Hydrothermal Synthesis of Co3O4/ZnO Hybrid Nanoparticles for Triethylamine Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ZnO and Co3O4-ZnO Nanoparticles
2.2. Characterizations
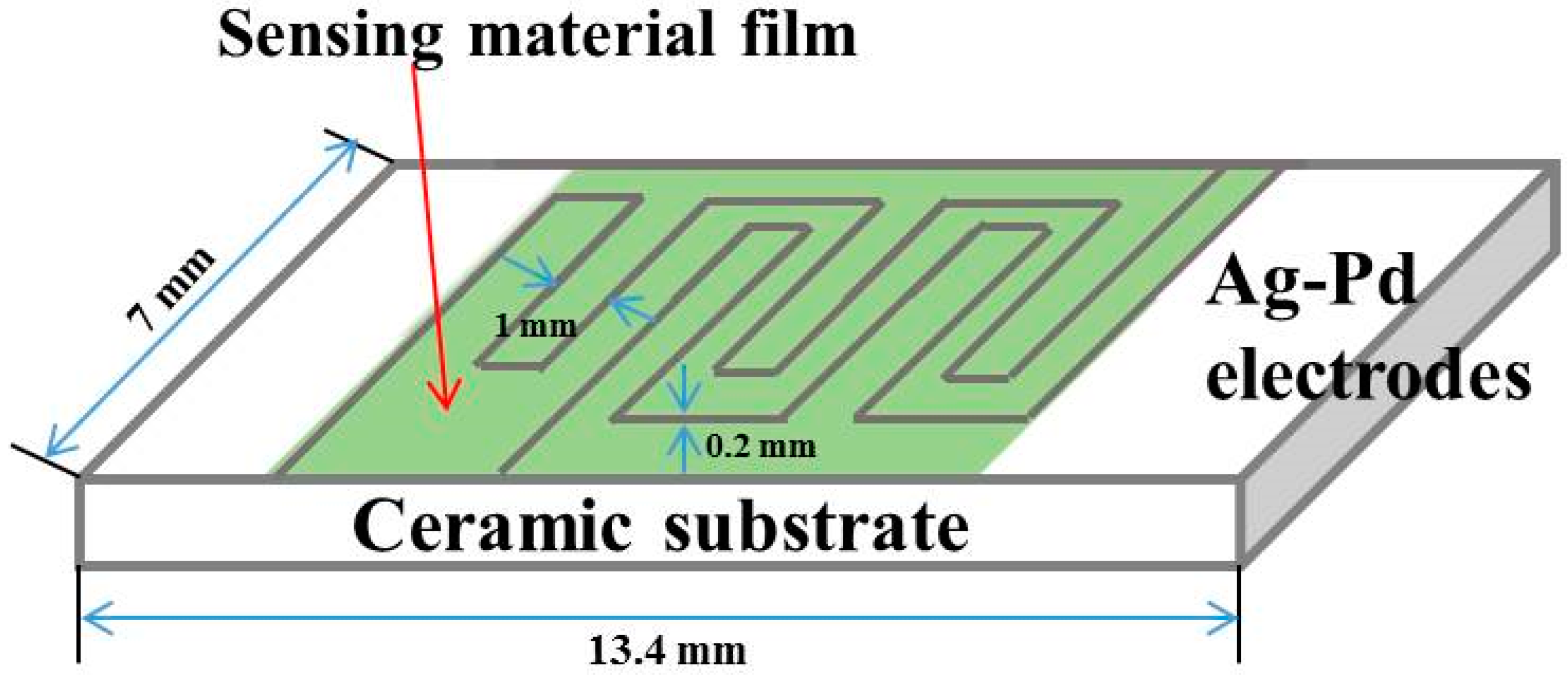
2.3. Construction of Gas Sensors
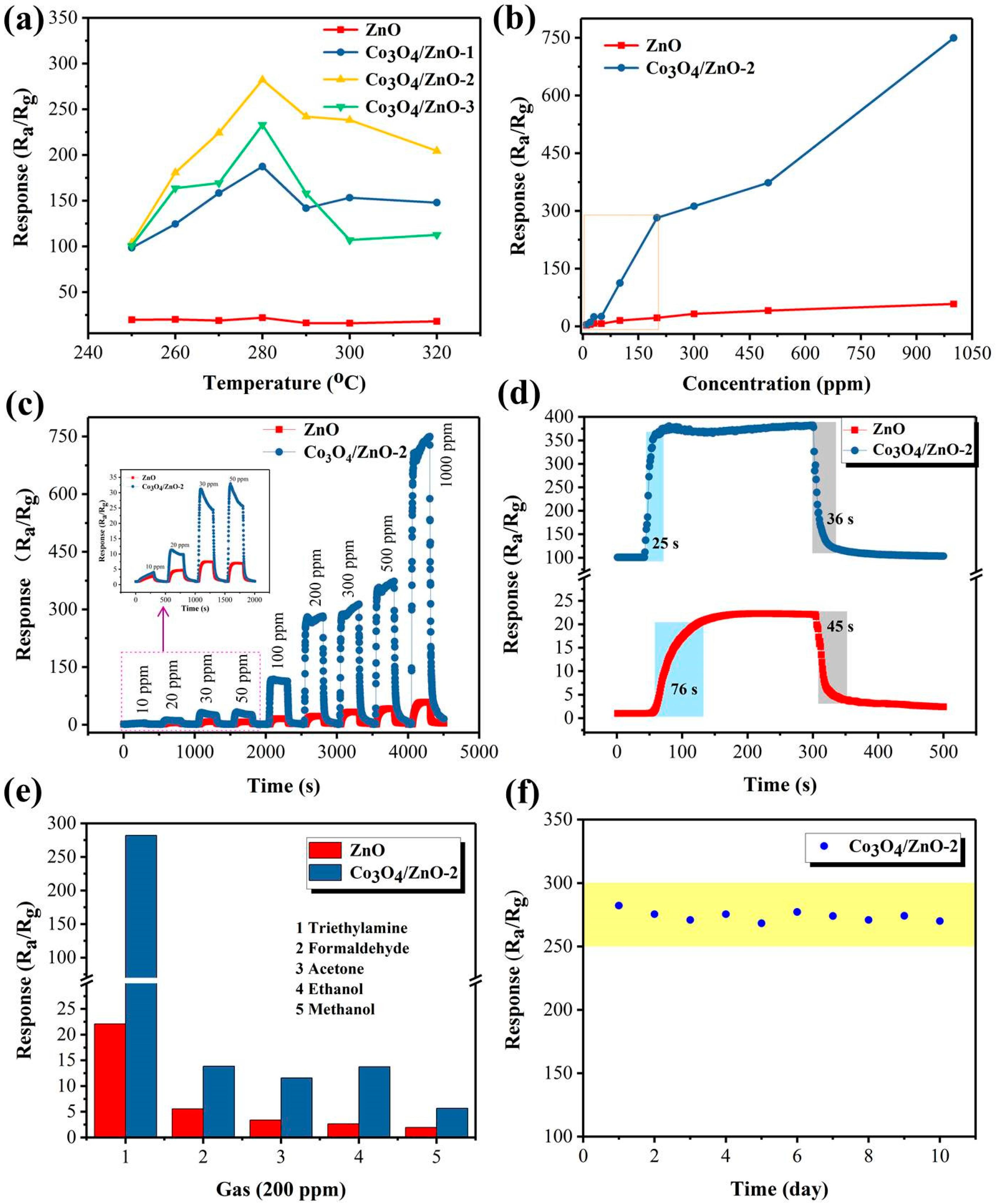
3. Results
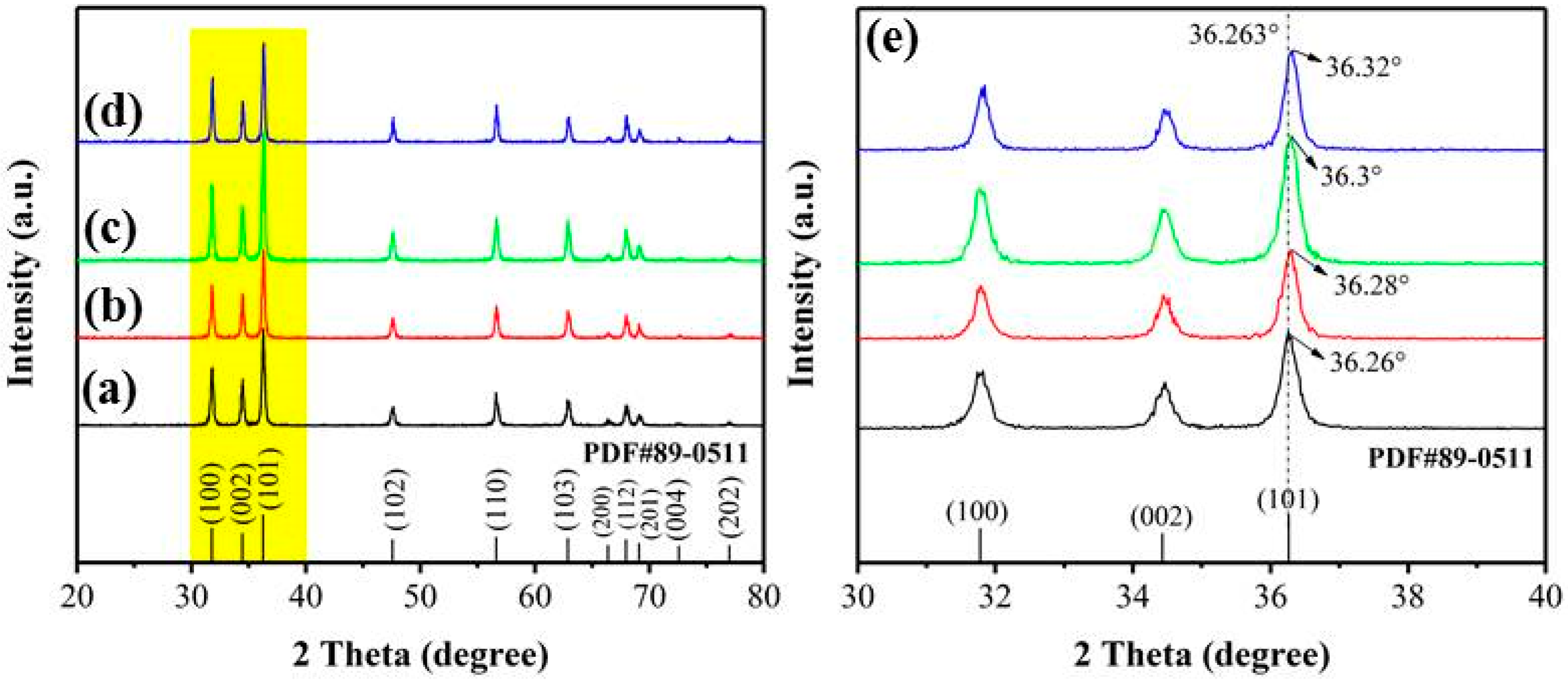
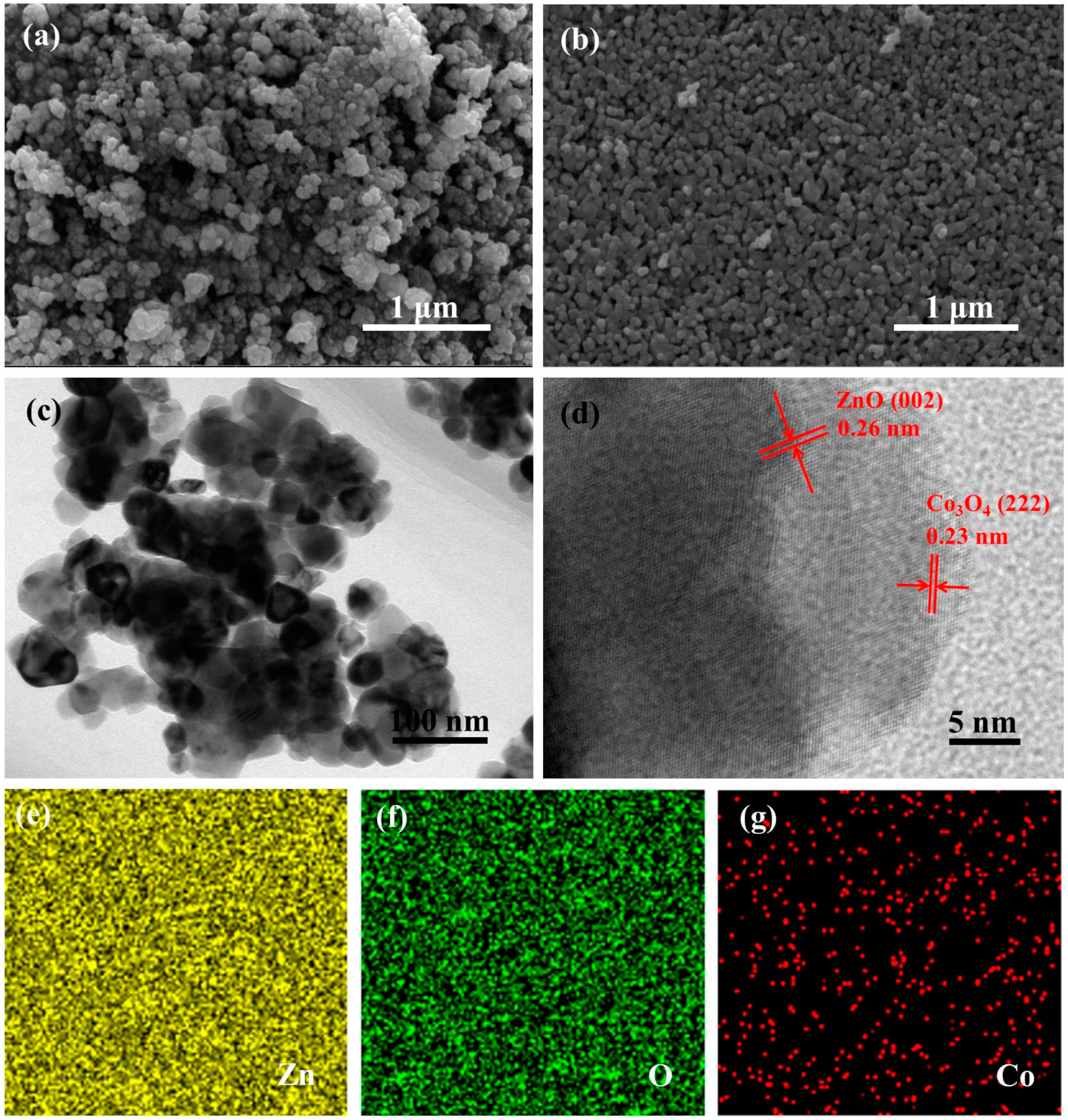
3.1. Materials Characterizations
3.2. Gas-Sensing Performances
3.3. Gas-Sensing Mechanisms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chu, X.F.; Jiang, D.L.; Zheng, C.M. The preparation and gas-sensing properties of NiFe2O4 nanocubes and nanorods. Sens. Actuators B 2007, 123, 793–797. [Google Scholar]
- Gu, F.B.; Cui, Y.Z.; Han, D.M.; Hong, S.; Flytzani-Stephanopoulos, M.; Wang, Z.H. Atomically dispersed Pt (II) on WO3 for highly selective sensing and catalytic oxidation of triethylamine. Appl. Catal. B 2019, 256, 117809. [Google Scholar] [CrossRef]
- Zhai, C.B.; Zhu, M.M.; Jiang, L.N.; Yang, T.Y.; Zhao, Q.; Luo, Y.; Zhang, M.Z. Fast triethylamine gas sensing response properties of nanosheets assembled WO3 hollow microspheres. Appl. Surf. Sci. 2019, 463, 1078–1084. [Google Scholar] [CrossRef]
- Ma, Q.; Chu, S.S.; Liu, Y.; Chen, Y.; Song, J.C.; Li, H.; Wang, J.P.; Che, Q.D.; Wang, G.; Fang, Y. Construction of SnS2/ZIF-8 derived flower-like porous SnO2/ZnO heterostructures with enhanced triethylamine gas sensing performance. Mater. Lett. 2019, 263, 452–455. [Google Scholar] [CrossRef]
- Lv, Y.Z.; Li, C.R.; Guo, L.; Wang, F.C.; Xu, Y.; Chu, X.F. Triethylamine gas sensor based on ZnO nanorods prepared by a simple solution route. Sens. Actuators B 2009, 141, 85–88. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Ma, C.; Yang, X.Y.; Song, Y.; Liang, X.S.; Zhao, X.; Wang, Y.L.; Gao, Y.; Liu, F.M.; Liu, F.M.; et al. NASICON-based gas sensor utilizing MMnO3 (M: Gd, Sm, La) sensing electrode for triethylamine detection. Sens. Actuators B 2019, 295, 56–64. [Google Scholar] [CrossRef]
