Preparation of Magnetic Fe3O4/MIL-88A Nanocomposite and Its Adsorption Properties for Bromophenol Blue Dye in Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Magnetic Fe3O4/MIL-88A Composite
2.3. Characterizations
2.4. Adsorption Experiments
3. Results and Discussion
3.1. Characterization of Magnetic Fe3O4/MIL-88A Composite
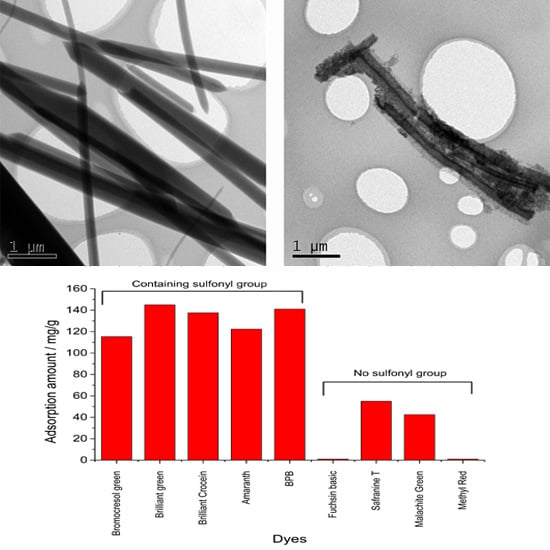
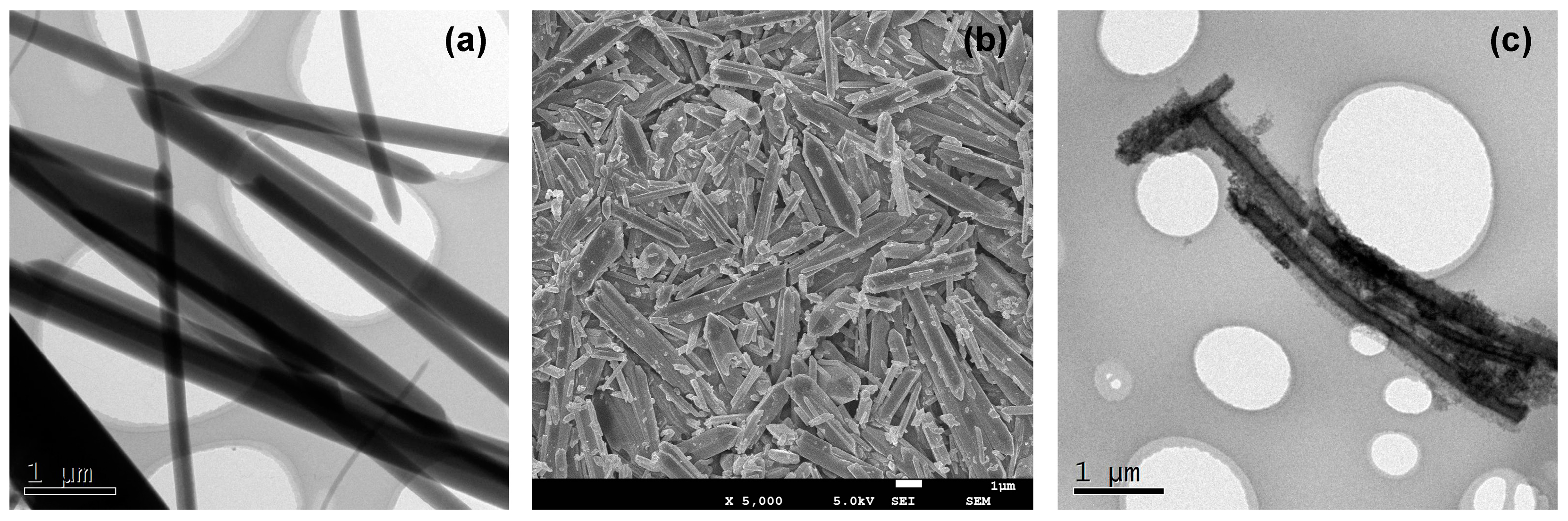
3.1.1. Transmission Electron Microscopy (TEM) and Field Emission Scanning Electron Microscopy (FESEM)
3.1.2. X-Ray Powder Diffraction (XRD)
3.1.3. Thermogravimetric Analysis (TGA)
3.1.4. Vibration Sample Magnetometer (VSM)
3.1.5. Adsorption Ability
3.2. Effects of Parameters on Dye Adsorption
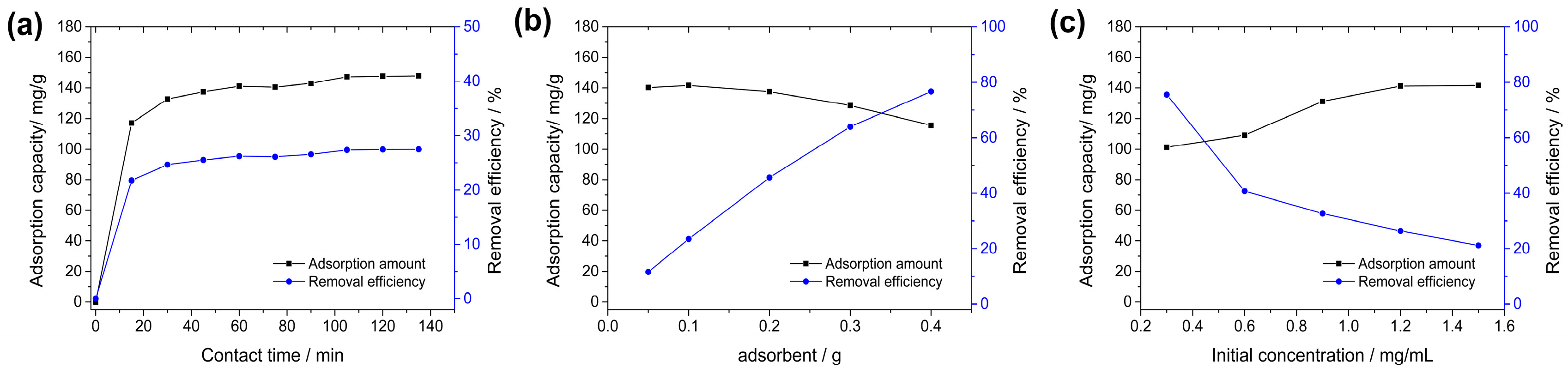
3.2.1. Effect of Contact Time
3.2.2. Effect of Adsorbent Dosage
3.2.3. Effects of Initial Dye Concentration
3.3. Adsorption Kinetics
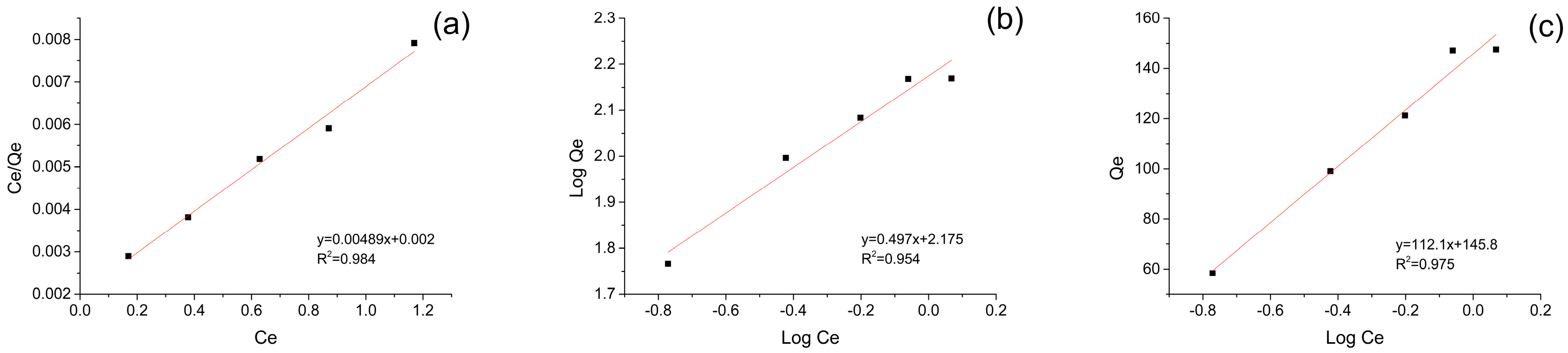
3.4. Adsorption Isotherms
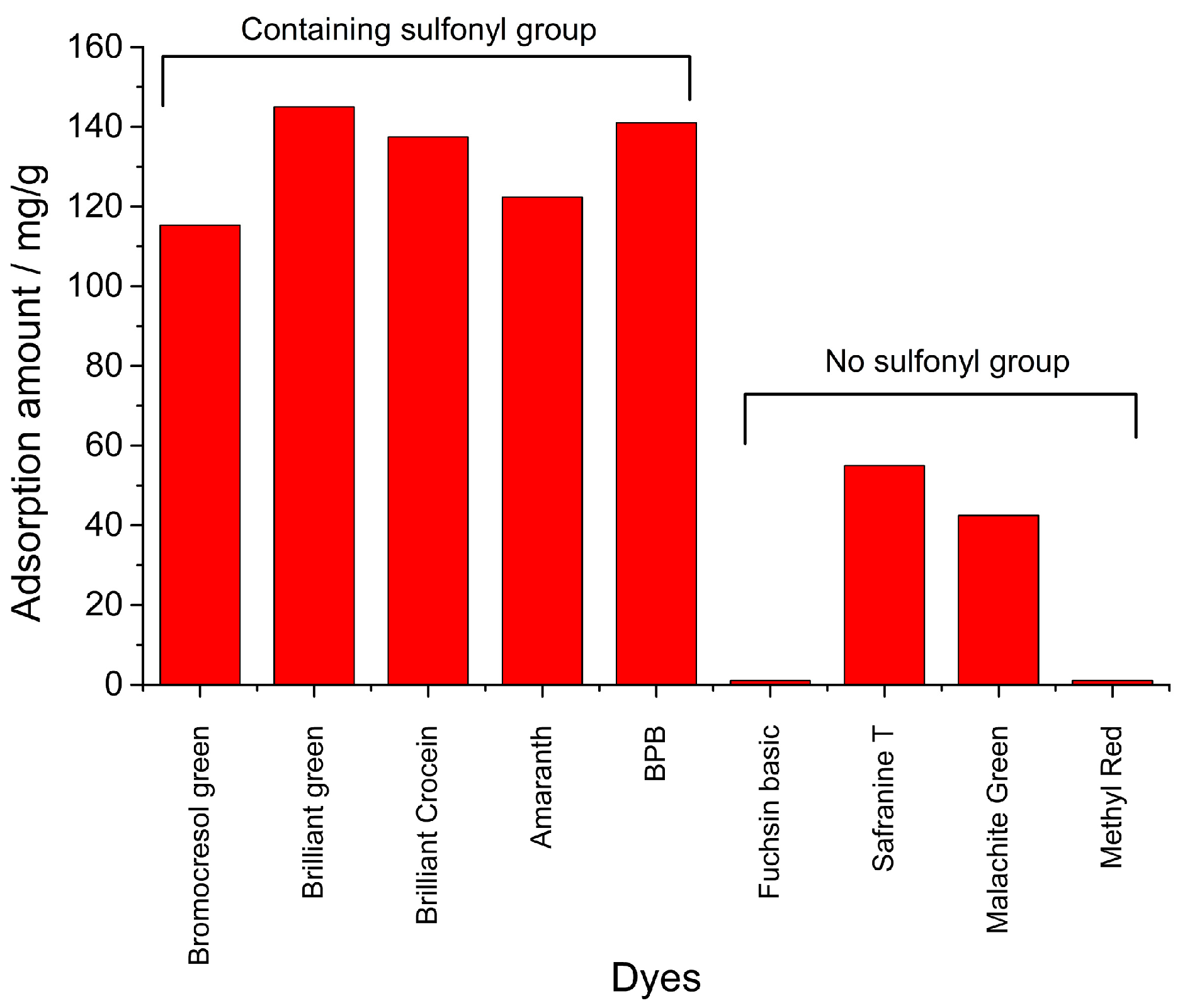
3.5. Comparison Study
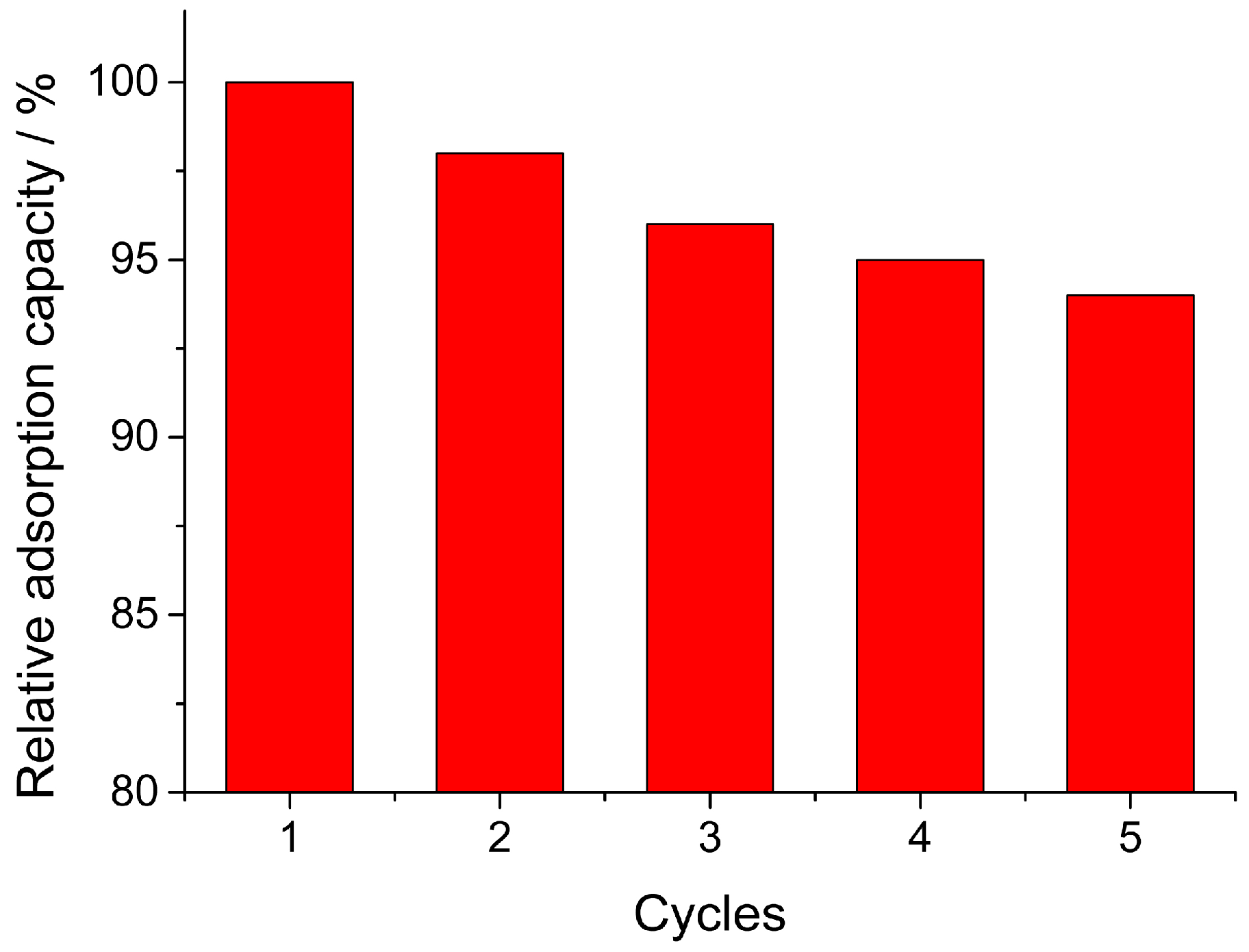
3.6. Recycling of Fe3O4/MIL-88A Composite
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ji, Y.; Ma, C.; Li, J.; Zhao, H.; Chen, Q.; Li, M.; Liu, H. A magnetic adsorbent for the removal of cationic dyes from wastewater. Nanomaterials 2018, 8, 710. [Google Scholar] [CrossRef] [PubMed]
- Matmin, J.; Affendi, I.; Ibrahim, S.; Endud, S. Additive-free rice starch-assisted synthesis of spherical nanostructured hematite for degradation of dye contaminant. Nanomaterials 2018, 8, 702. [Google Scholar] [CrossRef] [PubMed]
- Pajootan, E.; Arami, M.; Mahmoodi, N.M. Binary system dye removal by electrocoagulation from synthetic and real colored wastewaters. J. Taiwan Inst. Chem. Eng. 2012, 43, 282–290. [Google Scholar] [CrossRef]
