Vertically Aligned Carbon Nanotubes Grown on Copper Foil as Electrodes for Electrochemical Double Layer Capacitors
Abstract
1. Introduction
2. Experimental
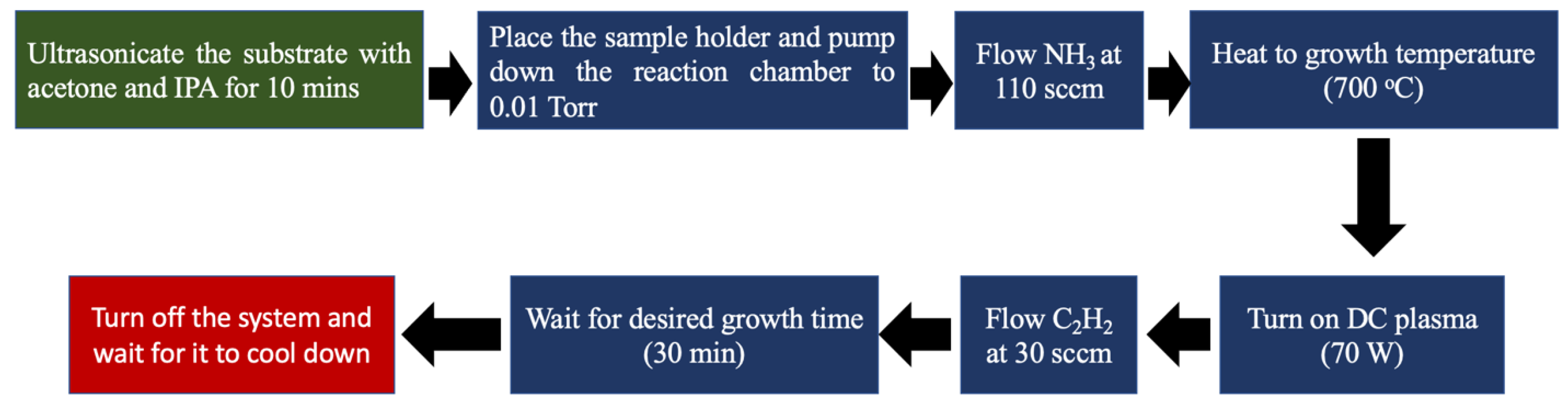
2.1. Synthesis of VACNTs
2.2. Material Characterization
2.2.1. Structural Characterization
2.2.2. Electrochemical Characterization
3. Results and Discussion
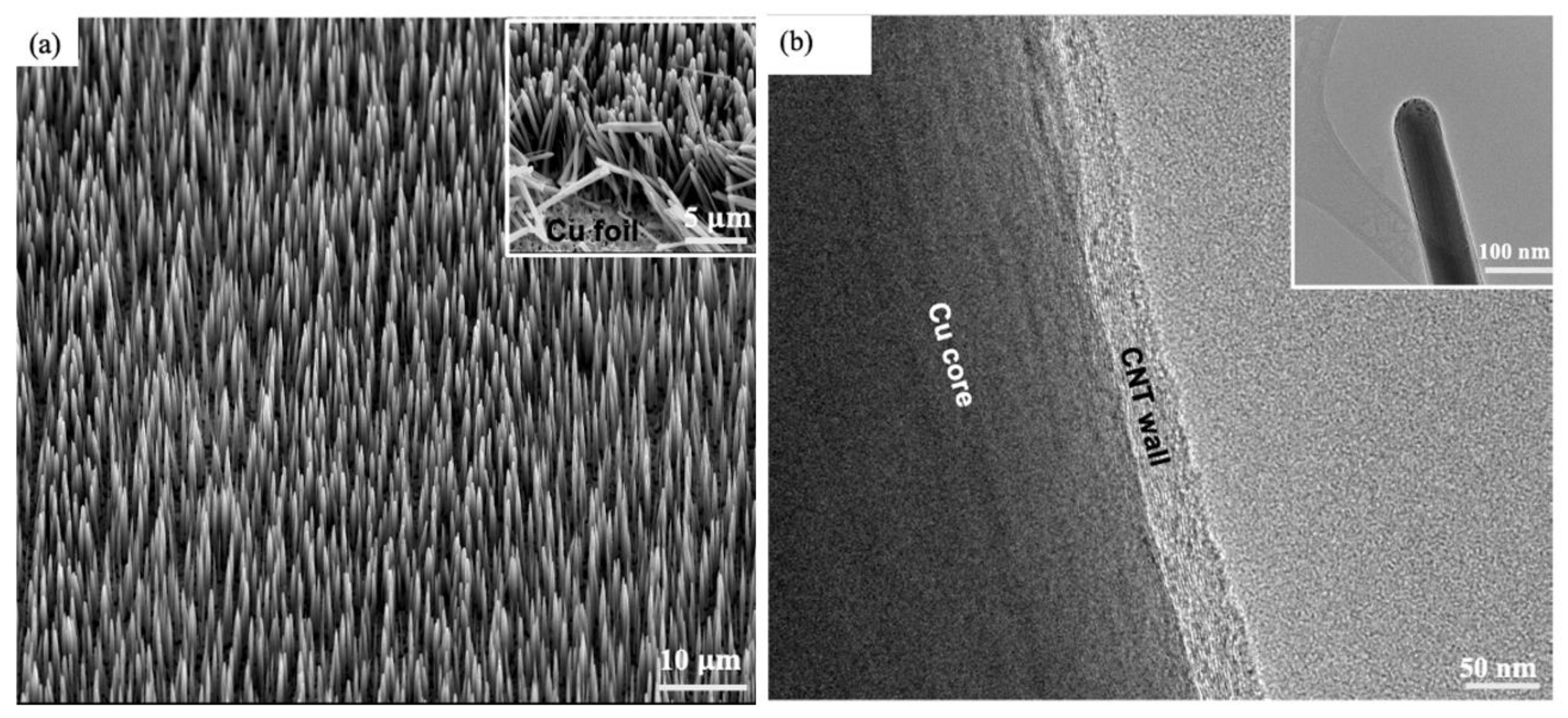
3.1. VACNT Morphology
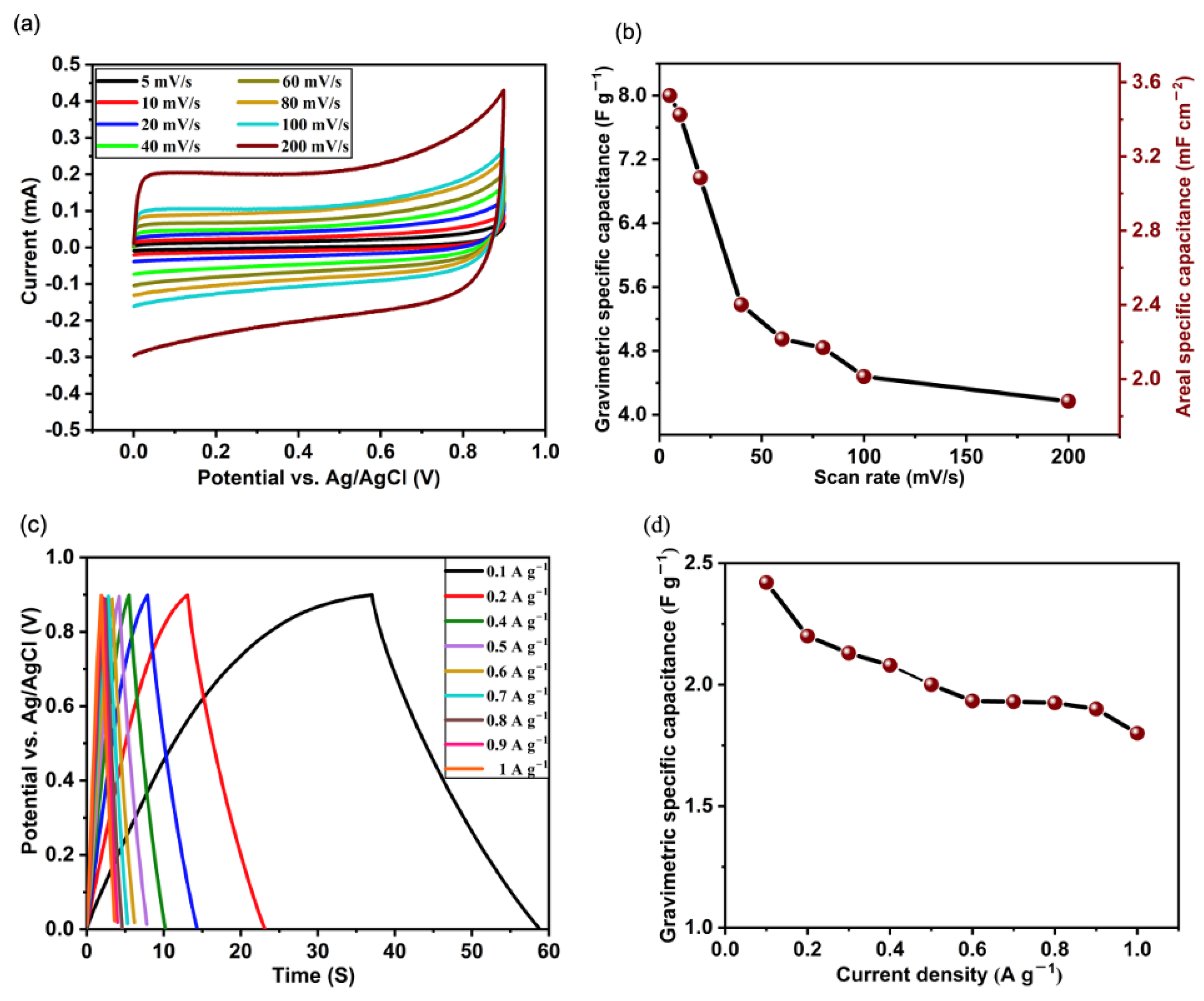
3.2. Electrochemical Properties of VACNT Electrodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Wu, Z.; Yuan, S.; Zhang, X.B. Advances and challenges for flexible energy storage and conversion devices and systems. Energy Environ. Sci. 2014, 7, 2101–2122. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Flexible supercapacitors based on carbon nanomaterials. J. Mater. Chem. A 2014, 2, 10756–10775. [Google Scholar] [CrossRef]
- Lu, Z.; Chang, Z.; Zhu, W.; Sun, X. Beta-phased Ni(OH)2 nanowall film with reversible capacitance higher than theoretical Faradic capacitance. Chem. Commun. 2011, 47, 9651–9653. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, Q.; Chen, J.; Chen, Y.; Guo, P.; Wei, C.; Cui, P.; Jiang, J.; Li, X.; Xu, Q. An intrinsically non-flammable organic electrolyte for wide temperature range supercapacitors. Chem. Eng. J. 2023, 457, 141265. [Google Scholar] [CrossRef]
- Gupta, N.; Mital, S.; Sharma, G.S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Inoue, Y.; Hayashi, K.; Karita, M.; Nakano, T.; Shimamura, Y.; Shirasu, K.; Yamamoto, G.; Hashida, T. Study on the mechanical and electrical properties of twisted CNT yarns fabricated from CNTs with various diameters. Carbon 2021, 176, 400–410. [Google Scholar] [CrossRef]
- Izadi-Najafabadi, A.; Futaba, D.N.; Iijima, S.; Hata, K. Ion diffusion and electrochemical capacitance in aligned and packed single-walled carbon nanotubes. J. Am. Chem. Soc. 2010, 132, 18017–18019. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous increase in carbon at pore sizes less than 1 nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, G.; Yang, Y.; Gu, Z. Comparison Between Electrochemical Properties of Aligned Carbon Nanotube Array and Entangled Carbon Nanotube Electrodes. J. Electrochem. Soc. 2008, 155, K19. [Google Scholar] [CrossRef]