- Tian, H.L.; Fan, H.Q.; Ma, J.W.; Liu, Z.Y.; Ma, L.T.; Lei, S.H.; Fang, J.W.; Long, C.B. Pt-decorated zinc oxide nanorod arrays with graphitic carbon nitride nanosheets for highly efficient dual-functional gas sensing. J. Hazard. Mater. 2018, 341, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kailasaganapathi, S.; Ramgir, N.; Datta, N.; Kumar, S.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Gas dependent sensing mechanism in ZnO nanobelt sensor. Appl. Surf. Sci. 2017, 394, 258–266. [Google Scholar] [CrossRef]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuators B 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Ma, Q.; Fang, Y.; Liu, Y.; Song, J.C.; Fu, X.H.; Li, H.; Chu, S.S.; Chen, Y. Facile synthesis of ZnO morphological evolution with tunable growth habits: Achieving superior gas-sensing properties of hemispherical ZnO/Au heterostructures for triethylamine. Phys. E 2019, 106, 180–186. [Google Scholar] [CrossRef]
- Agarwal, S.; Rai, P.; Gatell, E.N.; Llobet, E.; Güell, F.; Kumar, M.; Awasthi, K. Gas sensing properties of ZnO nanostructures (flowers/rods) synthesized by hydrothermal method. Sens. Actuators B 2019, 292, 24–31. [Google Scholar] [CrossRef]
- Navale, Y.H.; Navale, S.T.; Stadler, F.J.; Ramgir, N.S.; Patil, V.B. Enhanced NO2 sensing aptness of ZnO nanowire/CuO nanoparticle heterostructure-based gas sensors. Ceram. Int. 2019, 45, 1513–1522. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W.; Yang, J.D.; Li, Y.Q. One-step hydrothermal fabrication of nanosheet-assembled NiO/ZnO microflower and its ethanol sensing property. Ceram. Int. 2018, 44, 19825–19830. [Google Scholar] [CrossRef]