- Loos, R.; Niessner, R. Analysis of aromatic sulfonates in water by solid-phase extraction and capillary electrophoresis. J. Chromatogr. A 1998, 822, 291–303. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Z.; Yang, Q.; Huang, R. Efficient removal of lead, copper and cadmium ions from water by a porous calcium alginate/graphene oxide composite aerogel. Nanomaterials 2018, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Chen, X.; Shen, F.; Chen, G. Removal of chromium(VI) from wastewater by combined electrocoagulation–electroflotation without a filter. Sep. Purif. Technol. 2005, 43, 117–123. [Google Scholar] [CrossRef]
- Can, O.T.; Bayramoglu, M.; Kobya, M. Decolorization of reactive dye solutions by electrocoagulation using aluminum electrodes. Ind. Eng. Chem. Res. 2003, 42, 3391–3396. [Google Scholar] [CrossRef]
- Carmalin Sophia, A.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef]
- Jabbari, V.; Veleta, J.M.; Zarei-Chaleshtori, M.; Gardea-Torresdey, J.; Villagrán, D. Green synthesis of magnetic MOF@GO and MOF@CNT hybrid nanocomposites with high adsorption capacity towards organic pollutants. Chem. Eng. J. 2016, 304, 774–783. [Google Scholar] [CrossRef]
- Li, W.J.; Gao, S.Y.; Liu, T.F.; Han, L.W.; Lin, Z.J.; Cao, R. In situ growth of metal-organic framework thin films with gas sensing and molecule storage properties. Langmuir 2013, 29, 8657–8664. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Manos, G.; Dunne, L. Predicting the features of methane adsorption in large pore metal-organic frameworks for energy storage. Nanomaterials 2018, 8, 818. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Yao, W.; Wang, X.; Yu, S.; Yu, Z.; Wang, X. Synthesis of rod-like metal-organic framework (MOF-5) nanomaterial for efficient removal of U(VI): Batch experiments and spectroscopy study. Sci. Bull. 2018, 63, 831–839. [Google Scholar] [CrossRef]
- Ke, F.; Qiu, L.G.; Yuan, Y.P.; Jiang, X.; Zhu, J.F. Fe3O4@MOF core–shell magnetic microspheres with a designable metal–organic framework shell. J. Mater. Chem. 2012, 22, 9497–9500. [Google Scholar] [CrossRef]
