Nanotechnology in Oral Cavity Carcinoma: Recent Trends and Treatment Opportunities
Abstract
1. Introduction
2. Cancer Nanotechnology
3. Diagnostic Procedures
Lymphotropic Nanoparticles
4. Treatment Opportunities
4.1. Ongoing Clinical Trials
4.2. Sentinel Lymph Node Analysis
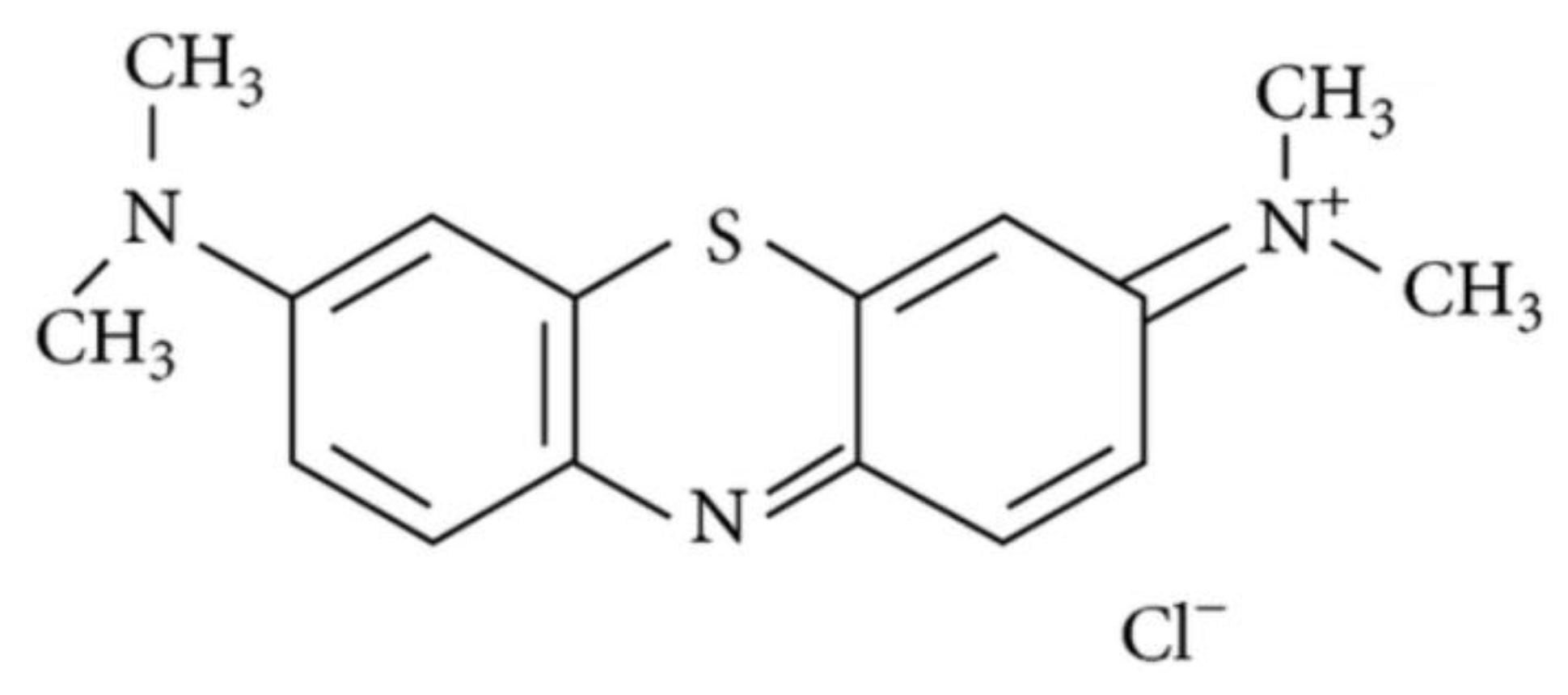
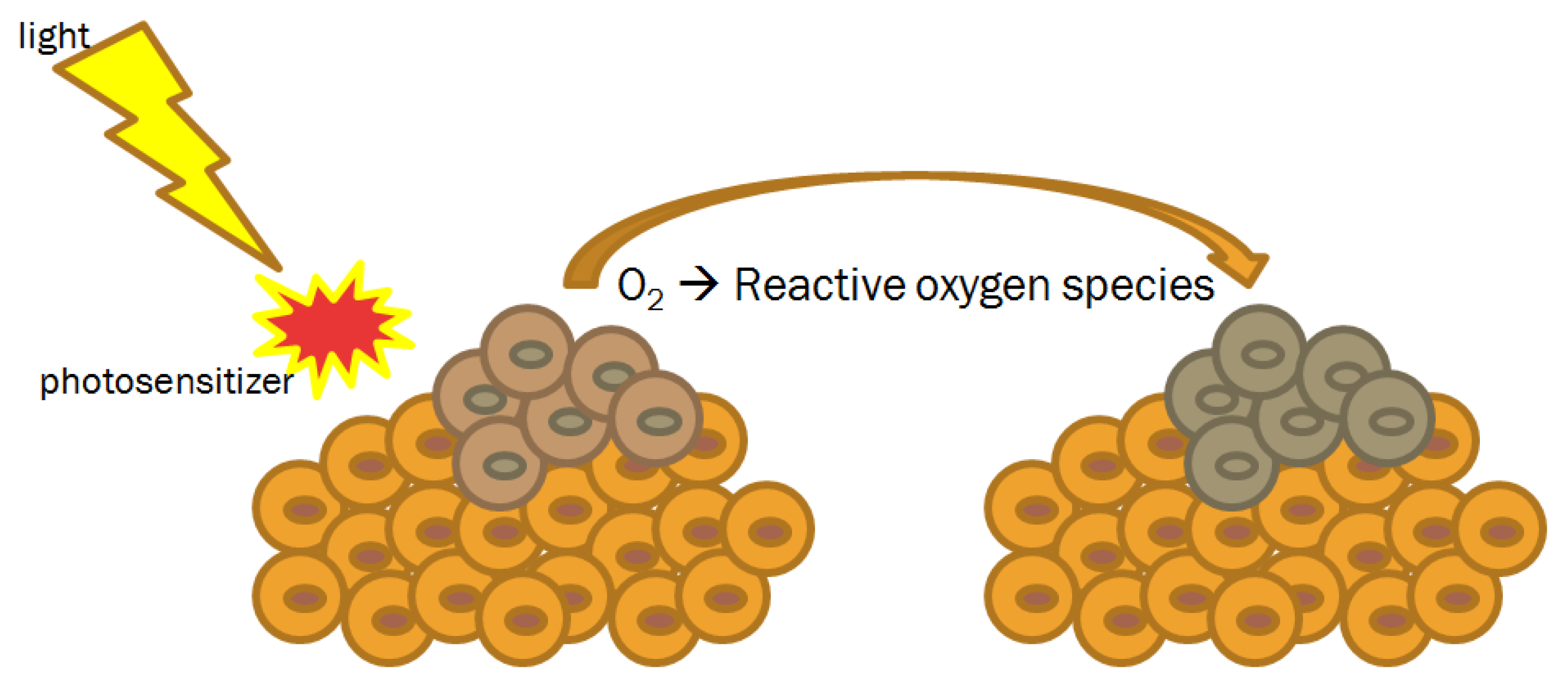