- Hussain, S.; Amade, R.; Moreno, H.; Bertran, E. RF-PECVD growth and nitrogen plasma functionalization of CNTs on copper foil for electrochemical applications. Diam. Relat. Mater. 2014, 49, 55–61. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, S.; Kim, S.; Park, K.W.; Cho, D.; Jeong, Y. Carbon nanotube film anodes for flexible lithium ion batteries. J. Power Sources 2015, 279, 495–501. [Google Scholar] [CrossRef]
- Atthipalli, G.; Epur, R.; Kumta, P.N.; Allen, B.L.; Tang, Y.; Star, A.; Gray, J.L. The effect of temperature on the growth of carbon nanotubes on copper foil using a nickel thin film as catalyst. Thin Solid Film. 2011, 519, 5371–5375. [Google Scholar] [CrossRef]
- Kavian, R.; Vicenzo, A.; Bestetti, M. Growth of carbon nanotubes on aluminium foil for supercapacitors electrodes. J. Mater Sci. 2011, 46, 1487–1493. [Google Scholar] [CrossRef]
- Masarapu, C.; Wei, B. Direct growth of aligned multiwalled carbon nanotubes on treated stainless steel substrates. Langmuir 2007, 23, 9046–9049. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.; Bastos, A.C.; Oliveira, F.J.; Conte, D.E.; Fan, Y.; Pinna, N.; Silva, R.F. Catalyst-free growth of carbon nanotube arrays directly on Inconel® substrates for electrochemical carbon-based electrodes. J. Mater. Chem. A 2015, 3, 17804–17810. [Google Scholar] [CrossRef]
- Sugime, H.; Esconjauregui, S.; Yang, J.; D’Arsié, L.; Oliver, R.A.; Bhardwaj, S.; Cepek, C.; Robertson, J. Low temperature growth of ultra-high mass density carbon nanotube forests on conductive supports. Appl. Phys. Lett. 2013, 103, 073116. [Google Scholar] [CrossRef]
- Lei, R.; Ni, H.; Chen, R.; Gu, H.; Zhang, H.; Dong, S. In situ growth of self-supported and defect-engineered carbon nanotube networks on 316L stainless steel as binder-free supercapacitors. J. Colloid Interface Sci. 2018, 532, 622–629. [Google Scholar] [CrossRef]