- Wang, L.L.; Lou, Z.; Zhang, R.; Zhou, T.T.; Deng, J.N.; Zhang, T. Hybrid Co3O4/SnO2 Core–Shell Nanospheres as Real-Time Rapid-Response Sensors for Ammonia Gas. ACS Appl. Mater. Interfaces 2016, 8, 6539–6545. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, W.; Yong, K. CuO/ZnO Heterostructured Nanorods: Photochemical Synthesis and the Mechanism of H2S Gas Sensing. J. Phys. Chem. 2012, 116, 15682–15691. [Google Scholar] [CrossRef]
- Zou, C.W.; Wang, J.; Xie, W. Synthesis and enhanced NO2 gas sensing properties of ZnO nanorods/TiO2 nanoparticles heterojunction composites. J. Colloid Interface Sci. 2016, 478, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Khodadadi, A.A.; Firooz, A.A.; Mortazavi, Y. In2O3-ZnO nanocomposites: High sensor response and selectivity to ethanol. Sens. Actuators B 2015, 212, 395–403. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, R.F.; Liu, S.H.; Song, J.; Chen, J.S.; Dong, B.; Song, H.W. NiO@ZnO Heterostructured Nanotubes: Coelectrospinning Fabrication, Characterization, and Highly Enhanced Gas Sensing Properties. Inorg. Chem. 2012, 51, 7733–7740. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xu, W.W.; Zhu, Z.Y.; Xue, Q.Z.; Lu, W.B.; Ding, D.G.; Zhu, L. ZIF-derived porous ZnO-Co3O4 hollow polyhedrons heterostructure with highly enhanced ethanol detection performance. Sens. Actuators B 2017, 253, 523–532. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhou, F.; Gao, L.; Duan, G.T. Co3O4 nanosheet-built hollow spheres containing ultrafine neck-connected grains templated by PS@Co-LDH and their ppb-level gas-sensing performance. Sens. Actuators B 2018, 261, 553–565. [Google Scholar] [CrossRef]