4.3. Photodynamic Therapy
5. Conclusions
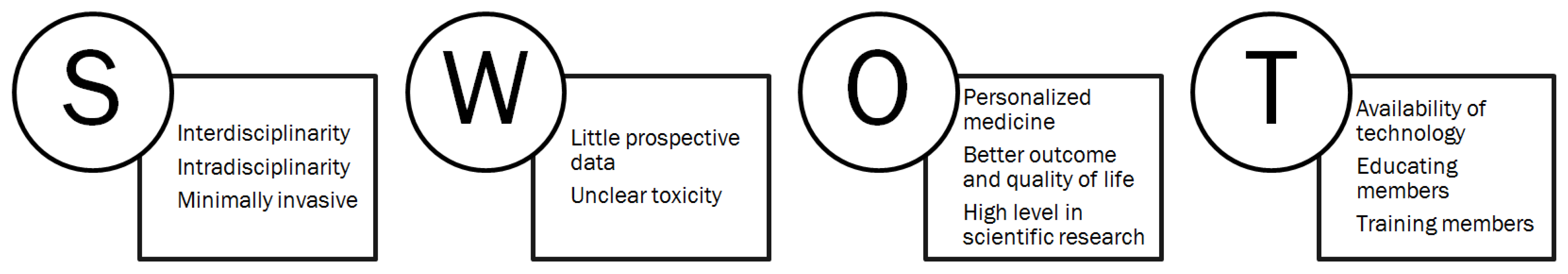
6. Direction for Future Research
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; et al. SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. Bethesda. Available online: https://seer.cancer.gov/archive/csr/1975_2014 (accessed on 7 October 2019).
