Facile Preparation of Self-Assembled Polydopamine-Modified Electrospun Fibers for Highly Effective Removal of Organic Dyes
Abstract
1. Introduction
2. Experimental Method
2.1. Materials
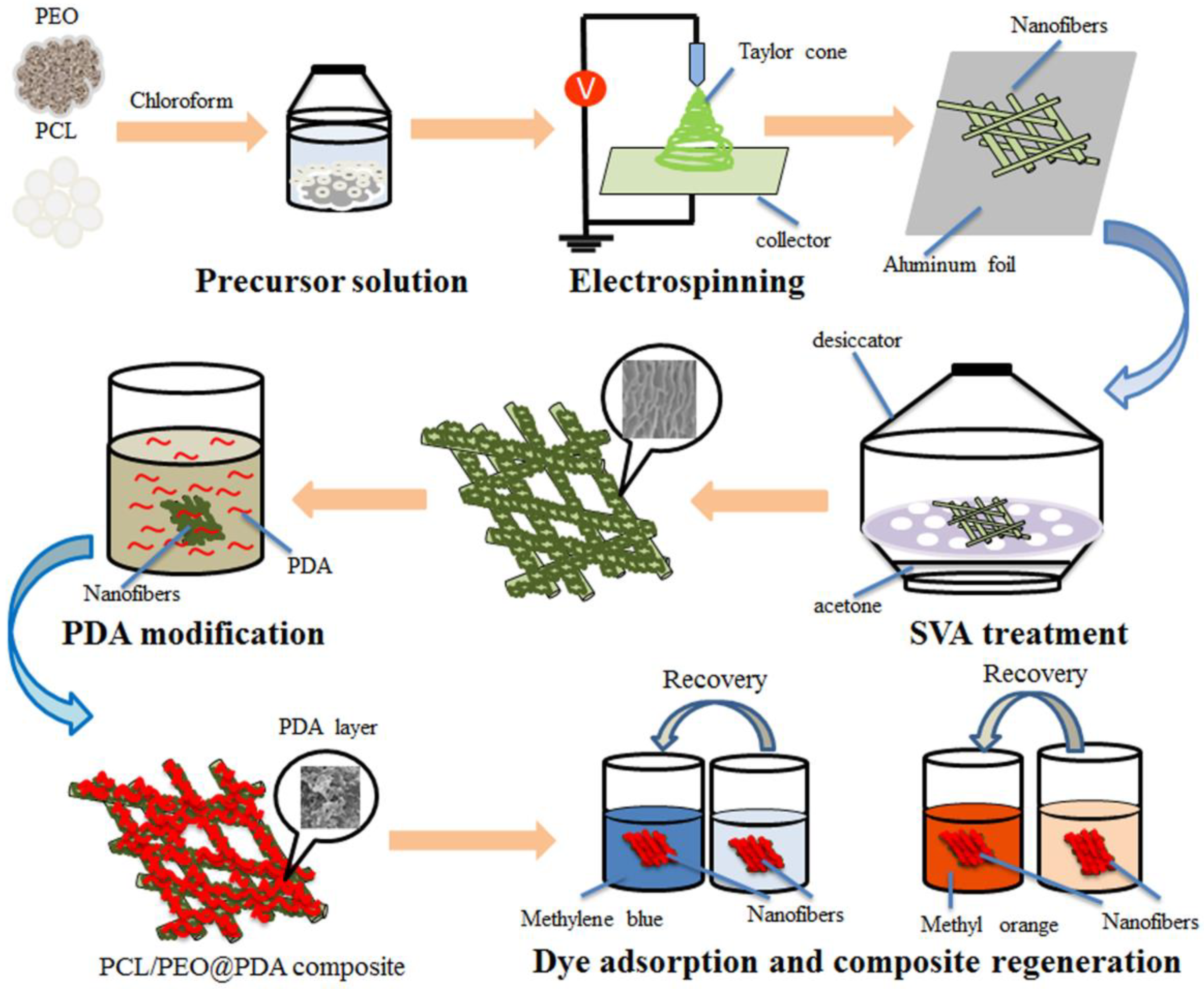
2.2. Preparation of PCL/PEO@PDA Nanocomposites
2.3. Adsorption Capacity Test
2.4. Characterization
3. Results and Discussion
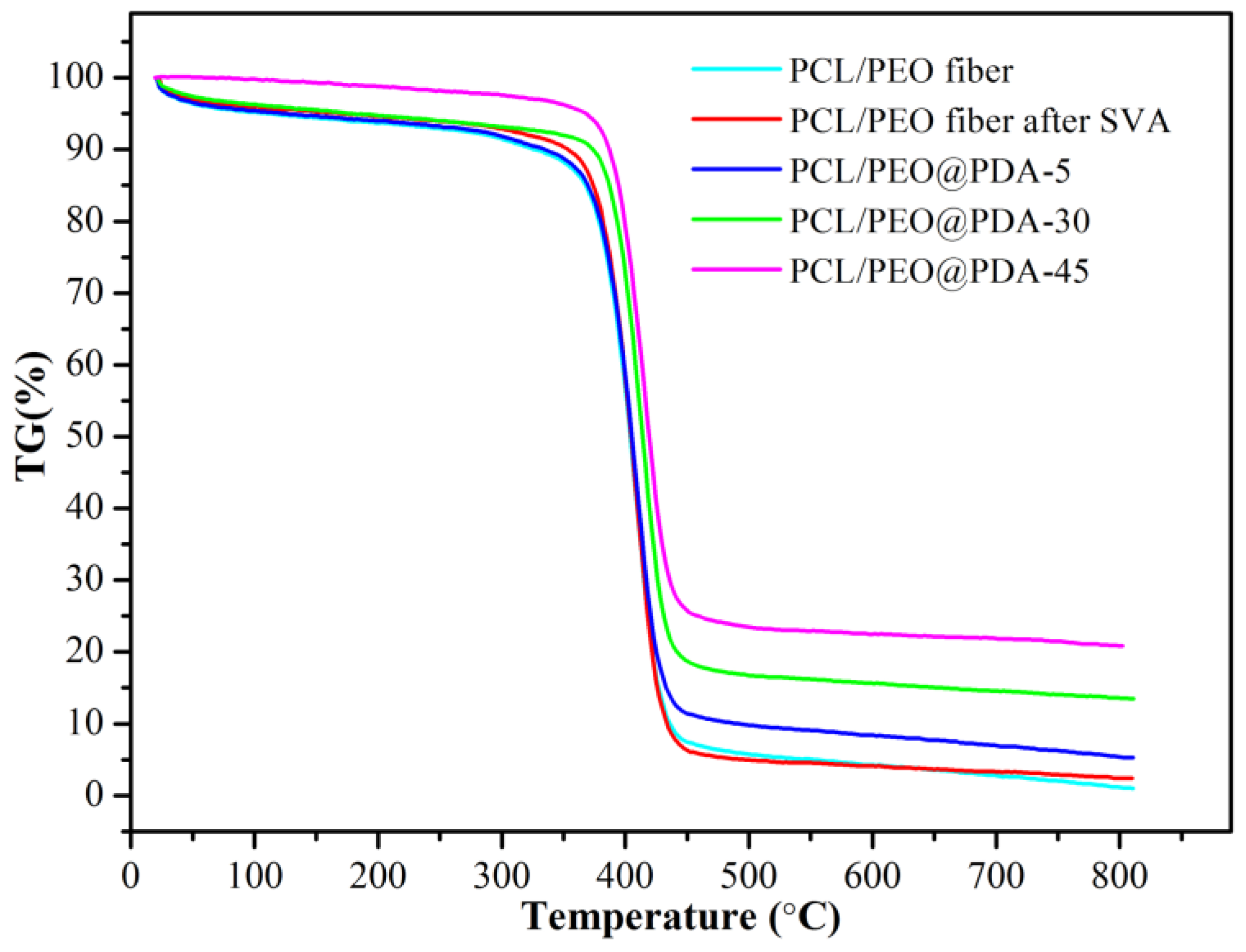
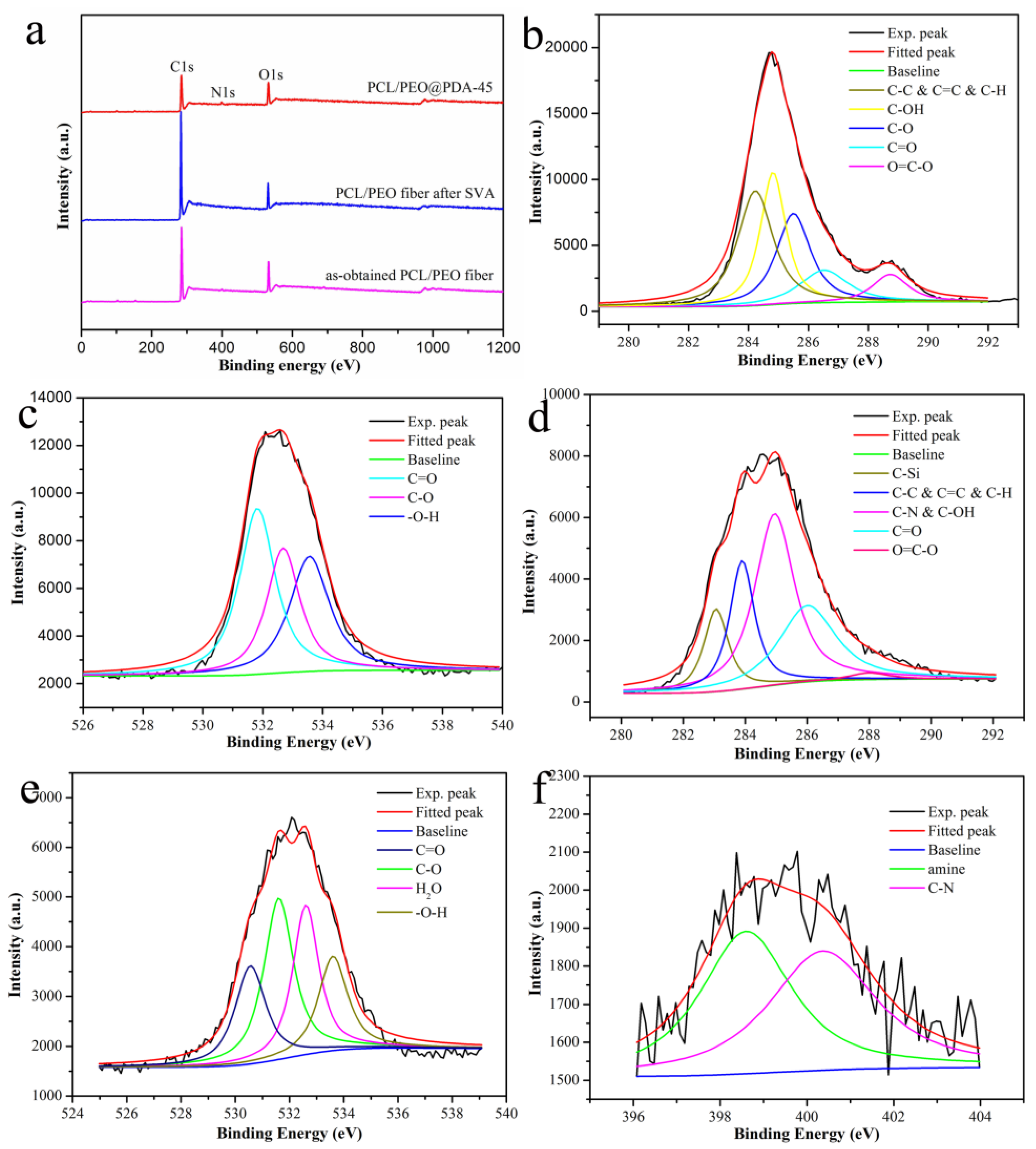
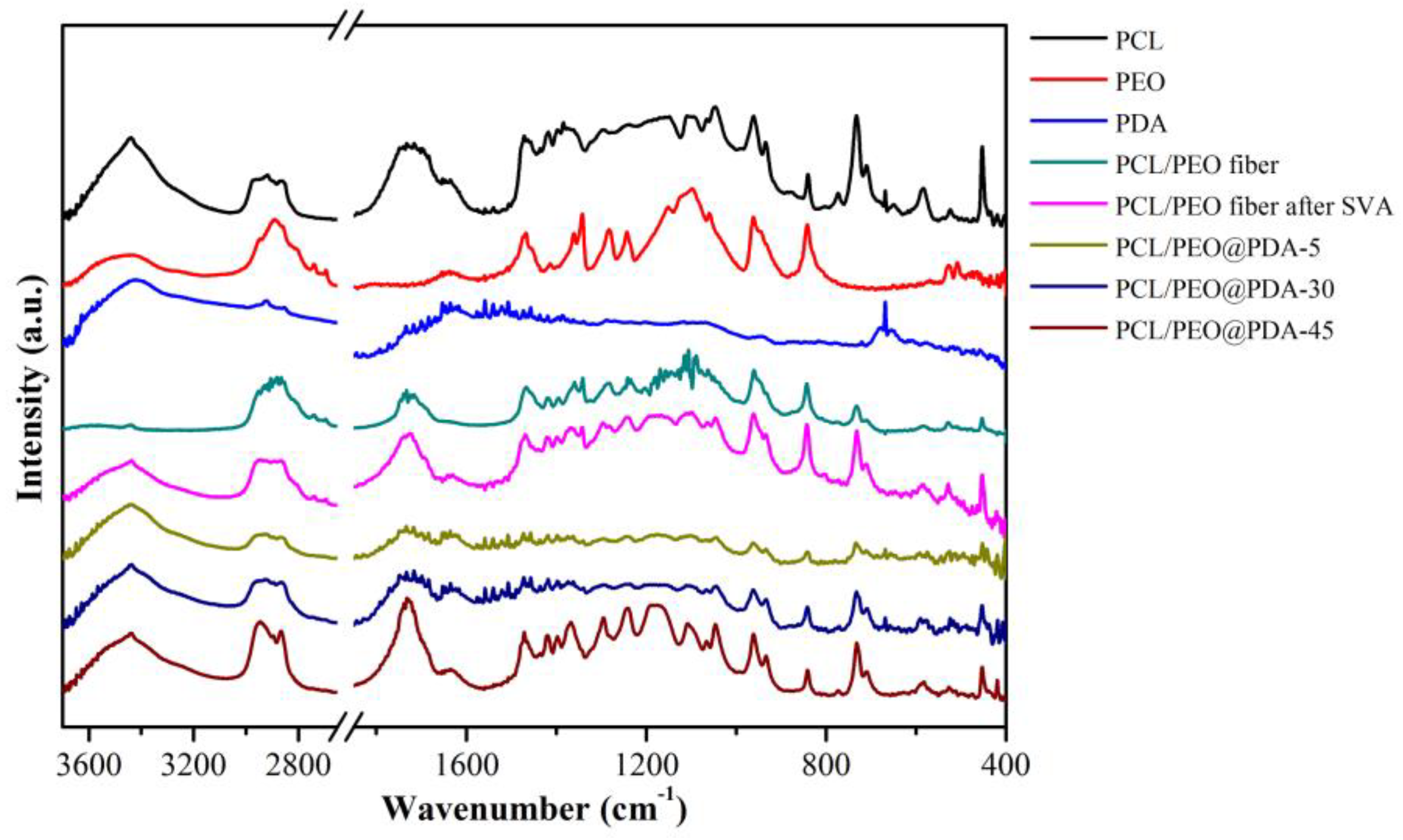
3.1. Characterization of PCL/PEO@PDA Composite Adsorbents
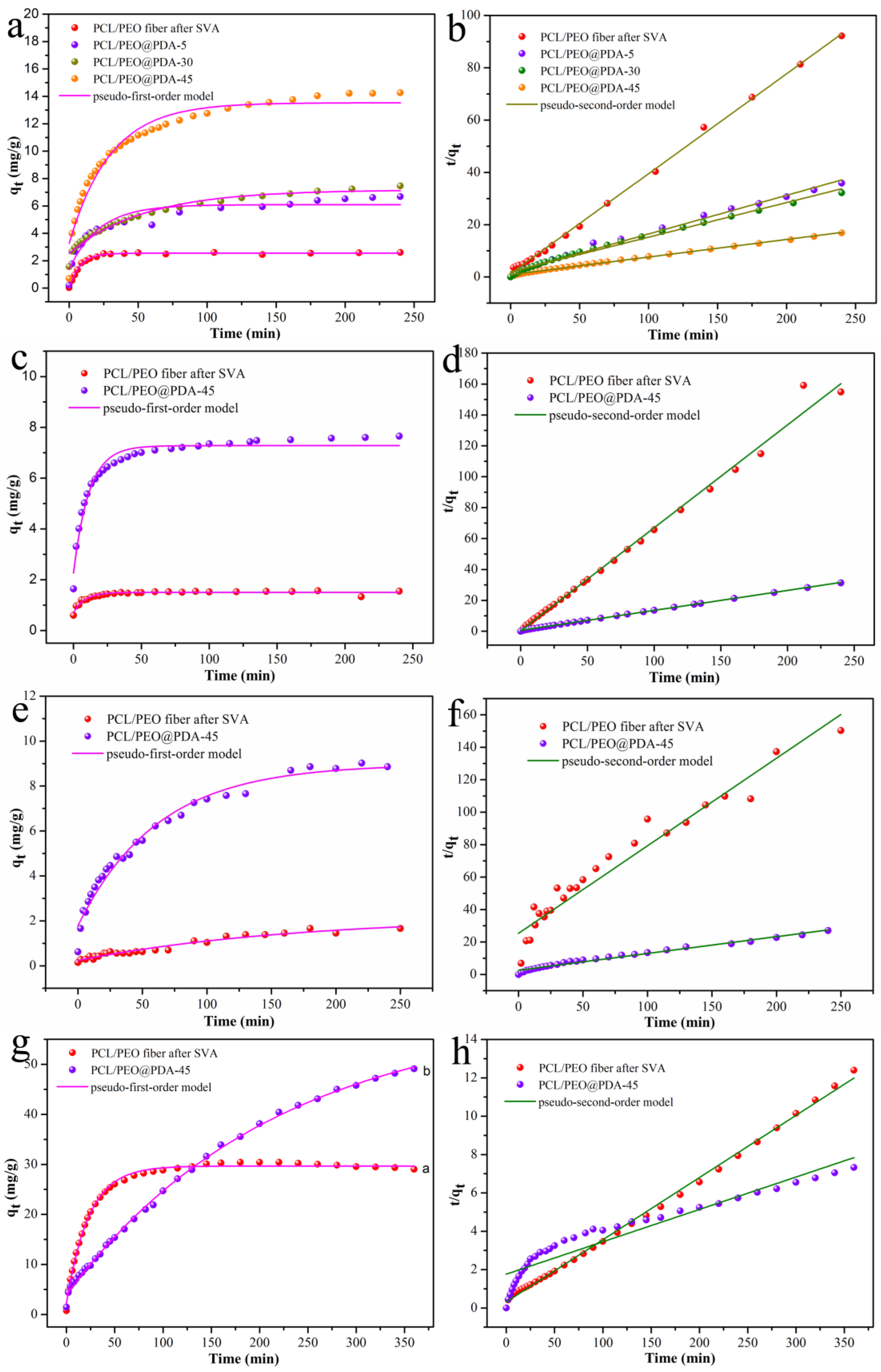
3.2. Adsorption Dye Performance of PCL/PEO@PDA Composite
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Szlinder-Richert, J.; Usydus, Z.; Malesa-Ciećwierz, M.; Polak-Juszczak, L.; Ruczynska, W. Marine and farmed fish on the Polish market: Comparison of the nutritive value and human exposure to PCDD/Fs and other contaminants. Chemosphere 2011, 85, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Dadfarnia, S.; Shabani, A.H.; Moradi, S.E.; Emami, S. Methyl red removal from water by iron based metal-organic frameworks loaded onto iron oxide nanoparticle adsorbent. Appl. Surf. Sci. 2015, 330, 85–93. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Shen, S.L.; Wang, S.Q.; Huang, J.; Su, P.; Wang, Q.R.; Zhao, B.X. A dual function magnetic nanomaterial modified with lysine for removal of organic dyes from water solution. Chem. Eng. J. 2014, 239, 250–256. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; He, Y.; Li, F.; Liu, X.Q.; Yang, J.B. Low-temperature solvothermal synthesis of graphene–TiO2 nanocomposite and its photocatalytic activity for dye degradation. Mater. Lett. 2014, 134, 115–118. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Ren, T.; Si, Y.; Yang, J.M.; Ding, B.; Yang, X.X.; Hong, F.; Yu, J.Y. Polyacrylonitrile/polybenzoxazine-based Fe3O4@carbon nanofibers: Hierarchical porous structure and magnetic adsorption property. J. Mater. Chem. 2012, 22, 15919–15927. [Google Scholar] [CrossRef]
- Rostamian, R.; Najafi, M.; Rafati, A.A. Synthesis and characterization of thiol-functionalized silica nano hollow sphere as a novel adsorbent for removal of poisonous heavy metal ions from water: Kinetics, isotherms and error analysis. Chem. Eng. J. 2011, 171, 1004–1011. [Google Scholar] [CrossRef]
