Covalent Organic Framework-Based Nanomembrane with Co-Immobilized Dual Enzymes for Micropollutant Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of Amorphous COF (aCOF)
2.3. Synthesis of HRP/GOx@aCOF
2.4. Synthesis of HRP/GOx@COF
2.5. Synthesis of Biocatalytic Membrane
2.6. Membrane Performance Evaluation
2.6.1. Pure Water Permeability
2.6.2. Dye Rejection
2.6.3. MP Removal Rate
2.7. Enzyme Activity of Biocatalytic Membrane
2.7.1. The Protein Immobilization Yield
2.7.2. The Membrane Surface Enzyme Activity
2.8. Stability and Reusability Test
2.9. Sample Preparation for Characterization
3. Results and Discussion
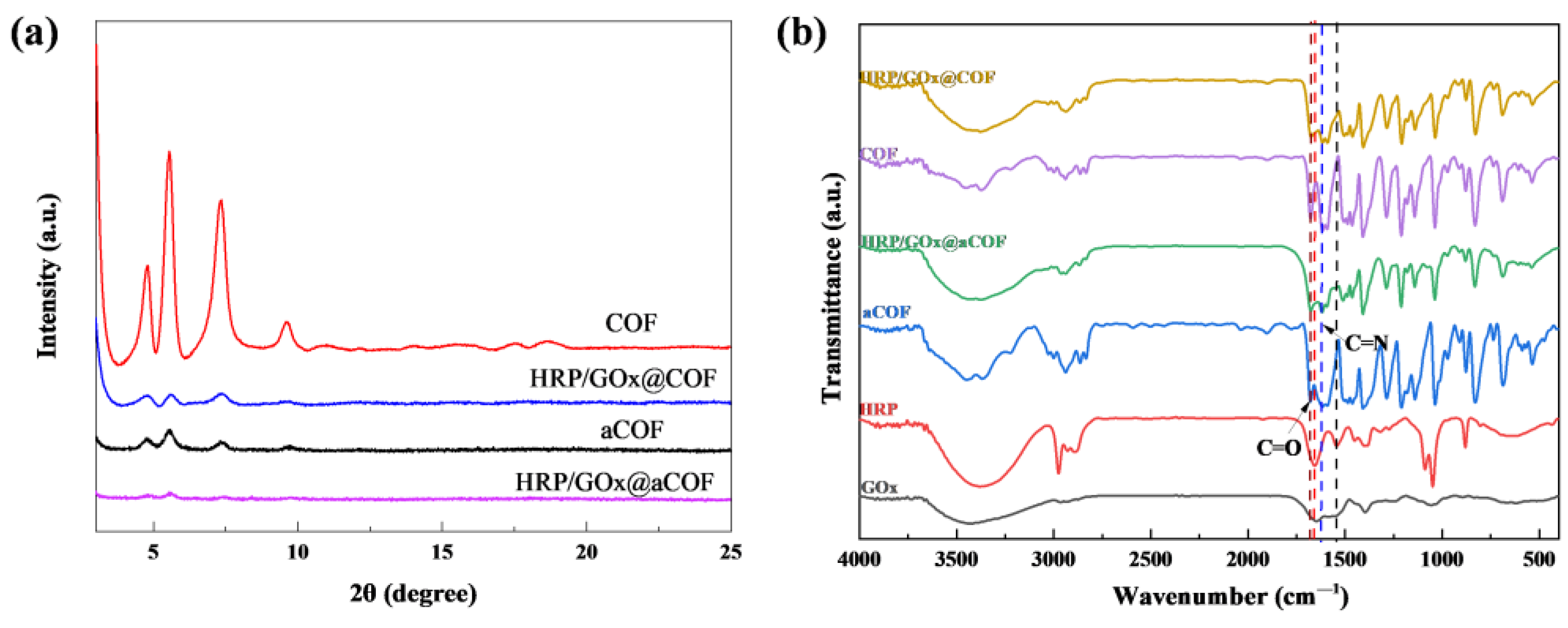
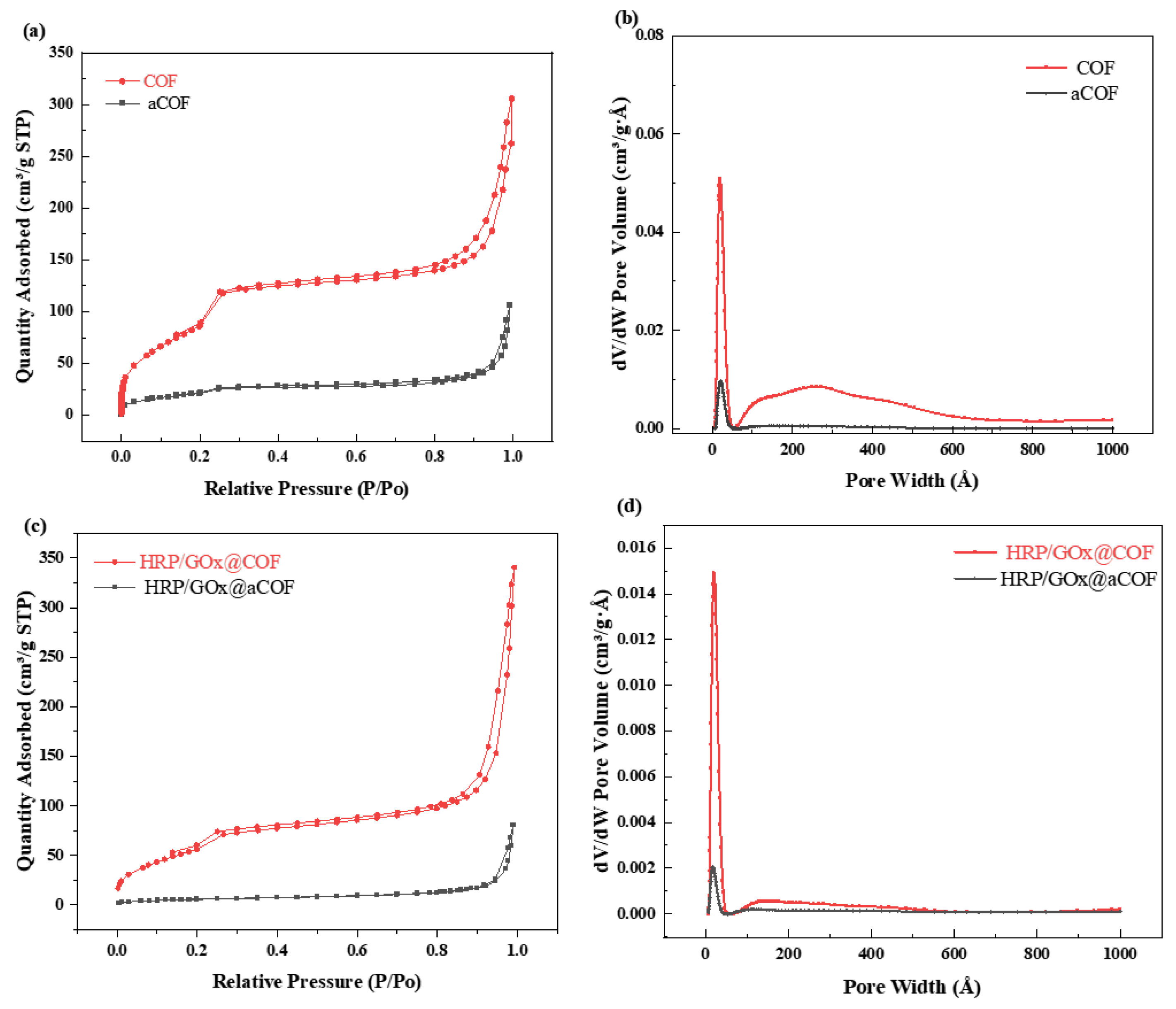
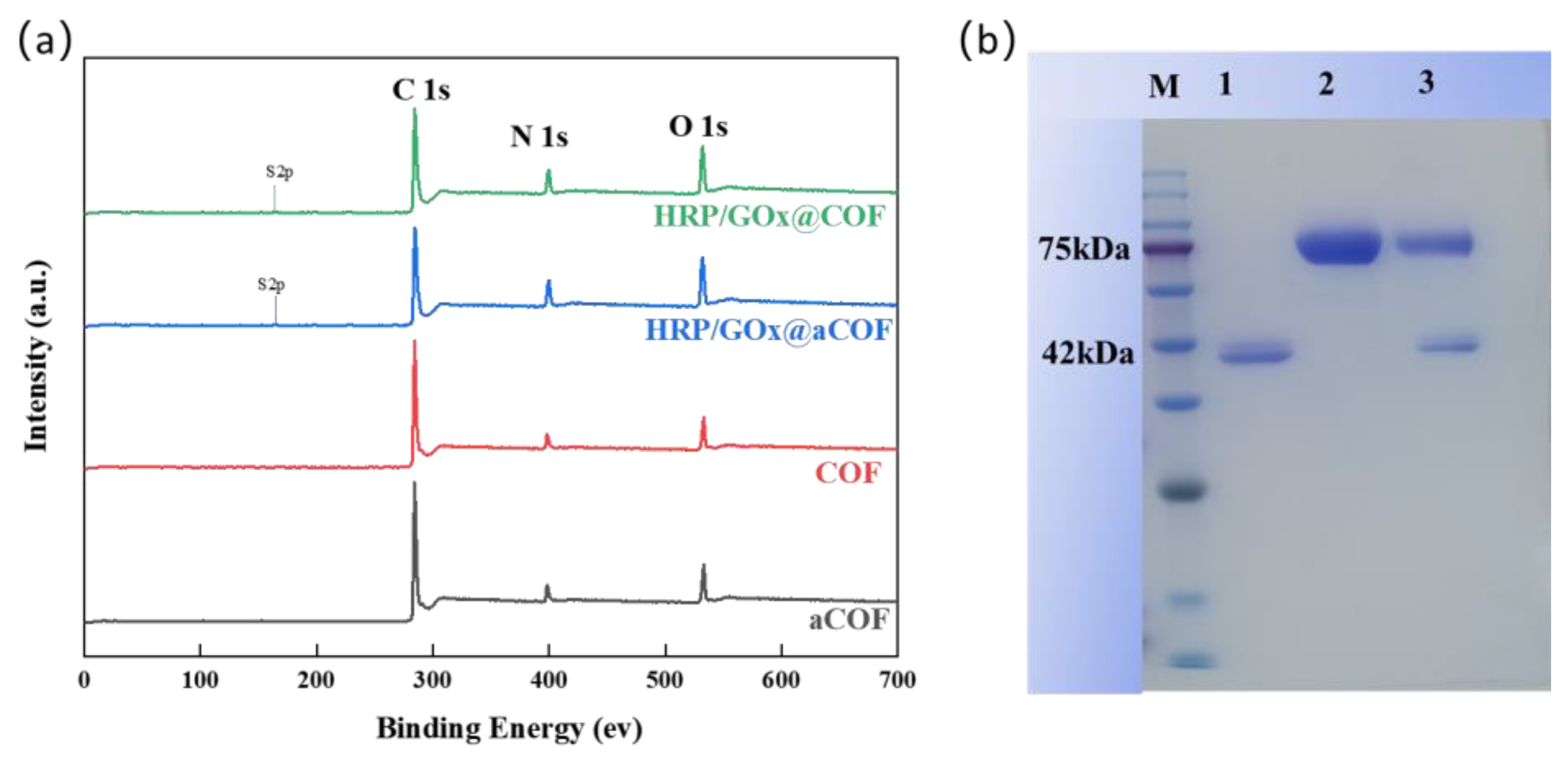
3.1. Characterization of COF Nanoparticles and Immobilized Enzyme