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Head and Neck Cancers, Version 2.2019. Available online: http://www.nccn.org (accessed on 7 October 2019).
- De Felice, F.; Musio, D.; Tombolini, V. Osteoradionecrosis and intensity modulated radiation therapy: An overview. Crit. Rev. Oncol. Hematol. 2016, 107, 39–43. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; de Vincentiis, M.; Luzzi, V.; Magliulo, G.; Tombolini, M.; Ruoppolo, G.; Polimeni, A. Late radiation-associated dysphagia in head and neck cancer patients: Evidence, research and management. Oral Oncol. 2018, 77, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Nanoparticle delivery platform for solid tumors. In Proceedings of the ASCO Annual Meeting 2014, Chicago, IL, USA, 30 May–3 June 2014. [Google Scholar]
- Calixto, G.; Bernegossi, J.; Fonseca-Santos, B.; Chorilli, M. Nanotechnology-based drug delivery systems for treatment of oral cancer: A review. Int. J. Nanomed. 2014, 9, 3719–3735. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Meng, H.; Xu, K.; Xu, Y.; Luo, P.; Luo, Y.; Lu, W.; Huang, J.; Liu, S.; Yu, J. CPT loaded nanoparticles based on beta-cyclodextrin-grafted poly(ethylene glycol)/poly (L-glutamic acid) diblock copolymer and their inclusion complexes with CPT. Coll. Surf. B Biointerfaces 2014, 113, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Min, K.H.; Lee, S.C.; Park, J.H.; Park, K.; Jeong, S.Y.; Choi, K.; Kwon, I.C.; Kim, K. Enhanced drug-loading and therapeutic efficacy of hydrotropic oligomer-conjugated glycol chitosan nanoparticles for tumor-targeted paclitaxel delivery. J. Control. Release 2013, 172, 823–831. [Google Scholar] [CrossRef]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine 2010, 6, 153–160. [Google Scholar] [CrossRef]
- Endo, K.; Ueno, T.; Kondo, S.; Wakisaka, N.; Murono, S.; Ito, M.; Kataoka, K.; Kato, Y.; Yoshizaki, T. Tumor-targeted chemotherapy with the nanopolymer-based drug NC-6004 for oral squamous cell carcinoma. Cancer Sci. 2013, 104, 369–374. [Google Scholar] [CrossRef]
- Kasahara, Y.; Endo, K.; Ueno, T.; Ueno, H.; Moriyama-Kita, M.; Odani, A.; Yoshizaki, T. Bone invasion-targeted chemotherapy with a novel anionic platinum complex (3Pt) for oral squamous cell carcinoma. Cancer Sci. 2019, 110, 3288–3295. [Google Scholar] [CrossRef]
- Mezei, M.; Gulasekharam, V. Liposomes—A selective drug delivery system for the topical route of administration. Lotion dosage form. Life Sci. 1980, 26, 1473–1477. [Google Scholar] [CrossRef]
- Manaia, E.B.; Abuçafy, M.P.; Chiari-Andréo, B.G.; Silva, B.L.; Oshiro Junior, J.A.; Chiavacci, L.A. Physicochemical characterization of drug nanocarriers. Int. J. Nanomed. 2017, 12, 4991–5011. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Hu, Q.; Liu, Z.; Gao, X.; Hu, R.; Song, Q.; Gu, G.; Xia, H.; Yao, L.; Pang, Z.; et al. Preparation and characterization of paclitaxel-loaded DSPE-PEG-liquid crystalline nanoparticles (LCNPs) for improved bioavailability. Int. J. Pharm. 2012, 424, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, L.; Sun, Y. Nanotechnology applied to overcome tumor drug resistance. J Control. Release 2012, 162, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P.H.