- Isacfranklin, M.; Rathinam, Y.; Ganesan, R.; Velauthapillai, D. Direct Growth of Binder-Free CNTs on a Nickel Foam Substrate for Highly Efficient Symmetric Supercapacitors. ACS Omega 2023, 8, 11700–11708. [Google Scholar] [CrossRef]
- Zhao, F.; Vicenzo, A.; Hashempour, M.; Bestetti, M. Supercapacitor electrodes by direct growth of multi-walled carbon nanotubes on Al: A study of performance versus layer growth evolution. Electrochim. Acta 2014, 150, 35–45. [Google Scholar] [CrossRef]
- Avasthi, P.; Kumar, A.; Balakrishnan, V. Aligned CNT Forests on Stainless Steel Mesh for Flexible Supercapacitor Electrode with High Capacitance and Power Density. ACS Appl. Nano Mater. 2019, 2, 1484–1495. [Google Scholar] [CrossRef]
- Querne, C.; Vignal, T.; Pinault, M.; Banet, P.; Mayne-L’Hermite, M.; Aubert, P.H. A comparative study of high density Vertically Aligned Carbon Nanotubes grown onto different grades of aluminum—Application to supercapacitors. J. Power Sources 2023, 553, 232258. [Google Scholar] [CrossRef]
- Raab, S.J.; Guschlbauer, R.; Lodes, M.A.; Körner, C. Thermal and Electrical Conductivity of 99.9% Pure Copper Processed via Selective Electron Beam Melting. Adv. Eng. Mater. 2016, 18, 1661–1666. [Google Scholar] [CrossRef]
- Kruszka, B.; Terzyk, A.P.; Wiśniewski, M.; Gauden, P.A.; Szybowicz, M. Synthesis of carbon nanotubes and nanotube forests on copper catalyst. Mater. Res. Express 2014, 1, 035040. [Google Scholar] [CrossRef]
- Atthipalli, G.; Epur, R.; Kumta, P.N.; Yang, M.; Lee, J.K.; Gray, J.L. Nickel catalyst-assisted vertical growth of dense carbon nanotube forests on bulk copper. J. Phys. Chem. C 2011, 115, 3534–3538. [Google Scholar] [CrossRef]
- Sepahvand, S.; Safaei, P.; Sanaee, Z. Growth of Carbon Nano Tubes on Copper Substrate Suitable for Lithium Ion Battery Anode. Procedia Mater. Sci. 2015, 11, 634–638. [Google Scholar] [CrossRef]
- Lahiri, I.; Oh, S.W.; Hwang, J.Y.; Cho, S.; Sun, Y.K.; Banerjee, R.; Choi, W. High Capacity and Excellent Stability of Lithium Ion Battery Anode Using Interface-Controlled Binder-Free Multiwall Carbon Nanotubes Grown on Copper. ACS Nano 2010, 4, 3440–3446. [Google Scholar] [CrossRef]