- Askari, H.; Ghaedi, M.; Dashtian, K.; Mha, A. Rapid and high-capacity ultrasonic assisted adsorption of ternary toxic anionic dyes onto MOF-5-activated carbon: Artificial neural networks, partial least squares, desirability function and isotherm and kinetic study. Ultrason. Sonochem. 2017, 37, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, X.; Zhang, C.; Yu, Q.; Zhao, Z.; Niu, X.; Sun, X.; Liu, Y.; Ma, L.; Li, Z. Enhanced adsorptive removal of anionic and cationic dyes from single or mixed dye solutions using MOF PCN-222. RSC Adv. 2017, 7, 16273–16281. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Zhi, T.; Niu, H.; Cai, Y.; Wei, M.; Wu, F.; Giesy, J.P. Synthesis of magnetic metal-organic framework (MOF) for efficient removal of organic dyes from water. Sci. Rep. 2015, 5, 11849. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, S.; Wang, L.; Zhu, W.; Xu, J.; Song, H. Adsorption of bromophenol blue from aqueous samples by novel supported ionic liquids. J. Chem. Technol. Biotechnol. 2014, 89, 230–238. [Google Scholar] [CrossRef]
- Chalati, T.; Horcajada, P.; Gref, R.; Couvreur, P.; Serre, C. Optimisation of the synthesis of MOF nanoparticles made of flexible porous iron fumarate MIL-88A. J. Mater. Chem. 2011, 21, 2220–2227. [Google Scholar] [CrossRef]
- Xu, W.-T.; Ma, L.; Ke, F.; Peng, F.-M.; Xu, G.-S.; Shen, Y.-H.; Zhu, J.-F.; Qiu, L.-G.; Yuan, Y.-P. Metal–organic frameworks MIL-88A hexagonal microrods as a new photocatalyst for efficient decolorization of methylene blue dye. Dalton Trans. 2014, 43, 3792–3798. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Xue, Y.; Wang, X.; Lin, T. Magnet-induced temporary superhydrophobic coatings from one-pot synthesized hydrophobic magnetic nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1449–1455. [Google Scholar] [CrossRef]
- Azha, S.F.; Sellaoui, L.; Shamsudin, M.S.; Ismail, S.; Bonilla-Petriciolet, A.; Ben Lamine, A.; Erto, A. Synthesis and characterization of a novel amphoteric adsorbent coating for anionic and cationic dyes adsorption: Experimental investigation and statistical physics modelling. Chem. Eng. J. 2018, 351, 221–229. [Google Scholar] [CrossRef]