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles—Known and unknown health risks. J. Nanobiotechnol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; le Maître, A.; Maillard, E.; Bourhis, J.; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Puyo, S.; Montaudon, D.; Pourquier, P. From old alkylating agents to new minor groove binders. Crit. Rev. Oncol. Hematol. 2014, 89, 43–61. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Liu, K.; Huo, Z.J.; Li, X.C.; Wang, M.; Liu, P.; Pang, B.; Wang, S.J. A cell-targeted chemotherapeutic nanomedicine strategy for oral squamous cell carcinoma therapy. J Nanobiotechnology 2015, 13, 63. [Google Scholar] [CrossRef]
- Caster, J.M.; Patel, A.N.; Zhang, T.; Wang, A. Investigational nanomedicines in 2016: A review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1416. [Google Scholar] [CrossRef]
- Robbins, K.T.; Medina, J.E.; Wolfe, G.T.; Levine, P.A.; Sessions, R.B.; Pruet, C.W. Standardizing neck dissection terminology. Official report of the academy’s committee for head and neck surgery and oncology. Arch. Otolaryngol. Head Neck Surg. 1991, 117, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.G.; Balzer, J.O.; Straub, R.; Eichler, K.; Vogl, T.J. Superparamagnetic iron oxide-enhanced MR imaging of head and neck lymph nodes. Radiology 2002, 222, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.G.; Rieger, J.; Baghi, M.; Bisdas, S.; Vogl, T.J. Cervical lymph nodes. Eur. J. Radiol. 2008, 66, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Anzai, Y.; Piccoli, C.W.; Outwater, E.K.; Stanford, W.; Bluemke, D.A.; Nurenberg, P.; Saini, S.; Maravilla, K.R.; Feldman, D.E.; Schmiedl, U.P.; et al. Evaluation of neck and body metastases to nodes with ferumoxtran 10-enhanced MR imaging: Phase III safety and efficacy study. Radiology 2003, 228, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, P.A.; Anzai, Y.; Morris, M.R.; Lucas, M.A. Ferumoxtran-10, a superparamagnetic iron oxide as a magnetic resonance enhancement agent for imaging lymph nodes: A phase 2 dose study. AJNR Am. J. Neuroradiol. 2002, 23, 649–656. [Google Scholar]
- Wang, Y.X.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef]
- Hoffman, H.T.; Quets, J.; Toshiaki, T.; Funk, G.F.; McCulloch, T.M.; Graham, S.M.; Robinson, R.A.; Schuster, M.E.; Yuh, W.T. Functional magnetic resonance imaging using iron oxide particles in characterizing head and neck adenopathy. Laryngoscope 2000, 110, 1425–1430. [Google Scholar] [CrossRef]
- Sigal, R.; Vogl, T.; Casselman, J.; Moulin, G.; Veillon, F.; Hermans, R.; Dubrulle, F.; Viala, J.; Bosq, J.; Mack, M.; et al. Lymph node metastases from head and neck squamous cell carcinoma: MR imaging with ultrasmall superparamagnetic iron oxide particles (Sinerem MR)—Results of a phase-III multicenter clinical trial. Eur. Radiol. 2002, 12, 1104–1113. [Google Scholar] [CrossRef]
- Curvo-Semedo, L.; Diniz, M.; Miguéis, J.; Julião, M.J.; Martins, P.; Pinto, A.; Caseiro-Alves, F. USPIO-enhanced magnetic resonance imaging for nodal staging in patients with head and neck cancer. J. Magn. Reson. Imaging 2006, 24, 123–131. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Moreno, V.; Salas, S.; Mirabel, X.; Calvo, E.; Doger, B.; Florescu, C.; Thariat, J.; Fijuth, J.; Rutkowski, T.; et al. Hafnium oxide nanoparticles NBTXR3 activated by radiotherapy as a new therapeutic option for elderly/frail HNSCC patients. J. Clin. Oncol. 2019, 37, 6069. [Google Scholar] [CrossRef]