- Xu, J.M.; Cheng, J.P. The advances of Co3O4 as gas sensing materials: A review. J. Alloys Compd. 2016, 686, 753–768. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, X.Y.; Liu, J.Y.; Wang, J.; Sun, Y.B. Facile synthesis of mesoporous ZnO/Co3O4 microspheres with enhanced gas-sensing for ethanol. Sens. Actuators B 2015, 221, 1492–1498. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Kheel, H.; Lee, C. Oxidizing gas sensing properties of the n-ZnO/p-Co3O4 composite nanoparticle network sensor. Sens. Actuators B 2016, 222, 1193–1200. [Google Scholar] [CrossRef]
- Xu, K.; Yang, L.; Yang, Y.; Yuan, C.L. Improved ethanol gas sensing performances of a ZnO/Co3O4 composite induced by its flytrap-like structure. Phys. Chem. Chem. Phys. 2017, 19, 29601–29607. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Gao, F.Q.; Zhao, Z.T.; Sang, S.B.; Li, P.W.; Zhang, W.D.; Zhou, X.T.; Chen, Y. Synthesis and characterization of Cobalt-doped ZnO microstructures for methane gas sensing. Appl. Surf. Sci. 2016, 363, 181–188. [Google Scholar] [CrossRef]
- Li, Y.W.; Luo, N.; Sun, G.; Zhang, B.; Jin, H.H.; Lin, L.; Bala, H.; Cao, J.L.; Zhang, Z.Y.; Wang, Y. Synthesis of porous nanosheets-assembled ZnO/ZnCo2O4 hierarchical structure for TEA detection. Sens. Actuators B 2019, 287, 199–208. [Google Scholar] [CrossRef]
- Zhang, S.S.; Sun, G.; Li, Y.W.; Zhang, B.; Wang, Y.; Zhang, Z.Y. Enhanced triethylamine gas sensing performance of the porous Zn2SnO4/SnO2 hierarchical microspheres. J. Alloys Compd. 2019, 785, 382–390. [Google Scholar] [CrossRef]
- Luo, N.; Sun, G.; Zhang, B.; Li, Y.W.; Jin, H.H.; Lin, L.; Bala, H.; Cao, J.L.; Zhang, Z.Y.; Wang, Y. Improved TEA sensing performance of ZnCo2O4 by structure evolution from porous nanorod to single-layer nanochain. Sens. Actuators B 2018, 277, 544–554. [Google Scholar] [CrossRef]