- Gao, Y.; Deng, S.-Q.; Jin, X.; Cai, S.-L.; Zheng, S.-R.; Zhang, W.-G. The construction of amorphous metal-organic cage-based solid for rapid dye adsorption and time-dependent dye separation from water. Chem. Eng. J. 2019, 357, 129–139. [Google Scholar] [CrossRef]
- Wu, H.; Ma, M.-D.; Gai, W.-Z.; Yang, H.; Zhou, J.-G.; Cheng, Z.; Xu, P.; Deng, Z.-Y. Arsenic removal from water by metal-organic framework MIL-88A microrods. Environ. Sci. Pollut. Res. 2018, 25, 27196–27202. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Y.; Chen, X.; Xiong, X.; Shi, S. Screening and identification of BSA bound ligands from Puerariae lobata flower by BSA functionalized Fe3O4 magnetic nanoparticles coupled with HPLC–MS/MS. J. Chromatogr. B 2012, 887–888, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, X.; Wang, Z.; Lü, M.; Wu, B.; Wang, Y.; Yan, C.; Yuan, A.; Yang, H. Controlled pyrolysis of MIL-88A to Fe2O3@C nanocomposites with varied morphologies and phases for advanced lithium storage. J. Mater. Chem. A 2017, 5, 25562–25573. [Google Scholar] [CrossRef]
- Hamza, W.; Dammak, N.; Hadjltaief, H.B.; Eloussaief, M.; Benzina, M. Sono-assisted adsorption of cristal violet dye onto tunisian smectite clay: Characterization, kinetics and adsorption isotherms. Ecotoxicol. Environ. Saf. 2018, 163, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, C.; Hou, B.; Wang, Y.; Hao, C.; Wu, J. Carbon composite lignin-based adsorbents for the adsorption of dyes. Chemosphere 2018, 206, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-Y.; Yu, P.; Tang, L.-S.; Wang, Q.-Y.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Tannic acid functionalized graphene hydrogel for organic dye adsorption. Ecotoxicol. Environ. Saf. 2018, 165, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Chaari, I.; Moussi, B.; Jamoussi, F. Interactions of the dye, c.I. Direct orange 34 with natural clay. J. Alloys Compd. 2015, 647, 720–727. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Kumar, A.; Rana, S.; Sharma, S.; Bhatnagar, A.; Florian, J.S.; Ghfar, A.A.; Khan, M.R. Efficient removal of coomassie brilliant blue r-250 dye using starch/poly(alginic acid-cl-acrylamide) nanohydrogel. Process Saf. Environ. Prot. 2017, 109, 301–310. [Google Scholar] [CrossRef]