- Satapathy, S.R.; Mohapatra, P.; Preet, R.; Das, D.; Sarkar, B.; Choudhuri, T.; Wyatt, M.D.; Kundu, C.N. Silver-based nanoparticles induce apoptosis in human colon cancer cells mediated through p53. Nanomedicine (Lond.) 2013, 8, 1307–1322. [Google Scholar] [CrossRef] [PubMed]
- Swanner, J.; Mims, J.; Carroll, D.L.; Akman, S.A.; Furdui, C.M.; Torti, S.V.; Singh, R.N. Differential cytotoxic and radiosensitizing effects of silver nanoparticles on triple-negative breast cancer and non-triple-negative breast cells. Int. J. Nanomed. 2015, 10, 3937–3953. [Google Scholar]
- Hsin, Y.H.; Chen, C.F.; Huang, S.; Shih, T.S.; Lai, P.S.; Chueh, P.J. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol. Lett. 2008, 179, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Moore, W.; Fattah, F.; Jiang, X.; Zheng, J.; Kurian, P.; Beg, M.S.; Khan, S.A. Activity and pharmacology of homemade silver nanoparticles in refractory metastatic head and neck squamous cell cancer. Head Neck 2019, 41, E11–E16. [Google Scholar]
- Alex, J.C.; Krag, D.N. Gamma-probe guided localization of lymph nodes. Surg. Oncol. 1993, 2, 137–143. [Google Scholar] [CrossRef]
- Morton, D.L.; Wen, D.R.; Wong, J.H.; Economou, J.S.; Cagle, L.A.; Storm, F.K.; Foshag, L.J.; Cochran, A.J. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch. Surg. 1992, 127, 392–399. [Google Scholar] [CrossRef]
- Ross, G.L.; Soutar, D.S.; MacDonald, D.G.; Shoaib, T.; Camilleri, I.G.; Robertson, A.G. Improved staging of cervical metastases in clinically node-negative patients with head and neck squamous cell carcinoma. Ann. Surg. Oncol. 2004, 11, 213–218. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.J.; Yang, X.; Peng, H. Diagnostic efficacy of sentinel lymph node biopsy in early oral squamous cell carcinoma: A meta-analysis of 66 studies. PLoS ONE 2017, 12, e0170322. [Google Scholar] [CrossRef]
- Hernando, J.; Villarreal, P.; Alvarez-Marcos, F.; Gallego, L.; García-Consuegra, L.; Junquera, L. Comparison of related complications: Sentinel node biopsy versus elective neck dissection. Int. J. Oral Maxillofac. Surg. 2014, 43, 1307–1312. [Google Scholar] [CrossRef]
- Murer, K.; Huber, G.F.; Haile, S.R.; Stoeckli, S.J. Comparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the n0 neck in patients with oral squamous cell carcinoma. Head Neck 2011, 33, 1260–1264. [Google Scholar] [CrossRef]
- Schiefke, F.; Akdemir, M.; Weber, A.; Akdemir, D.; Singer, S.; Frerich, B. Function, postoperative morbidity, and quality of life after cervical sentinel node biopsy and after selective neck dissection. Head Neck 2009, 31, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Constant, C.R.; Murley, A.H. A clinical method of functional assessment of the shoulder. Clin. Orthop. Relat. Res. 1987, 214, 160–164. [Google Scholar] [CrossRef]
- Mølstrøm, J.; Grønne, M.; Green, A.; Bakholdt, V.; Sørensen, J.A. Topographical distribution of sentinel nodes and metastases from T1-T2 oral squamous cell carcinomas. Eur. J. Cancer 2019, 107, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Chone, C.T.; Aniteli, M.B.; Magalhães, R.S.; Freitas, L.L.; Altemani, A.; Ramos, C.D.; Etchebehere, E.; Crespo, A.N. Impact of immunohistochemistry in sentinel lymph node biopsy in head and neck cancer. Eur. Arch. Otorhinolaryngol. 2013, 270, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, J.R.; Kumar, V.; Gupta, S.; Chaturvedi, A.; Misra, S.; Akhtar, N.; Agarwal, P.; Jamal, N.; Pareek, P. Outcome of sentinel lymph node biopsy in early-stage squamous cell carcinoma of the oral cavity with methylene blue dye alone: A prospective validation study. Br. J. Oral Maxillofac. Surg. 2019, 57, 755–759. [Google Scholar] [CrossRef]