- Mahdavi, S.; Jalali, M.; Afkhami, A. Heavy metals removal from aqueous solutions using TiO2, MgO, and Al2O3 nanoparticles. Chem. Eng. Commun. 2013, 200, 448–470. [Google Scholar] [CrossRef]
- Aditya, D.; Rohan, P.; Suresh, G. Nano-adsorbents for wastewater treatment: A review. Res. J. Chem. Environ. 2011, 15, 1033–1040. [Google Scholar]
- Luo, X.G.; Zhang, L. High effective adsorption of organic dyes on magnetic cellulose beads entrapping activated carbon. J. Hazard. Mater. 2009, 171, 340–347. [Google Scholar] [CrossRef]
- Fu, J.W.; Xin, Q.Q.; Wu, X.C.; Chen, Z.H.; Yan, Y.; Liu, S.J.; Wang, M.H.; Xu, Q. Selective adsorption and separation of organic dyes from aqueous solution on polydopamine microspheres. J. Colloid Interface Sci. 2016, 461, 292–304. [Google Scholar] [CrossRef]
- Wang, X.F.; Yu, J.Y.; Sun, G.; Ding, B. Electrospun nanofibrous materials: A versatile medium for effective oil/water separation. Mater. Today 2016, 19, 403–414. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Hu, X.L.; Liu, S.; Zhou, G.Y.; Huang, Y.B.; Xie, Z.G.; Jing, X.B. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Liu, J.Z.; Bauer, A.J.P.; Li, B.B. Solvent vapor annealing: An efficient approach for inscribing secondary nanostructures onto electrospun fibers. Macromol. Rapid Commun. 2014, 35, 1503–1508. [Google Scholar] [CrossRef]
- Lu, Q.P.; Yu, Y.F.; Ma, Q.L.; Chen, B.; Zhang, H. 2D Transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef]
- Lin, J.Y.; Ding, B.; Yang, J.M.; Yu, J.Y.; Sun, G. Subtle regulation of the micro-and nanostructures of electrospun polystyrene fibers and their application in oil absorption. Nanoscale 2012, 4, 176–182. [Google Scholar] [CrossRef]
- Si, Y.; Wang, X.Q.; Li, Y.; Chen, K.; Wang, J.Q.; Yu, J.Y.; Wang, H.J.; Ding, B. Optimized colorimetric sensor strip for mercury (II) assay using hierarchical nanostructured conjugated polymers. J. Mater. Chem. A 2014, 2, 645–652. [Google Scholar] [CrossRef]
- Miao, Y.E.; Wang, R.Y.; Chen, D.; Liu, Z.Y.; Liu, T.X. Electrospun self-standing membrane of hierarchical SiO2@ γ-AlOOH (Boehmite) core/sheath fibers for water remediation. ACS Appl. Mater. Interfaces 2012, 4, 5353–5359. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Liu, R. Fe3O4@ polydopamine and derived Fe3O4@ carbon core–shell nanoparticles: Comparison in adsorption for cationic and anionic dyes. Colloid Surf. A-Physicochem. Eng. Asp. 2017, 522, 260–265. [Google Scholar] [CrossRef]