- Saad, M.; Tahir, H.; Khan, J.; Hameed, U.; Saud, A. Synthesis of polyaniline nanoparticles and their application for the removal of crystal violet dye by ultrasonicated adsorption process based on response surface methodology. Ultrason. Sonochem. 2017, 34, 600–608. [Google Scholar] [CrossRef]
- Al-Hussain, S.; Atta, A.; Al-Lohedan, H.; Ezzat, A.; Tawfeek, A. Application of new sodium vinyl sulfonate-co-2-acrylamido-2-me[thylpropane sulfonic acid sodium salt-magnetite cryogel nanocomposites for fast methylene blue removal from industrial waste water. Nanomaterials 2018, 8, 878. [Google Scholar] [CrossRef]
- Tekin, N.; Şafaklı, A.; Bingöl, D. Process modeling and thermodynamics and kinetics evaluation of basic yellow 28 adsorption onto sepiolite. Desalin. Water Treat. 2015, 54, 2023–2035. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of methylene blue from aqueous solutions by adsorption on kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Gupta, A.K. Determination of thermodynamic parameters from langmuir isotherm constant-revisited. J. Mol. Liq. 2017, 225, 137–146. [Google Scholar] [CrossRef]
- Tanhaei, B.; Ayati, A.; Lahtinen, M.; Sillanpää, M. Preparation and characterization of a novel chitosan/Al2O3/magnetite nanoparticles composite adsorbent for kinetic, thermodynamic and isotherm studies of methyl orange adsorption. Chem. Eng. J. 2015, 259, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Zhao, Y.; Xia, K.; Guo, Y.; Qu, Z.; Bai, R. A mild and facile synthesis of amino functionalized CoFe2O4@SiO2 for Hg(II) removal. Nanomaterials 2018, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Carazo, E.; Borrego-Sánchez, A.; Sánchez-Espejo, R.; García-Villén, F.; Cerezo, P.; Aguzzi, C.; Viseras, C. Kinetic and thermodynamic assessment on isoniazid/montmorillonite adsorption. Appl. Clay Sci. 2018, 165, 82–90. [Google Scholar] [CrossRef]