- Ramamurthy, R.; Kottayasamy Seenivasagam, R.; Shanmugam, S.; Palanivelu, K. A prospective study on sentinel lymph node biopsy in early oral cancers using methylene blue dye alone. Indian J. Surg. Oncol. 2014, 5, 178–183. [Google Scholar] [CrossRef]
- Troyan, S.L.; Kianzad, V.; Gibbs-Strauss, S.L.; Gioux, S.; Matsui, A.; Oketokoun, R.; Ngo, L.; Khamene, A.; Azar, F.; Frangioni, J.V. The FLARE intraoperative near-infrared fluorescence imaging system: A first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann. Surg. Oncol. 2009, 16, 2943–2952. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, Z.; Ngandeu Neubi, G.M.; Cheng, H.; Zhang, C.; Zhang, H.; Wang, R.; Zhou, J.; Ding, Y. Lipoprotein-inspired penetrating nanoparticles for deep tumor-targeted shuttling of indocyanine green and enhanced photo-theranostics. Biomater. Sci. 2019, 7, 3425–3437. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Tse, B.W.; Yang, H.; Thorling, C.A.; Liu, Y.; Touraud, M.; Chouane, J.B.; Liu, X.; Roberts, M.S.; et al. Indocyanine green-incorporating nanoparticles for cancer theranostics. Theranostics 2018, 8, 1227–1242. [Google Scholar] [CrossRef]
- Hutteman, M.; Mieog, J.S.; van der Vorst, J.R.; Liefers, G.J.; Putter, H.; Löwik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. Randomized, double-blind comparison of indocyanine green with or without albumin premixing for near-infrared fluorescence imaging of sentinel lymph nodes in breast cancer patients. Breast Cancer Res. Treat. 2011, 127, 163–170. [Google Scholar] [CrossRef]
- Hutteman, M.; van der Vorst, J.R.; Gaarenstroom, K.N.; Peters, A.A.; Mieog, J.S.; Schaafsma, B.E.; Löwik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. Optimization of near-infrared fluorescent sentinel lymph node mapping for vulvar cancer. Am. J. Obstet. Gynecol. 2012, 206, 89.e1–89.e5. [Google Scholar] [CrossRef] [PubMed]
- van der Vorst, J.R.; Hutteman, M.; Gaarenstroom, K.N.; Peters, A.A.; Mieog, J.S.; Schaafsma, B.E.; Kuppen, P.J.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. Optimization of near-infrared fluorescent sentinel lymph node mapping in cervical cancer patients. Int. J. Gynecol. Cancer 2011, 21, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Lee, C.M.; Cheong, S.J.; Kim, E.M.; Hwang, H.; Na, K.S.; Lim, S.T.; Sohn, M.H.; Jeong, H.J. The effect of mannosylation of liposome-encapsulated indocyanine green on imaging of sentinel lymph node. J. Liposome Res. 2013, 23, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Bredell, M.G. Sentinel lymph node mapping by indocyanin green fluorescence imaging in oropharyngeal cancer—Preliminary experience. Head Neck Oncol. 2010, 2, 31. [Google Scholar] [CrossRef]
- van der Vorst, J.R.; Schaafsma, B.E.; Verbeek, F.P.; Keereweer, S.; Jansen, J.C.; van der Velden, L.A.; Langeveld, A.P.; Hutteman, M.; Löwik, C.W.; van de Velde, C.J.; et al. Near-infrared fluorescence sentinel lymph node mapping of the oral cavity in head and neck cancer patients. Oral Oncol. 2013, 49, 15–19. [Google Scholar] [CrossRef]