- García-Céspedes, J.; Thomasson, S.; Teo, K.B.K.; Kinloch, I.A.; Milne, W.I.; Pascual, E.; Bertran, E. Efficient diffusion barrier layers for the catalytic growth of carbon nanotubes on copper substrates. Carbon 2009, 47, 613–621. [Google Scholar] [CrossRef]
- Li, G.; Chakrabarti, S.; Schulz, M.; Shanov, V. Growth of aligned multiwalled carbon nanotubes copper substrates by chemical vapor deposition. J. Mater. Res. 2009, 24, 2813–2820. [Google Scholar] [CrossRef]
- Thapa, A.; Wang, X.; Li, W. Synthesis and field emission properties of Cu-filled vertically aligned carbon nanotubes. Appl. Surf. Sci. 2021, 537, 148086. [Google Scholar] [CrossRef]
- Nwanno, C.E.; Thapa, A.; Watt, J.; Simkins Bendayan, D.; Li, W. Field Emission Properties of Cu-Filled Vertically Aligned Carbon Nanotubes Grown Directly on Thin Cu Foils. Nanomaterials 2024, 14, 988. [Google Scholar] [CrossRef]
- Zilli, D.; Bonelli, P.R.; Cukierman, A.L. Effect of alignment on adsorption characteristics of self-oriented multi-walled carbon nanotube arrays. Nanotechnology 2006, 17, 5136–5141. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, G.; Yang, Y. Electrochemical properties of ultra-long, aligned, carbon nanotube array electrode in organic electrolyte. J. Power Sources 2007, 172, 476–480. [Google Scholar] [CrossRef]
- Arcila-Velez, M.R.; Zhu, J.; Childress, A.; Karakaya, M.; Podila, R.; Rao, A.M.; Roberts, M.E. Roll-to-roll synthesis of vertically aligned carbon nanotube electrodes for electrical double layer capacitors. Nano Energy 2014, 8, 9–16. [Google Scholar] [CrossRef]
- Hussain, S.; Amade, R.; Jover, E.; Bertran, E. Nitrogen plasma functionalization of carbon nanotubes for supercapacitor applications. J. Mater. Sci. 2013, 48, 7620–7628. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Guedes, A.; Szefczyk, M.E.; Pereira, A.M.; Araújo, J.P.; Freire, C. Progress in the Raman spectra analysis of covalently functionalized multiwalled carbon nanotubes: Unraveling disorder in graphitic materials. Phys. Chem. Chem. Phys. 2016, 18, 12784–12796. [Google Scholar] [CrossRef]