- Wong, S.; Tumari, H.H.; Ngadi, N.; Mohamed, N.B.; Hassan, O.; Mat, R.; Saidina Amin, N.A. Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL). J. Clean. Prod. 2019, 206, 394–406. [Google Scholar] [CrossRef]
- Heimann, S.; Ndé-Tchoupé, A.I.; Hu, R.; Licha, T.; Noubactep, C. Investigating the suitability of Fe0 packed-beds for water defluoridation. Chemosphere 2018, 209, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Dhananasekaran, S.; Palanivel, R.; Pappu, S. Adsorption of methylene blue, bromophenol blue, and coomassie brilliant blue by α-chitin nanoparticles. J. Adv. Res. 2016, 7, 113–124. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Ashiq, M.N. Adsorption of dyes from aqueous solutions on activated charcoal. J. Hazard. Mater. 2007, 139, 57–66. [Google Scholar] [CrossRef]
- Shapkin, N.P.; Maiorov, V.I.; Leont’ev, L.B.; Shkuratov, A.L.; Shapkina, V.Y.; Khal’chenko, I.G.J.C.J. A study of the adsorption properties of modified layered silicate. Colloid J. 2014, 76, 746–752. [Google Scholar] [CrossRef]
- El-Gamal, S.M.A.; Amin, M.S.; Ahmed, M.A. Removal of methyl orange and bromophenol blue dyes from aqueous solution using sorel’s cement nanoparticles. J. Environ. Chem. Eng. 2015, 3, 1702–1712. [Google Scholar] [CrossRef]
- El-Zahhar, A.A.; Awwad, N.S.; El-Katori, E.E. Removal of bromophenol blue dye from industrial waste water by synthesizing polymer-clay composite. J. Mol. Liq. 2014, 199, 454–461. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Abou-Gamra, Z.M. Mesoporous mgo nanoparticles as a potential sorbent for removal of fast orange and bromophenol blue dyes. Nanotechnol. Environ. Eng. 2016, 1, 10. [Google Scholar] [CrossRef]
- You, L.; Wu, Z.; Kim, T.; Lee, K. Kinetics and thermodynamics of bromophenol blue adsorption by a mesoporous hybrid gel derived from tetraethoxysilane and bis(trimethoxysilyl)hexane. J. Colloid Interface Sci. 2006, 300, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, R.; Khatun, E.; Pal, M.; Talukdar, S.; Mandal, D.; Saha, P.; Mandal, K. Influence of functional group of dye on the adsorption behaviour of CoFe2O4 nano-hollow spheres. New J. Chem. 2017, 41, 9095–9102. [Google Scholar] [CrossRef]