- Mieog, J.S.; Troyan, S.L.; Hutteman, M.; Donohoe, K.J.; van der Vorst, J.R.; Stockdale, A.; Liefers, G.J.; Choi, H.S.; Gibbs-Strauss, S.L.; Putter, H.; et al. Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann. Surg. Oncol. 2011, 18, 2483–2491. [Google Scholar] [CrossRef]
- van der Poel, H.G.; Buckle, T.; Brouwer, O.R.; Valdés Olmos, R.A.; van Leeuwen, F.W. Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: Clinical proof of concept of an integrated functional imaging approach using a multimodal tracer. Eur. Urol. 2011, 60, 826–833. [Google Scholar] [CrossRef]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Hopper, C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2000, 1, 212–219. [Google Scholar] [CrossRef]
- Jeskdnex, A.; Tappeiner, V.H. Zut behandlung der hautcarcinomit mit fluoresciercnden stoffen. Muench. Med. Wochneshr. 1903, 47, 2042–2044. [Google Scholar]
- von Tappeiner, H.; Jodlbauer, A. Ueber die Wukung der photodynamischen (fluores- cierenden) Stoffe auf Protozoen und Enzyme. Arch. Klin. Med. 1904, 80, 427–487. [Google Scholar]
- Pass, H.I. Photodynamic therapy in oncology: Mechanisms and clinical use. J. Natl. Cancer Inst. 1993, 85, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar] [PubMed]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and type II photosensitized oxidation reactions: Guidelines and mechanistic pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Wondrak, G.T.; Jacobson, M.K.; Jacobson, E.L. Endogenous UVA-photosensitizers: Mediators of skin photodamage and novel targets for skin photoprotection. Photochem. Photobiol. Sci. 2006, 5, 215–237. [Google Scholar] [CrossRef]
- Gondivkar, S.M.; Gadbail, A.R.; Choudhary, M.G.; Vedpathak, P.R.; Likhitkar, M.S. Photodynamic treatment outcomes of potentially-malignant lesions and malignancies of the head and neck region: A systematic review. J. Investig. Clin. Dent. 2018, 9. [Google Scholar] [CrossRef]
- Karakullukcu, B.; van Oudenaarde, K.; Copper, M.P.; Klop, W.M.; van Veen, R.; Wildeman, M.; Bing Tan, I. Photodynamic therapy of early stage oral cavity and oropharynx neoplasms: An outcome analysis of 170 patients. Eur. Arch. Otorhinolaryngol. 2011, 268, 281–288. [Google Scholar] [CrossRef]
- Biel, M.A. Photodynamic therapy treatment of early oral and laryngeal cancers. Photochem. Photobiol. 2007, 83, 1063–1068. [Google Scholar] [CrossRef]
- Schweitzer, V.G. PHOTOFRIN-mediated photodynamic therapy for treatment of early stage oral cavity and laryngeal malignancies. Lasers Surg. Med. 2001, 29, 305–313. [Google Scholar] [CrossRef]
- Grant, W.E.; Hopper, C.; Speight, P.M.; Macrobert, A.J.; Bown, S.G. Photodynamic therapy of malignant and premalignant lesions in patients with ‘field cancerization’ of the oral cavity. J. Laryngol. Otol. 1993, 107, 1140–1145. [Google Scholar] [CrossRef]
- Hopper, C.; Kübler, A.; Lewis, H.; Tan, I.B.; Putnam, G. mTHPC-mediated photodynamic therapy for early oral squamous cell carcinoma. Int. J. Cancer 2004, 111, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Mimikos, C.; Shafirstein, G.; Arshad, H. Current state and future of photodynamic therapy for the treatment of head and neck squamous cell carcinoma. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef] [PubMed]