- Wang, D.; Chu, X.F.; Gong, M.L. Gas-sensing properties of sensors based on single-crystalline SnO2 nanorods prepared by a simple molten-salt method. Sens. Actuators B 2006, 117, 183–187. [Google Scholar] [CrossRef]
- Ju, D.X.; Xu, H.Y.; Qiu, Z.W.; Guo, J.; Zhang, J.; Cao, B.Q. Highly sensitive and selective triethylamine-sensing properties of nanosheets directly grown on ceramic tube by forming NiO/ZnO PN heterojunction. Sens. Actuators B 2014, 200, 288–296. [Google Scholar] [CrossRef]
- Chu, X.F.; Jiang, D.L.; Guo, Y.; Zheng, C.M. Ethanol gas sensor based on CoFe2O4 nano-crystallines prepared by hydrothermal method. Sens. Actuators B 2006, 120, 177–181. [Google Scholar]
- Sun, G.; Chen, H.L.; Li, Y.W.; Ma, G.Z.; Zhang, S.S.; Jia, T.K.; Cao, J.L.; Wang, X.D.; Bala, H.; Zhang, Z.Y. Synthesis and triethylamine sensing properties of mesoporous α-Fe2O3 microrods. Mater. Lett. 2016, 178, 213–216. [Google Scholar] [CrossRef]
- Xu, H.Y.; Ju, D.X.; Li, W.R.; Zhang, J.; Wang, J.Q.; Cao, B.Q. Superior triethylamine-sensing properties based on TiO2/SnO2 n-n heterojunction nanosheets directly grown on ceramic tubes. Sens. Actuators B 2016, 228, 634–642. [Google Scholar] [CrossRef]
- Yang, X.L.; Yu, Q.; Zhang, S.F.; Sun, P.; Lu, H.Y.; Yan, X.; Liu, F.M.; Zhou, X.; Liang, X.S.; Gao, Y.; et al. Highly sensitive and selective triethylamine gas sensor based on porous SnO2/Zn2SnO4 composites. Sens. Actuators B 2018, 266, 213–220. [Google Scholar] [CrossRef]
- Yang, H.Y.; Cheng, X.L.; Zhang, X.F.; Zheng, Z.K.; Tang, X.F.; Xu, Y.M.; Gao, S.; Zhao, H.; Huo, L.H. A novel sensor for fast detection of triethylamine based on rutile TiO2 nanorod arrays. Sens. Actuators B 2014, 205, 322–328. [Google Scholar] [CrossRef]