- Kokai, F.; Shimazu, T.; Adachi, K.; Koshio, A.; Takahashi, Y. Fabrication of completely filled carbon nanotubes with copper nanowires in a confined space. Appl. Phys. A Mater. Sci. Process. 2009, 97, 55–62. [Google Scholar] [CrossRef]
- Cao, A.; Xu, C.; Liang, J.; Wu, D.; Wei, B. X-ray diffraction characterization on the alignment degree of carbon nanotubes. Chem. Phys. Lett. 2001, 344, 13–17. [Google Scholar] [CrossRef]
- Kumarathasan, P.; Breznan, D.; Das, D.; Salam, M.A.; Siddiqui, Y.; MacKinnon-Roy, C.; Guan, J.; de Silva, N.; Simard, B.; Vincent, R. Cytotoxicity of carbon nanotube variants: A comparative in vitro exposure study with A549 epithelial and J774 macrophage cells. Nanotoxicology 2015, 9, 148–161. [Google Scholar] [CrossRef]
- Smith, B. Infrared spectral interpretation: A systematic approach. In Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–304. [Google Scholar]
- Osswald, S.; Havel, M.; Gogotsi, Y. Monitoring oxidation of multiwalled carbon nanotubes by Raman spectroscopy. J. Raman Spectrosc. 2007, 38, 728–736. [Google Scholar] [CrossRef]
- Mehra, P.; Paul, A. Decoding Carbon-Based Materials’ Properties for High CO2Capture and Selectivity. ACS Omega 2022, 7, 34538–34546. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Viswanathan, B. Template synthesis and characterization of well-aligned nitrogen containing carbon nanotubes. Mater. Chem. Phys. 2005, 93, 291–295. [Google Scholar] [CrossRef]
- Misra, A.; Tyagi, P.K.; Singh, M.K.; Misra, D.S. FTIR studies of nitrogen doped carbon nanotubes. Diam. Relat. Mater. 2006, 15, 385–388. [Google Scholar] [CrossRef]
- Hadianfard, M.J.; Alizadeh, M.; Moradzaman, M. Effects of chemical and mechanical funtionalization of carbon nanotubes on the behavior of a CNT/Phenolic nanocomposite. Boletín Grup Español Carbón 2019, 51, 20–25. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=6956801&info=resumen&idioma=ENG%0Ahttps://dialnet.unirioja.es/servlet/articulo?codigo=6956801 (accessed on 22 September 2025).