- Dong, Z.H.; Wang, D.; Liu, X.; Pei, X.F.; Chen, L.W.; Jin, J. Bio-inspired surface-functionalization of graphene oxide for the adsorption of organic dyes and heavy metal ions with a superhigh capacity. J. Mater. Chem. A 2014, 2, 5034–5040. [Google Scholar] [CrossRef]
- Wang, C.R.; Sun, S.X.; Zhang, L.X.; Yin, J.J.; Jiao, T.F.; Zhang, L.; Xu, Y.L.; Zhou, J.X.; Peng, Q.M. Facile preparation and catalytic performance characterization of AuNPs-loaded hierarchical electrospun composite fibers by solvent vapor annealing treatment. Colloid Surf. A-Physicochem. Eng. Asp. 2019, 561, 283–291. [Google Scholar] [CrossRef]
- Bauer, A.J.P.; Grim, Z.B.; Li, B. Hierarchical polymer blend fibers of high structural regularity prepared by facile solvent vapor annealing treatment. Macromol. Mater. Eng. 2018, 303, 1700489. [Google Scholar] [CrossRef]
- Huang, X.X.; Jiao, T.F.; Liu, Q.Q.; Zhang, L.X.; Zhou, J.X.; Li, B.B.; Peng, Q.M. Hierarchical electrospun nanofibers treated by solvent vapor annealing as air filtration mat for high-efficiency PM2.5 capture. Sci. China Mater. 2019, 62, 423–436. [Google Scholar] [CrossRef]
- Farnad, N.; Farhadi, K.; Voelcker, N.H. Polydopamine nanoparticles as a new and highly selective biosorbent for the removal of copper(II) ions from aqueous solutions. Water Air Soil Pollut. 2012, 223, 3535–3544. [Google Scholar] [CrossRef]
- Yu, X.; Fan, H.L.; Liu, Y.; Shi, Z.J.; Jin, Z.X. Characterization of carbonized polydopamine nanoparticles suggests ordered supramolecular structure of polydopamine. Langmuir 2014, 30, 5497–5505. [Google Scholar] [CrossRef]
- Gan, L.; Shang, S.M.; Hu, E.L.; Yuen, C.W.M.; Jiang, S.X. Konjac glucomannan/graphene oxide hydrogel with enhanced dyes adsorption capability for methyl blue and methyl orange. Appl. Surf. Sci. 2015, 357, 866–872. [Google Scholar] [CrossRef]
- Cheng, C.; Li, S.; Zhao, W.H.; Wei, Q.; Nie, S.Q.; Sun, S.D.; Zhao, C.S. The hydrodynamic permeability and surface property of polyethersulfone ultrafiltration membranes with mussel-inspired polydopamine coatings. J. Membr. Sci. 2012, 417, 228–236. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef]
- Xing, R.R.; Wang, W.; Jiao, T.F.; Ma, K.; Zhang, Q.R.; Hong, W.; Qiu, H.; Zhou, J.X.; Zhang, L.X.; Peng, Q.M. Bioinspired polydopamine sheathed nanofibers containing carboxylate graphene oxide nanosheet for high-efficient dyes scavenger. ACS Sustain. Chem. Eng. 2017, 5, 4948–4956. [Google Scholar] [CrossRef]