- Wu, M.Z.; Zhang, X.F.; Gao, S.; Cheng, X.L.; Rong, Z.M.; Xu, Y.M.; Zhao, H.; Huo, L.H. Construction of monodisperse vanadium pentoxide hollow spheres via a facile route and triethylamine sensing property. CrystEngComm 2013, 15, 10123–10131. [Google Scholar] [CrossRef]
- Li, W.R.; Xu, H.Y.; Zhai, T.; Yu, H.Q.; Chen, Z.R.; Qiu, Z.W.; Song, X.P.; Wang, J.Q.; Cao, B.Q. Enhanced triethylamine sensing properties by designing Au@SnO2/MoS2 nanostructure directly on alumina tubes. Sens. Actuators B 2017, 253, 97–107. [Google Scholar] [CrossRef]
- Zhai, T.; Xu, H.Y.; Li, W.R.; Yu, H.Q.; Chen, Z.R.; Wang, J.Q.; Cao, B.Q. Low-temperature in-situ growth of SnO2 nanosheets and its high triethylamine sensing response by constructing Au-loaded ZnO/SnO2 heterostructure. J. Alloys Compd. 2018, 737, 603–612. [Google Scholar] [CrossRef]
- Xue, D.P.; Wang, Y.; Cao, J.L.; Zhang, Z.Y. Hydrothermal Synthesis of CeO2-SnO2 Nanoflowers for Improving Triethylamine Gas Sensing Property. Nanomaterials 2018, 8, 1025. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Wang, C.; Guo, H.; Sun, P.; Liu, F.M.; Liang, X.S.; Lu, G.Y. Double-Shell Architectures of ZnFe2O4 Nanosheets on ZnO Hollow Spheres for High-Performance Gas Sensors. ACS Appl. Mater. Interfaces 2015, 7, 17811–17818. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, X.N.; Yao, M.X.; Sun, G.; Zhang, Z.Y. Enhanced CH4 sensing properties of Pd modified ZnO nanosheets. Ceram. Int. 2019, 45, 13150–13157. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, S.M.; Hao, P.; Tian, J.; Cui, H.Z.; Wang, X.Z. Soft-templated formation of double-shelled ZnO hollow microspheres for acetone gas sensing at low concentration/near room temperature. Sens. Actuators B 2018, 273, 751–759. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Yang, Z.M.; Wu, Z.L.; Dong, G.K. Metal-organic frameworks-derived hollow zinc oxide/cobalt oxide nanoheterostructure for highly sensitive acetone sensing. Sens. Actuators B 2019, 283, 42–51. [Google Scholar] [CrossRef]