- Fayazfar, H.; Afshar, A.; Dolati, A. Controlled Growth of Well-Aligned Carbon Nanotubes, Electrochemical Modification and Electrodeposition of Multiple Shapes of Gold Nanostructures. Mater. Sci. Appl. 2013, 4, 667–678. [Google Scholar] [CrossRef]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy Storage Data Reporting in Perspective—Guidelines for Interpreting the Performance of Electrochemical Energy Storage Systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from “supercapacitor” to “battery” behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539. [Google Scholar] [CrossRef]
- Dogru, I.B.; Durukan, M.B.; Turel, O.; Unalan, H.E. Flexible supercapacitor electrodes with vertically aligned carbon nanotubes grown on aluminum foils. Prog. Nat. Sci. Mater. Int. 2016, 26, 232–236. [Google Scholar] [CrossRef]
- Zhang, C.; Bhoyate, S.; Zhao, C.; Kahol, P.K.; Kostoglou, N.; Mitterer, C.; Hinder, S.J.; Baker, M.A.; Constantinides, G.; Polychronopoulou, K.; et al. Electrodeposited nanostructured CoFe2O4 for overall water splitting and supercapacitor applications. Catalysts 2019, 9, 176. [Google Scholar] [CrossRef]
- Velmurugan, V.; Srinivasarao, U.; Ramachandran, R.; Saranya, M.; Grace, A.N. Synthesis of tin oxide/graphene (SnO2/G) nanocomposite and its electrochemical properties for supercapacitor applications. Mater. Res. Bull. 2016, 84, 145–151. [Google Scholar] [CrossRef]
- Gujar, T.P.; Kim, W.Y.; Puspitasari, I.; Jung, K.D.; Joo, O.S. Electrochemically deposited nanograin ruthenium oxide as a pseudocapacitive electrode. Int. J. Electrochem. Sci. 2007, 2, 666–673. [Google Scholar] [CrossRef]
- Zhu, J.; He, J. Facile synthesis of graphene-wrapped honeycomb MnO2 nanospheres and their application in supercapacitors. ACS Appl. Mater. Interfaces 2012, 4, 1770–1776. [Google Scholar] [CrossRef]
- Ren, S.; Yang, Y.; Xu, M.; Cai, H.; Hao, C.; Wang, X. Hollow SnO2 microspheres and their carbon-coated composites for supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 26–32. [Google Scholar] [CrossRef]
- Huq, M.M.; Hsieh, C.-T.; Ho, C.Y. Preparation of carbon nanotube-activated carbon hybrid electrodes by electrophoretic deposition for supercapacitor applications. Diam. Relat. Mater. 2016, 62, 58–64. [Google Scholar] [CrossRef]
- Fan, W.; Wang, Q.; Rong, K.; Shi, Y.; Peng, W.; Li, H.; Guo, Z.; Xu, B.B.; Hou, H.; Algadi, H.; et al. MXene Enhanced 3D Needled Waste Denim Felt for High-Performance Flexible Supercapacitors. Nano-Micro Lett. 2024, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Ghai, V.; Chatterjee, K.; Agnihotri, P.K. Vertically aligned carbon nanotubes-coated aluminium foil as flexible supercapacitor electrode for high power applications. Carbon Lett. 2021, 31, 473–481. [Google Scholar] [CrossRef]
- Gupta, S.; Tai, N.H. Carbon materials as oil sorbents: A review on the synthesis and performance. J. Mater. Chem. A 2016, 4, 1550–1565. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; Mckay, G.; Manawi, Y.; Malaibari, Z.; Hussien, M.A. Outstanding adsorption performance of high aspect ratio and super-hydrophobic carbon nanotubes for oil removal. Chemosphere 2016, 164, 142–155. [Google Scholar] [CrossRef]
- Sianipar, M.; Kim, S.H.; Khoiruddin Iskandar, F.; Wenten, I.G. Functionalized carbon nanotube (CNT) membrane: Progress and challenges. RSC Adv. 2017, 7, 51175–51198. [Google Scholar] [CrossRef]