- Tang, L.; Livi, K.J.T.; Chen, K.L. Polysulfone membranes modified with bioinspired polydopamine and silver nanoparticles formed in situ to mitigate biofouling. Environ. Sci. Technol. Lett. 2015, 2, 59–65. [Google Scholar] [CrossRef]
- Ji, Y.; Ghosh, K.; Shu, X.Z.; Li, B.Q.; Sokolov, J.C.; Prestwich, G.D.; Clark, R.A.F.; Rafailovich, M.H. Electrospun three-dimensional hyaluronic acid nanofibrous scaffolds. Biomaterials 2006, 27, 3782–3792. [Google Scholar] [CrossRef]
- Yang, X.H.; Shao, C.L.; Guan, H.Y.; Li, X.L.; Gong, J. Preparation and characterization of ZnO nanofibers by using electrospun PVA/zinc acetate composite fiber as precursor. Inorg. Chem. Commun. 2004, 7, 176–178. [Google Scholar] [CrossRef]
- Konwer, S.; Boruah, R.; Dolui, S.K. Studies on conducting polypyrrole/graphene oxide composites as supercapacitor electrode. J. Electron. Mater. 2011, 40, 2248. [Google Scholar] [CrossRef]
- Contarini, S.; Howlett, S.P.; Rizzo, C.; De Angelis, B.A. XPS study on the dispersion of carbon additives in silicon carbide powders. Appl. Surf. Sci. 1991, 51, 177–183. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.B.; Li, C.Y.; Li, J.S.; Xu, F.J.; Mao, C.; Yang, W.T.; Shen, J. Improvement of hemocompatibility of polycaprolactone film surfaces with zwitterionic polymer brushes. Langmuir 2011, 27, 11575–11581. [Google Scholar] [CrossRef]
- Manakhov, A.; Kedroňová, E.; Medalová, J.; Černochová, P.; Obrusník, A.; Michlíček, M.; Shtansky, D.V.; Zajíčková, L. Carboxyl-anhydride and amine plasma coating of PCL nanofibers to improve their bioactivity. Mater. Des. 2017, 132, 257–265. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Wu, P.; Liu, G.J.; He, X.F.; Qi, B.Y.; Zeng, G.F.; Wang, W.; Sun, Y.H.; Cui, F.Y. Ultrahigh adsorption capacity of anionic dyes with sharp selectivity through the cationic charged hybrid nanofibrous membranes. Chem. Eng. J. 2017, 313, 957–966. [Google Scholar] [CrossRef]
- Ye, W.C.; Chen, Y.; Zhou, Y.X.; Fu, J.J.; Wu, W.C.; Gao, D.Q.; Zhou, F.; Wang, C.M.; Xue, D.S. Enhancing the catalytic activity of flowerike Pt nanocrystals using polydopamine functionalized graphene supports for methanol electrooxidation. Electrochim. Acta 2014, 142, 18–24. [Google Scholar] [CrossRef]