- Li, B.; Liu, J.Y.; Liu, Q.; Chen, R.R.; Zhang, H.S.; Yu, J.; Song, D.L.; Li, J.Q.; Zhang, M.L.; Wang, J. Core-shell structure of ZnO/Co3O4 composites derived from bimetallicorganic frameworks with superior sensing performance for ethanol gas. Appl. Surf. Sci. 2019, 475, 700–709. [Google Scholar] [CrossRef]
- Song, Z.H.; Chen, W.G.; Zhang, H.; Li, Y.Q.; Zeng, W.; Tang, S.R.; Zhu, C.Z. Highly sensitive and selective acetylene sensors based on p-n heterojunction of NiO nanoparticles on flower-like ZnO structures. Ceram. Int. 2019, 45, 19635–19643. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.P.; Wang, B.Q.; Sun, P.; Wang, Q.J.; Gao, Y.; Liang, X.S.; Zhang, T.; Lu, G.Y. Acetone gas sensor based on NiO/ZnO hollow spheres: Fast response and recovery, and low (ppb) detection limit. J. Colloid Interface Sci. 2017, 495, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Yin, J.; Li, L.; Zhang, L.X.; Bie, L.J. Enhanced ethanol gas-sensing properties of flower-like p-CuO/n-ZnO heterojunction nanorods. Sens. Actuators B 2014, 202, 500–507. [Google Scholar] [CrossRef]
- Poloju, M.; Jayababu, N.; Ramana Reddy, M.V. Improved gas sensing performance of Al doped ZnO/CuO nanocomposite based ammonia gas sensor. Mater. Sci. Eng. B 2018, 227, 61–67. [Google Scholar] [CrossRef]

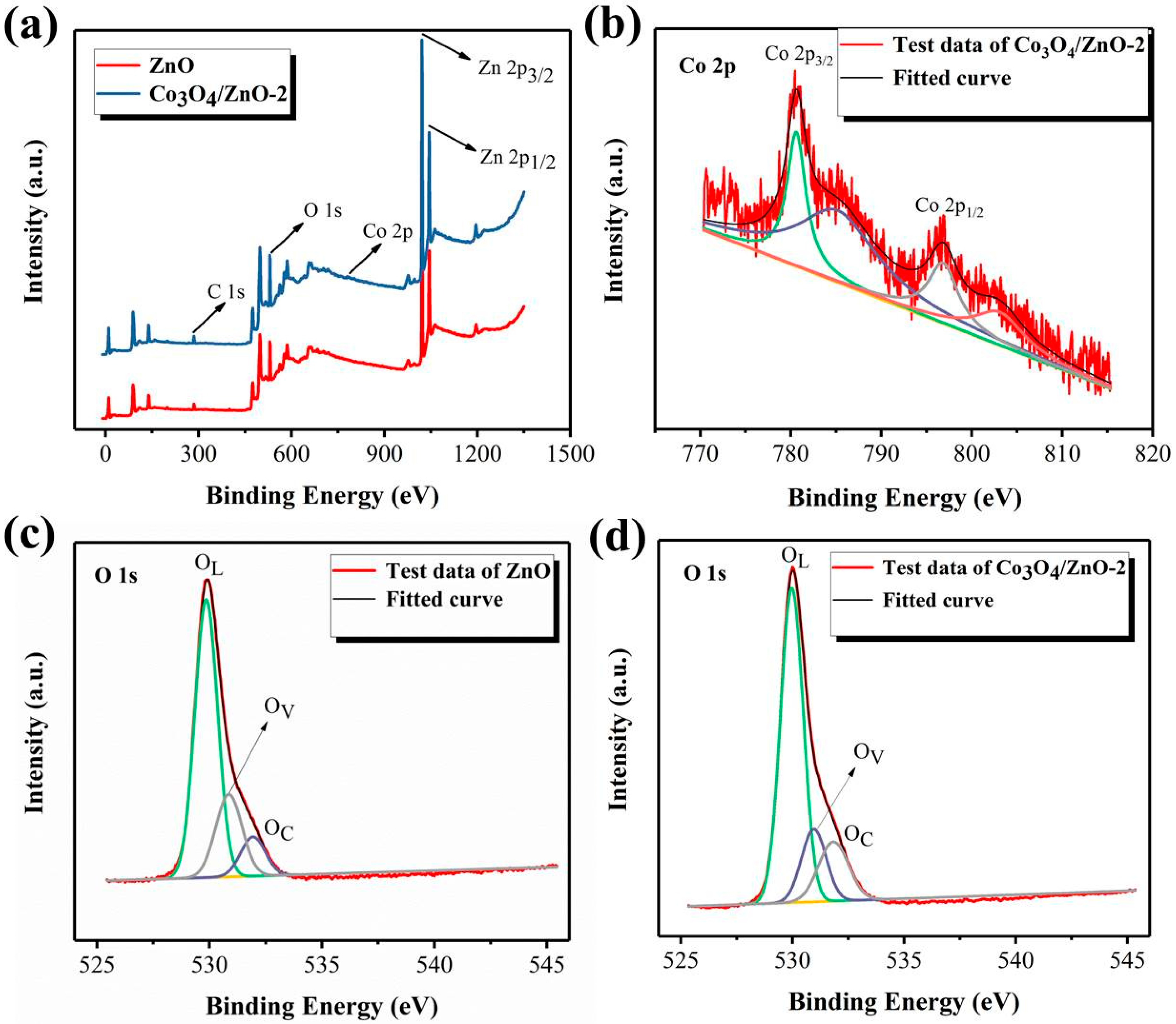

| Materials | OL (%) | OV (%) | OC (%) | Ene. OL (eV) | Ene. OV (eV) | Ene. OC (eV) |
|---|---|---|---|---|---|---|
| ZnO | 66.8 | 22.99 | 10.21 | 529.85 | 530.83 | 531.93 |