- Byon, H.R.; Gallant, B.M.; Lee, S.W.; Shao-Horn, Y. Role of oxygen functional groups in carbon nanotube/graphene freestanding electrodes for high performance lithium batteries. Adv. Funct. Mater. 2013, 23, 1037–1045. [Google Scholar] [CrossRef]
- Kim, B.; Chung, H.; Kim, W. High-performance supercapacitors based on vertically aligned carbon nanotubes and nonaqueous electrolytes. Nanotechnology 2012, 23, 155401. [Google Scholar] [CrossRef]
- Park, J.; Yoo, Y.E.; Mai, L.; Kim, W. Rational Design of a Redox-Active Nonaqueous Electrolyte for a High-Energy-Density Supercapacitor Based on Carbon Nanotubes. ACS Sustain. Chem. Eng. 2019, 7, 7728–7735. [Google Scholar] [CrossRef]
- Thillaikkarasi, D.; Karthikeyan, S.; Ramesh, R.; Sengodan, P.; Kavitha, D.; Muthubalasubramanian, M. Electrochemical performance of various activated carbon-multi-walled carbon nanotubes symmetric supercapacitor electrodes in aqueous electrolytes. Carbon Lett. 2022, 32, 1481–1505. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, G.P.; Yang, Y.S. Using a cut-paste method to prepare a carbon nanotube fur electrode. Nanotechnology 2007, 18, 195607. [Google Scholar] [CrossRef]
- Yan, L.; Issaka Alhassan, S.; Gang, H.; Wu, B.; Wei, D.; Cao, Y.; Chen, P.; Wang, H. Enhancing charge transfer utilizing ternary composite slurry for high-efficient flow-electrode capacitive deionization. Chem. Eng. J. 2023, 468, 143413. [Google Scholar] [CrossRef]
- Azam, M.A.; Talib, E.; Seman, R.N.A.R. Direct deposition of multi-walled carbon nanotubes onto stainless steel and YEF foils using a simple electrophoretic deposition for electrochemical capacitor electrode. Mater. Res. Express 2019, 6, 015501. [Google Scholar] [CrossRef]
- Aval, L.F.; Ghoranneviss, M.; Pour, G.B. High-performance supercapacitors based on the carbon nanotubes, graphene and graphite nanoparticles electrodes. Heliyon 2018, 4, e00862. [Google Scholar] [CrossRef]
- Aboutalebi, S.H.; Chidembo, A.T.; Salari, M.; Konstantinov, K.; Wexler, D.; Liu, H.-K.; Dou, S.X. Comparison of GO, GO/MWCNTs composite and MWCNTs as potential electrode materials for supercapacitors. Energy Environ. Sci. 2011, 4, 1855–1865. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwanno, C.E.; Gotame, R.C.; Watt, J.; Kuo, W.; Li, W. Vertically Aligned Carbon Nanotubes Grown on Copper Foil as Electrodes for Electrochemical Double Layer Capacitors. Nanomaterials 2025, 15, 1506. https://doi.org/10.3390/nano15191506
Nwanno CE, Gotame RC, Watt J, Kuo W, Li W. Vertically Aligned Carbon Nanotubes Grown on Copper Foil as Electrodes for Electrochemical Double Layer Capacitors. Nanomaterials. 2025; 15(19):1506. https://doi.org/10.3390/nano15191506
Chicago/Turabian StyleNwanno, Chinaza E., Ram Chandra Gotame, John Watt, Winson Kuo, and Wenzhi Li. 2025. "Vertically Aligned Carbon Nanotubes Grown on Copper Foil as Electrodes for Electrochemical Double Layer Capacitors" Nanomaterials 15, no. 19: 1506. https://doi.org/10.3390/nano15191506
APA StyleNwanno, C. E., Gotame, R. C., Watt, J., Kuo, W., & Li, W. (2025). Vertically Aligned Carbon Nanotubes Grown on Copper Foil as Electrodes for Electrochemical Double Layer Capacitors. Nanomaterials, 15(19), 1506. https://doi.org/10.3390/nano15191506