- Sohni, S.; Gul, K.; Ahmad, F.; Ahmad, I.; Khan, A.; Khan, N.; Bahadar Khan, S. Highly efficient removal of acid red-17 and bromophenol blue dyes from industrial wastewater using graphene oxide functionalized magnetic chitosan composite. Polym. Compos. 2018, 39, 3317–3328. [Google Scholar] [CrossRef]
- Mazaheri, H.; Ghaedi, M.; Asfaram, A.; Hajati, S. Performance of cus nanoparticle loaded on activated carbon in the adsorption of methylene blue and bromophenol blue dyes in binary aqueous solutions: Using ultrasound power and optimization by central composite design. J. Mol. Liq. 2016, 219, 667–676. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Ramezani, M.; Ghaedi, A.M. Synthesis and characterization of Fe2O3–ZnO–ZnFe2O4/carbon nanocomposite and its application to removal of bromophenol blue dye using ultrasonic assisted method: Optimization by response surface methodology and genetic algorithm. J. Taiwan Inst. Chem. Eng. 2016, 59, 275–284. [Google Scholar] [CrossRef]
- Saha, B.; Das, S.; Saikia, J.; Das, G. Preferential and enhanced adsorption of different dyes on iron oxide nanoparticles: A comparative study. J. Phys. Chem. C 2011, 115, 8024–8033. [Google Scholar] [CrossRef]

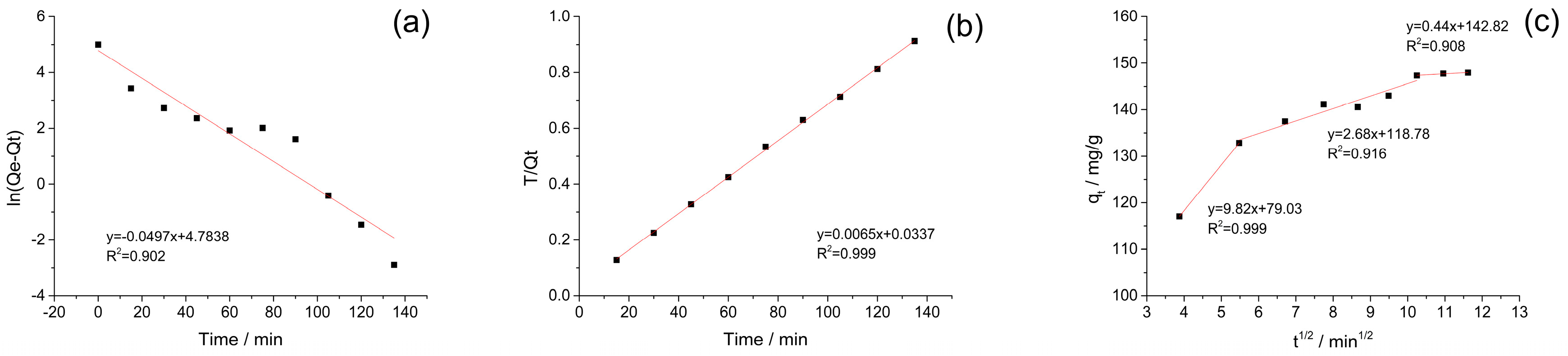
| Exp | Pseudo-First-Order Model | Pseudo-Second-Order Model | Intraparticle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qexp | qe | k1 | R2 | qe | kad | R2 | k1 | k2 | k3 |
| 148.0 | 119.56 | 0.0497 | 0.902 | 153.85 | 0.00125 | 0.999 | 9.82 | 2.68 | 0.44 |
| Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|
| qm | b | R2 | 1/n | k | R2 | B | AT | R2 |
| 204.50 | 2.445 | 0.984 | 0.497 | 149.62 | 0.954 | 112.1 | 19.98 | 0.975 |
| Dyes | TPSA/A2 | pKa |
|---|---|---|
| BPB | 92.2 | 3.85 |
| Bromocresol Green | 92.2 | 4.7 |
| Brilliant Green | 92.1 | 2.5 |