| Co3O4/ZnO-2 | 66.6 | 16.75 | 16.65 | 529.97 | 530.95 | 531.84 |
| Materials | Tem. (°C) | Con. (ppm) | Res. | Tres/Trec. (s) | Ref. |
|---|---|---|---|---|---|
| SnO2 nanorods | 350 | 50 | 64.8 | - | [29] |
| NiO-ZnO nanosheets | 320 | 100 | 300 | - | [30] |
| Nano-CoFe2O4 | 190 | 10 | 2 | 100/120 | [31] |
| α-Fe2O3 microrods | 275 | 100 | 11.8 | - | [32] |
| TiO2-SnO2 nanosheets | 260 | 100 | 52.3 | - | [33] |
| SnO2-Zn2SnO4 spheres | 250 | 100 | 48 | - | [34] |
| TiO2 nanorods | 290 | 100 | 14.2 | - | [35] |
| V2O5 hollow spheres | 370 | 100 | 7.2 | 20/96 | [36] |
| Au@SnO2/MoS2 | 300 | 100 | 96 | 12/66 | [37] |
| Au@ZnO/SnO2 | 300 | 100 | 115 | 7/30 | [38] |
| CeO2-SnO2 nanoflowers | 310 | 200 | 252.2 | - | [39] |
| Co3O4-ZnO | 280 | 100 | 112.5 | 22/26 | This work |
| 280 | 200 | 282.3 | 25/36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, X.; Yi, G.; Li, H.; Shi, C.; Sun, G.; Zhang, Z. Hydrothermal Synthesis of Co3O4/ZnO Hybrid Nanoparticles for Triethylamine Detection. Nanomaterials 2019, 9, 1599. https://doi.org/10.3390/nano9111599
Yang Y, Wang X, Yi G, Li H, Shi C, Sun G, Zhang Z. Hydrothermal Synthesis of Co3O4/ZnO Hybrid Nanoparticles for Triethylamine Detection. Nanomaterials. 2019; 9(11):1599. https://doi.org/10.3390/nano9111599
Chicago/Turabian StyleYang, Yanqiong, Xiaodong Wang, Guiyun Yi, Huimin Li, Chuang Shi, Guang Sun, and Zhanying Zhang. 2019. "Hydrothermal Synthesis of Co3O4/ZnO Hybrid Nanoparticles for Triethylamine Detection" Nanomaterials 9, no. 11: 1599. https://doi.org/10.3390/nano9111599
APA StyleYang, Y., Wang, X., Yi, G., Li, H., Shi, C., Sun, G., & Zhang, Z. (2019). Hydrothermal Synthesis of Co3O4/ZnO Hybrid Nanoparticles for Triethylamine Detection. Nanomaterials, 9(11), 1599. https://doi.org/10.3390/nano9111599