- Zhao, X.N.; Jiao, T.F.; Xing, R.R.; Huang, H.; Hu, J.; Qu, Y.; Zhou, J.X.; Zhang, L.X.; Peng, Q.M. Preparation of diamond-based AuNP-modified nanocomposites with elevated catalytic performances. RSC Adv. 2017, 7, 49923–49930. [Google Scholar] [CrossRef]
- Zhao, X.N.; Jiao, T.F.; Ma, X.L.; Huang, H.; Hu, J.; Qu, Y.; Zhou, J.X.; Zhang, L.X.; Peng, Q.M. Facile fabrication of hierarchical diamond-based AuNPs-modified nanocomposites via layer-by-layer assembly with enhanced catalytic capacities. J. Taiwan Inst. Chem. Eng. 2017, 80, 614–623. [Google Scholar] [CrossRef]
- Duan, B.; Dong, C.H.; Yuan, X.Y.; Yao, K.D. Electrospinning of chitosan solutions in acetic acid with poly (ethylene oxide). J. Biomater. Sci. Polym. Ed. 2004, 15, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Chiang, S.W.; Du, H.D.; Li, J.; Gan, L.; Zhang, X.; Chu, X.D.; Yao, Y.W.; Li, B.H.; Kang, F.Y. Thermal conductivity of electrospinning chain-aligned polyethylene oxide (PEO). Polymer 2017, 115, 52–59. [Google Scholar] [CrossRef]
- Canbolat, M.F.; Celebioglu, A.; Uyar, T. Drug delivery system based on cyclodextrin-naproxen inclusion complex incorporated in electrospun polycaprolactone nanofibers. Colloids Surf. B Biointerfaces 2014, 115, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.R.; de Menezes, L.R.; Dutra Filho, J.C.D.; Cabral, L.M.; Tavares, M.I.B. Surface-coated polycaprolactone nanoparticles with pharmaceutical application: Structural and molecular mobility evaluation by TD-NMR. Polym. Test. 2017, 60, 39–48. [Google Scholar] [CrossRef]
- Zhu, B.; Edmondson, S. Polydopamine-melanin initiators for surface-initiated ATRP. Polymer 2011, 52, 2141–2149. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.Z.; Wu, Z.T.; Wu, Y.; Gao, T.T.; Yao, J.S. Efficient removal of methyl orange and alizarin red S from pH-unregulated aqueous solution by the catechol–amine resin composite using hydrocellulose as precursor. ACS Sustain. Chem. Eng. 2017, 5, 1871–1880. [Google Scholar] [CrossRef]
- Guo, R.; Jiao, T.F.; Li, R.F.; Chen, Y.; Guo, W.C.; Zhang, L.X.; Zhou, J.X.; Zhang, Q.R.; Peng, Q.M. Sandwiched Fe3O4/carboxylate graphene oxide nanostructures constructed by layer-by-layer assembly for highly efficient and magnetically recyclable dye removal. ACS Sustain. Chem. Eng. 2018, 6, 1279–1288. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, C.; Jiao, T.; Song, J.; Zhang, X.; Xing, R.; Zhou, J.; Zhang, L.; Peng, Q. Self-assembled AgNP-containing nanocomposites constructed by electrospinning as efficient dye photocatalyst materials for wastewater treatment. Nanomaterials 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jiao, T.; Xing, R.; Zou, G.; Zhou, J.; Zhang, L.; Peng, Q. Fabrication of tunable hierarchical MXene@AuNPs nanocomposites constructed by self-reduction reactions with enhanced catalytic performances. Sci. China Mater. 2018, 61, 728–736. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, F.; Jiao, T.; Xing, R.; Zhang, L.; Zhang, Q.; Peng, Q. Selective Cu(II) ion removal from wastewater via surface charged self-assembled polystyrene-Schiff base nanocomposites. Colloid Surf. A-Physicochem. Eng. Asp. 2018, 545, 60–67. [Google Scholar] [CrossRef]
- Chen, K.; Li, J.; Zhang, L.; Xing, R.; Jiao, T.; Gao, F.; Peng, Q. Facile synthesis of self-assembled carbon nanotubes/dye composite films for sensitive electrochemical determination of Cd(II) ions. Nanotechnology 2018, 29, 445603. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ma, K.; Jiao, T.; Xing, R.; Zhang, L.; Zhou, J.; Li, B. Graphene oxide-polymer composite Langmuir films constructed by interfacial thiol-ene photopolymerization. Nanoscale Res. Lett. 2017, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Duan, P.; Jiao, T.; Peng, Q.; Liu, M. Self-assembled luminescent quantum dots to generate full-color and white circularly polarized light. Angew. Chem. Int. Ed. 2017, 56, 12174–12178. [Google Scholar] [CrossRef]
- Xing, R.; Liu, K.; Jiao, T.; Zhang, N.; Ma, K.; Zhang, R.; Zou, Q.; Ma, G.; Yan, X. An injectable self-assembling collagen-gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv. Mater. 2016, 28, 3669–3676. [Google Scholar] [CrossRef]
- Guo, H.; Jiao, T.; Zhang, Q.; Guo, W.; Peng, Q.; Yan, X. Preparation of graphene oxide-based hydrogels as efficient dye adsorbents for wastewater treatment. Nanoscale Res. Lett. 2015, 10, 272. [Google Scholar] [CrossRef]
- Zhan, F.; Wang, R.; Yin, J.; Han, Z.; Zhang, L.; Jiao, T.; Zhou, J.; Zhang, L.; Peng, Q. Facile solvothermal preparation of Fe3O4–Ag nanocomposite with excellent catalytic performance. RSC Adv. 2019, 9, 878–883. [Google Scholar] [CrossRef]