| Nanomaterial | Structure | Advantages | Limitations |
|---|---|---|---|
| Nanoparticle | polysaccharides, proteins and biocompatible/biodegradable polymers | biocompatibility, biodegradability | toxicity? |
| Liposome | membrane-like lipid layers, often phospholipids and cholesterol | permeability, charge density, steric hindrance | Less stable than nanoparticles |
| Hydrogel | hydrophilic polymeric chains dispersed in water | hydrophilicity, flexibility, versatility, high water absorptivity, biocompatibility; it interacts with saliva glycoproteins, causing mucoadhesion | slow response time |
| Liquid crystal | materials in mesophase state | it can be stored for long periods because thermodynamically stable | toxicity? |
| Diagnosis | Patterns Post Contrast | Characteristic |
|---|---|---|
| Non-pathologic node |  | Node having an overall dark signal intensity; homogenous architecture |
| Non-pathologic node |  | Node having an overall dark signal with subtle granularities; homogenous architecture |
| Non-pathologic node |  | Node having an overall dark signal other than a central or hilar area of fat seen on T1 sequence; heterogenous architecture |
| Possibly pathologic node |  | Less than 50% of node has high signal intensity; heterogenous architecture |
| Pathologic node |  | Partial darkening whereby more than 50% of the node has area of high signal intensity; heterogenous architecture |
| Pathologic node |  | Node has central high signal with darkening along the peripheral rim; heterogenous architecture |
| Pathologic node |  | No blackening of node or node hyperintense to surrounding tissue; is heterogenous or homogenous architecture |
| Author | Year | Study Type | OCC Patients | Photosensitizers | Outcomes |
|---|---|---|---|---|---|
| Karakullukcu [70] | 2011 | Retrospective | 105 | Temoporfin | OR: 91.4%; CR: 68.6%; 2-y DFS: 74%; 5-yDFS: 61% |
| Biel [71] | 2007 | Retrospective | 161 | Photofrin | CR: 93.2% |
| Schweitzer [72] | 2001 | Retrospective | 10 | Photofrin | CR: 80% |
| Grant [73] | 1993 | Retrospective | 11 | Photofrin | CR: 90.9% |
| Hopper [74] | 2004 | Phase IIb | 121 | Meta-tetrahydroxyphenylchlorin | CR: 85%; 1-y OS: 89%; 2-y OS: 75% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Felice, F.; Cavallini, C.; Barlattani, A.; Tombolini, M.; Brugnoletti, O.; Tombolini, V.; Polimeni, A. Nanotechnology in Oral Cavity Carcinoma: Recent Trends and Treatment Opportunities. Nanomaterials 2019, 9, 1546. https://doi.org/10.3390/nano9111546
De Felice F, Cavallini C, Barlattani A, Tombolini M, Brugnoletti O, Tombolini V, Polimeni A. Nanotechnology in Oral Cavity Carcinoma: Recent Trends and Treatment Opportunities. Nanomaterials. 2019; 9(11):1546. https://doi.org/10.3390/nano9111546
Chicago/Turabian StyleDe Felice, Francesca, Costanza Cavallini, Alberta Barlattani, Mario Tombolini, Orlando Brugnoletti, Vincenzo Tombolini, and Antonella Polimeni. 2019. "Nanotechnology in Oral Cavity Carcinoma: Recent Trends and Treatment Opportunities" Nanomaterials 9, no. 11: 1546. https://doi.org/10.3390/nano9111546
APA StyleDe Felice, F., Cavallini, C., Barlattani, A., Tombolini, M., Brugnoletti, O., Tombolini, V., & Polimeni, A. (2019). Nanotechnology in Oral Cavity Carcinoma: Recent Trends and Treatment Opportunities. Nanomaterials, 9(11), 1546. https://doi.org/10.3390/nano9111546






