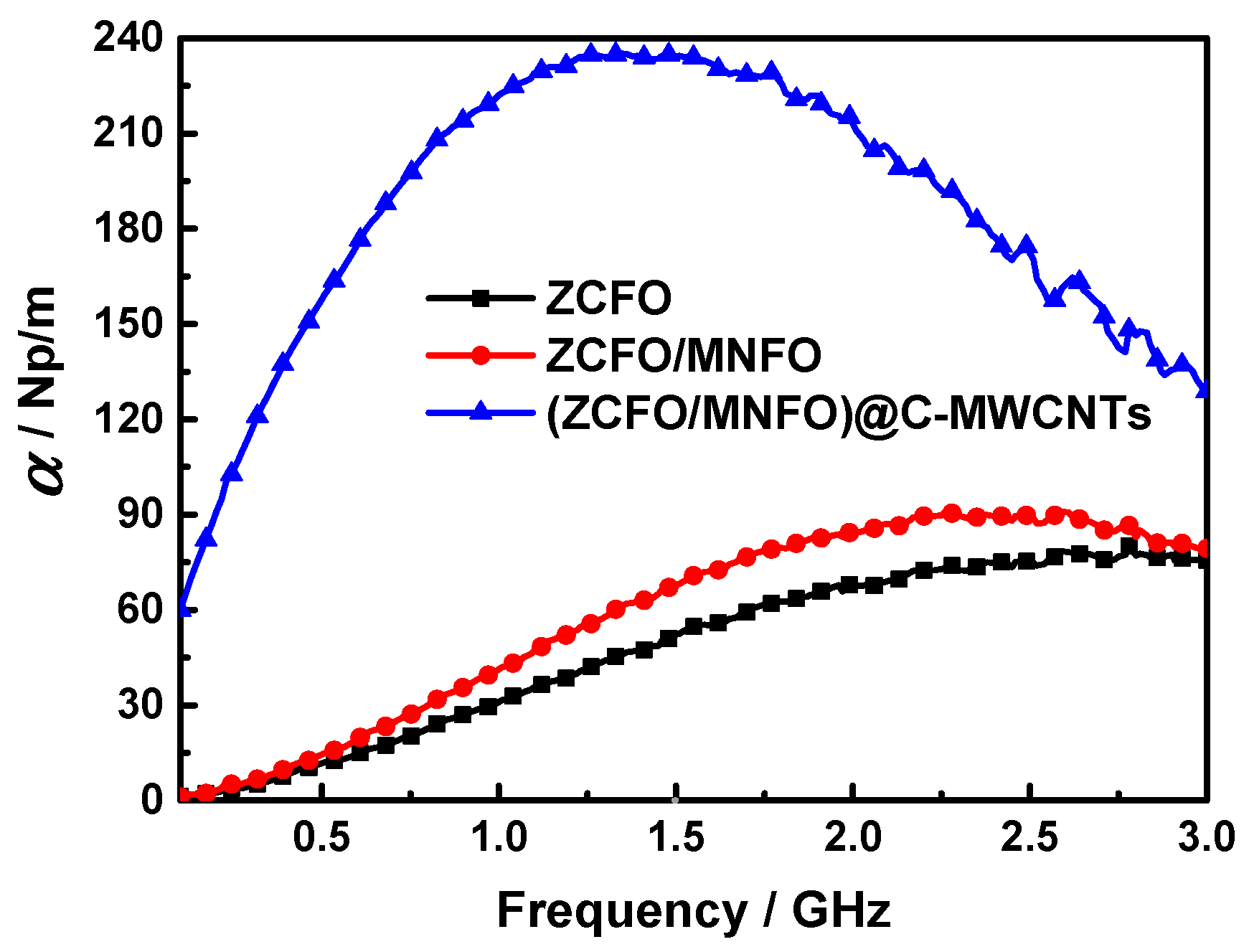
| Materials | Thickness | Minimum RL | Position | RL< −10 dB | Refs. |
|---|---|---|---|---|---|
| (ZCFO/MNFO)@C-MWCNTs | 5 mm | −35.14 dB | 0.56 GHz | 0.27–1.01 GHz | This work |
| CoFe2O4 | 2 mm | −55 dB | 9.25 GHz | 8.2–10.8 GHz | [65] |
| ZnFe2O4@rGO@TiO2 | 2.5 mm | −55.6 dB | 3.8 GHz | 2.8–5.4 GHz | [66] |
| Fe@ZrO2 | 1.97 mm | −45.36 dB | 6.2 GHz | / | [67] |
| CoFe2O4/LPA-SWCNT | 2 mm | −30.7 dB | 12.9 GHz | 10.1–17.3 GHz | [68] |
| CoFe2O4/NiFe2O4 | 4.5 mm | −20.1 dB | 9.7 GHz | 7.8–16.2 GHz | [69] |
| SiCnw/GA-S | 3.63 mm | −54.8 dB | 5.3 GHz | / | [70] |
| CoFe2O4/rGO@PVP | 1.96 mm | −56.8 dB | 15.7 GHz | 10.64–17.44 GHz | [71] |
| Ni0.6Zn0.3Mn0.1Fe2O4 | 10 mm | -20 dB | 1.8 GHz | / | [72] |
| MWCNTs/Co-Ni/Fe3O4 | 3 mm | −13.57 dB | 1.51 GHz | / | [73] |
| SnS/SnO2@C-16V | 5 mm | < −12 dB | / | 1.5–2 GHz | [74] |
| Fe@Fe3C@C | 2.4 mm | −58.0 dB | 8.68 GHz | / | [75] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, P.; Zhang, L.; Wu, H.; Feng, X.; Wang, J.; Rao, H.; Wang, Y.; Dai, J.; Tang, Y. Two-Step Solvothermal Synthesis of (Zn0.5Co0.5Fe2O4/Mn0.5Ni0.5Fe2O4)@C-MWCNTs Hybrid with Enhanced Low Frequency Microwave Absorbing Performance. Nanomaterials 2019, 9, 1601. https://doi.org/10.3390/nano9111601
Yin P, Zhang L, Wu H, Feng X, Wang J, Rao H, Wang Y, Dai J, Tang Y. Two-Step Solvothermal Synthesis of (Zn0.5Co0.5Fe2O4/Mn0.5Ni0.5Fe2O4)@C-MWCNTs Hybrid with Enhanced Low Frequency Microwave Absorbing Performance. Nanomaterials. 2019; 9(11):1601. https://doi.org/10.3390/nano9111601
Chicago/Turabian StyleYin, Pengfei, Limin Zhang, Hongjing Wu, Xing Feng, Jian Wang, Hanbing Rao, Yanying Wang, Jianwu Dai, and Yuting Tang. 2019. "Two-Step Solvothermal Synthesis of (Zn0.5Co0.5Fe2O4/Mn0.5Ni0.5Fe2O4)@C-MWCNTs Hybrid with Enhanced Low Frequency Microwave Absorbing Performance" Nanomaterials 9, no. 11: 1601. https://doi.org/10.3390/nano9111601
APA StyleYin, P., Zhang, L., Wu, H., Feng, X., Wang, J., Rao, H., Wang, Y., Dai, J., & Tang, Y. (2019). Two-Step Solvothermal Synthesis of (Zn0.5Co0.5Fe2O4/Mn0.5Ni0.5Fe2O4)@C-MWCNTs Hybrid with Enhanced Low Frequency Microwave Absorbing Performance. Nanomaterials, 9(11), 1601. https://doi.org/10.3390/nano9111601