| Brilliant Crocein | 197 | - a |
| Amaranth | 238 | - |
| Fuchsin Basic | 75.9 | - |
| Safranine T | 68.8 | 6.4 |
| Malachite Green | 6.2 | 6.9 |
| Methyl Red | 65.3 | 4.95 |
| Adsorbent | Adsorbate | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|
| α-Chitin nanoparticles | BPB | 22.72 | [48] |
| Activated charcoal | BPB | About 9.0 × 10−3 | [49] |
| SiO2·Bth+·PF6− ionic liquids | BPB | 238.10 | [24] |
| Modified layered silicate | BPB | 184.5 | [50] |
| Sorel’s cement nanoparticles | BPB | 4.88 | [51] |
| Polymer-clay composite | BPB | About 7.5 | [52] |
| Mesoporous MgO nanoparticles | BPB | 40 | [53] |
| Mesoporous hybrid gel | BPB | 18.43 | [54] |
| CoFe2O4 nano-hollow spheres | BPB | 29.3 | [55] |
| Graphene oxide functionalized magnetic chitosan composite | BPB | 9.5 | [56] |
| CuS-NP-AC | BPB | 106.4 | [57] |
| Fe2O3-ZnO-ZnFe2O4/carbon nanocomposite | BPB | 90.91 | [58] |
| Iron oxide nanoparticles | BPB | About 110 | [59] |
| Fe3O4/MIL-88A | BPB | 141.9–167.2 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Huang, Y.; Xiao, A.; Qiu, H.; Liu, L. Preparation of Magnetic Fe3O4/MIL-88A Nanocomposite and Its Adsorption Properties for Bromophenol Blue Dye in Aqueous Solution. Nanomaterials 2019, 9, 51. https://doi.org/10.3390/nano9010051
Liu Y, Huang Y, Xiao A, Qiu H, Liu L. Preparation of Magnetic Fe3O4/MIL-88A Nanocomposite and Its Adsorption Properties for Bromophenol Blue Dye in Aqueous Solution. Nanomaterials. 2019; 9(1):51. https://doi.org/10.3390/nano9010051
Chicago/Turabian StyleLiu, Yi, Yumin Huang, Aiping Xiao, Huajiao Qiu, and Liangliang Liu. 2019. "Preparation of Magnetic Fe3O4/MIL-88A Nanocomposite and Its Adsorption Properties for Bromophenol Blue Dye in Aqueous Solution" Nanomaterials 9, no. 1: 51. https://doi.org/10.3390/nano9010051
APA StyleLiu, Y., Huang, Y., Xiao, A., Qiu, H., & Liu, L. (2019). Preparation of Magnetic Fe3O4/MIL-88A Nanocomposite and Its Adsorption Properties for Bromophenol Blue Dye in Aqueous Solution. Nanomaterials, 9(1), 51. https://doi.org/10.3390/nano9010051