- Sun, S.; Wang, C.; Han, S.; Jiao, T.; Wang, R.; Yin, J.; Li, Q.; Wang, Y.; Geng, L.; Yu, X.; et al. Interfacial nanostructures and acidichromism behaviors in self-assembled terpyridine derivatives Langmuir-Blodgett films. Colloid Surf. A-Physicochem. Eng. Asp. 2019, 564, 1–9. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, B.; Wang, R.; Zhang, L.; Jiao, T.; Liu, Z. Facile preparation of rod-like MnO nanomixtures via hydrothermal approach and highly efficient removal of methylene blue for wastewater Treatment. Nanomaterials 2019, 9, 10. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, C.Q.; Zou, Q.L.; Xie, Z.C.; Yan, X.H. Self-assembled Zinc/cystine-based chloroplast mimics capable of photoenzymatic reactions for sustainable fuel synthesis. Angew. Chem. Int. Ed. 2017, 56, 7876–7880. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xing, R.R.; Li, Y.X.; Zou, Q.L.; Möhwald, H.; Yan, X.H. Mimicking primitive photobacteria: Sustainable hydrogen evolution based on peptide-porphyrin co-assemblies with self-mineralized reaction center. Angew. Chem. Int. Ed. 2016, 55, 12503–12507. [Google Scholar] [CrossRef]
- Liu, K.; Xing, R.R.; Chen, C.J.; Shen, G.Z.; Yan, L.Y.; Zou, Q.L.; Ma, G.H.; Möhwald, H.; Yan, X.H. Peptide-induced hierarchical long-range order and photocatalytic activity of porphyrin assemblies. Angew. Chem. Int. Ed. 2015, 54, 500–505. [Google Scholar] [CrossRef]


| Samples | Specific Surface Area (m2g−1) | Average Pore Diameter (nm) | Pore Volume (cm3g−1) |
|---|---|---|---|
| As-obtained PCL/PEO fiber | 8.5467 | 24.1263 | 0.008736 |
| PCL/PEO fiber after SVA | 15.3133 | 47.0420 | 0.012631 |
| PCL/PEO@PDA-45 | 9.0741 | 27.1665 | 0.008920 |
| NO. | Materials | qe (mg/g) | Characteristics | Ref. | |
|---|---|---|---|---|---|
| MB | MO | ||||
| 1 | Magnetic cellulose beads (MCB-AC) | 1.8 | 1.3 | Selective magnetic response, environmentally friendly process, reusable spherical beads. | [12] |
| 2 | PDA microspheres | 147.0 | almost zero | Selective adsorption of cationic dyes, economical adsorption and separation. | [13] |
| 3 | Fe3O4@PDA NPs | 10.0 | 2.1 | Selective adsorption capacity for cationic dyes, magnetic core-shell structure, easily magnetic separation. | [22] |
| 4 | PDA/GO | 1800.0 | 30.0 | Controllable PDA layer thickness, high surface area structure, excellent adsorption performance. | [23] |
| 5 | poly(vinyl alcohol)/poly(acrylic acid)/carboxylate graphene oxide@polydopamine(PVA/PAA/GO-COOH@PDA) | 31.3 | - | Environmentally friendly and controllable preparation method, larger specific surface area, excellent reusability. | [33] |
| 6 | poly(catechol-tetraethylenepentamine-cyanuric chloride)@hydrocellulose (PCEC-C) | - | 37.2 | Selective adsorption of anionic dyes, simple method and better adsorption stability. | [50] |
| 7 | PCL/PEO@PDA-45 | 14.8 | 60.2 | Selective adsorption of MO, convenient and controllable method, excellent stability and reuse. | Present work |
| MB | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | R2 | K1 (min−1) | qe (mg/g) | R2 | K2 (g/mg⋅min) | |
| PCL/PEO after SVA | 2.5533 | 0.9949 | 0.1270 | 2.6183 | 0.9987 | 0.3809 |
| PCL/PEO@PDA-5 | 6.0916 | 0.8968 | 0.0459 | 6.7700 | 0.9931 | 0.1457 |
| PCL/PEO@PDA-30 | 7.1766 | 0.9738 | 0.0184 | 7.5614 | 0.9904 | 0.1297 |
| PCL/PEO@PDA-45 | 13.5342 | 0.9576 | 0.0335 | 14.8522 | 0.9966 | 0.0669 |
| RhB | ||||||
| PCL/PEO after SVA | 1.5026 | 0.9344 | 0.1217 | 1.5018 | 0.9923 | 1.1646 |
| PCL/PEO@PDA-45 | 7.2790 | 0.9668 | 0.0878 | 7.7316 | 0.9997 | 0.0286 |
| ST | ||||||
| PCL/PEO after SVA | 2.1838 | 0.9566 | 0.0059 | 1.8543 | 0.9358 | 0.0115 |
| PCL/PEO@PDA-45 | 8.9755 | 0.9788 | 0.0162 | 9.6628 | 0.9826 | 0.0041 |
| MO | ||||||
| PCL/PEO after SVA | 29.6755 | 0.9963 | 0.0432 | 30.8547 | 0.9980 | 0.0323 |
| PCL/PEO@PDA-45 | 60.2260 | 0.9983 | 0.0046 | 59.2417 | 0.8960 | 0.0136 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Yin, J.; Wang, R.; Jiao, T.; Huang, H.; Zhou, J.; Zhang, L.; Peng, Q. Facile Preparation of Self-Assembled Polydopamine-Modified Electrospun Fibers for Highly Effective Removal of Organic Dyes. Nanomaterials 2019, 9, 116. https://doi.org/10.3390/nano9010116
Wang C, Yin J, Wang R, Jiao T, Huang H, Zhou J, Zhang L, Peng Q. Facile Preparation of Self-Assembled Polydopamine-Modified Electrospun Fibers for Highly Effective Removal of Organic Dyes. Nanomaterials. 2019; 9(1):116. https://doi.org/10.3390/nano9010116
Chicago/Turabian StyleWang, Cuiru, Juanjuan Yin, Ran Wang, Tifeng Jiao, Haiming Huang, Jingxin Zhou, Lexin Zhang, and Qiuming Peng. 2019. "Facile Preparation of Self-Assembled Polydopamine-Modified Electrospun Fibers for Highly Effective Removal of Organic Dyes" Nanomaterials 9, no. 1: 116. https://doi.org/10.3390/nano9010116
APA StyleWang, C., Yin, J., Wang, R., Jiao, T., Huang, H., Zhou, J., Zhang, L., & Peng, Q. (2019). Facile Preparation of Self-Assembled Polydopamine-Modified Electrospun Fibers for Highly Effective Removal of Organic Dyes. Nanomaterials, 9(1), 116. https://doi.org/10.3390/nano9010116