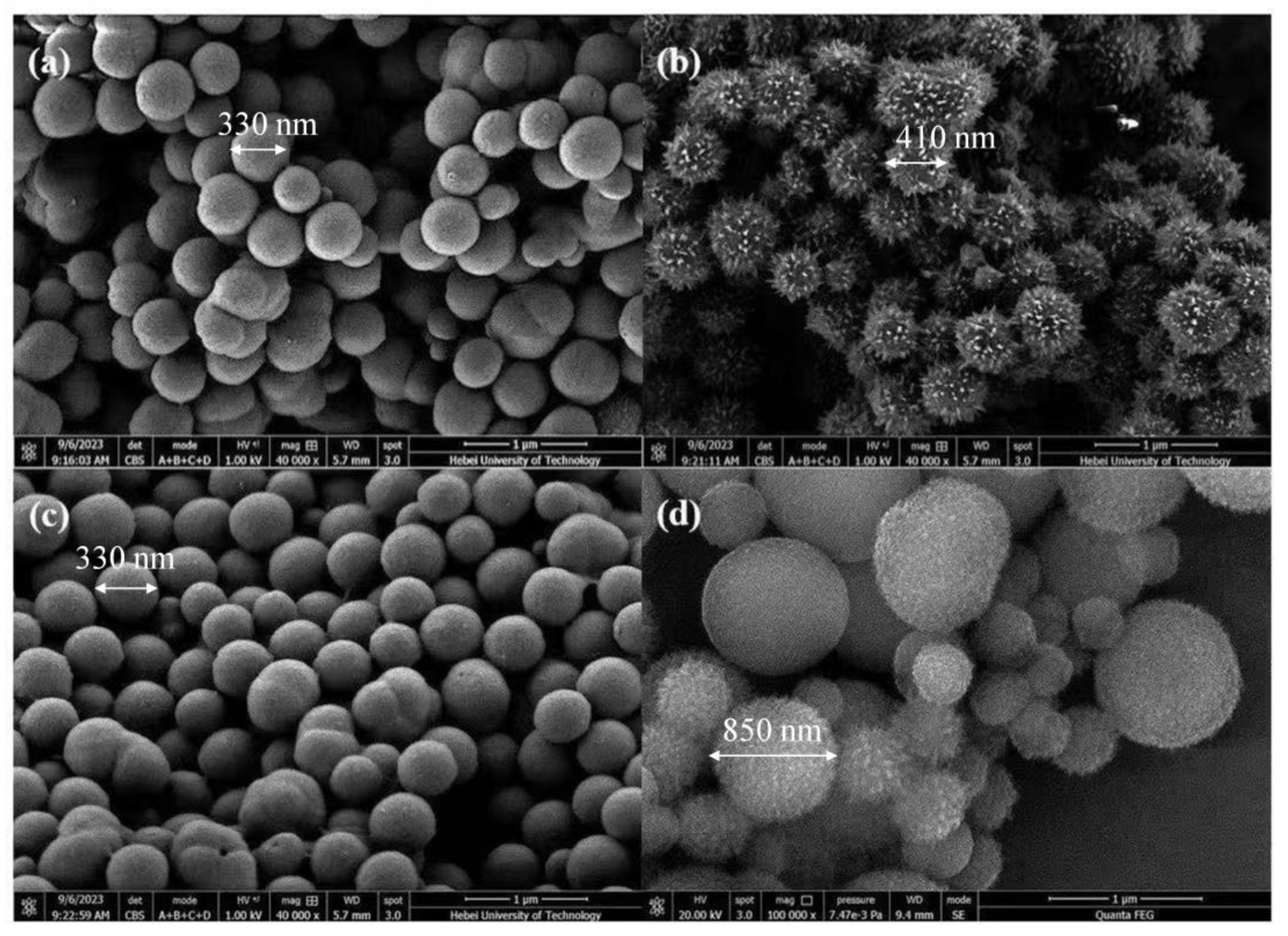
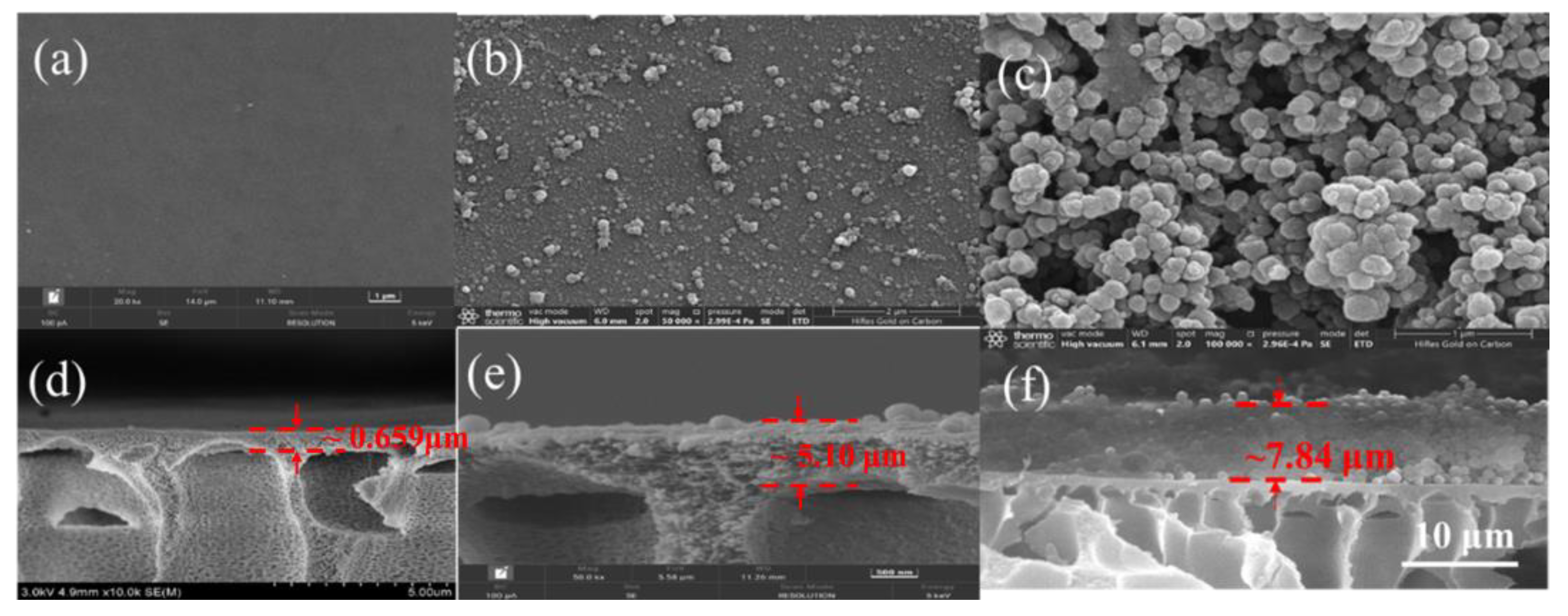
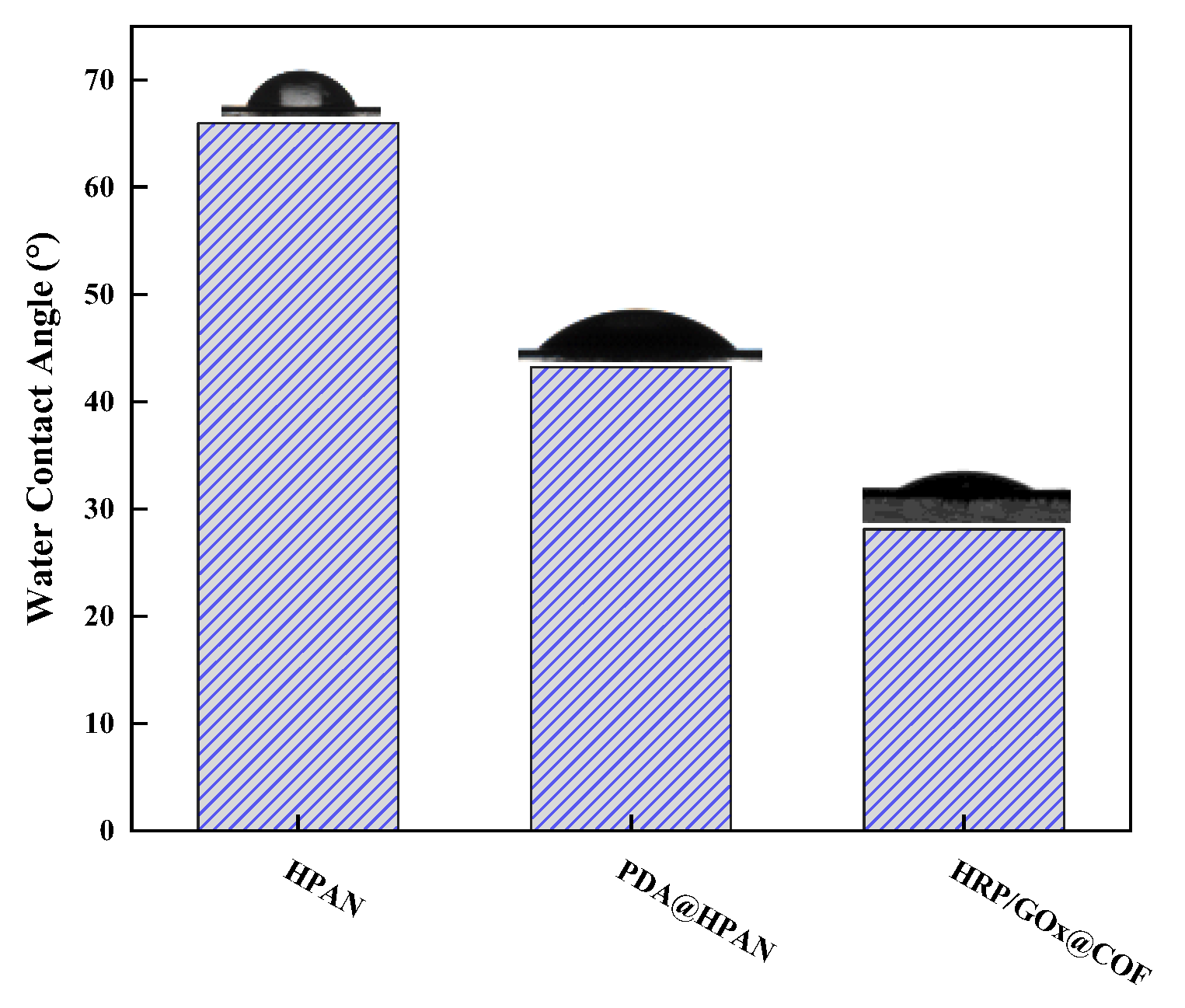
3.2. Characterization of Biocatalytic Membranes
3.3. Separation Performance of Biocatalytic Membranes
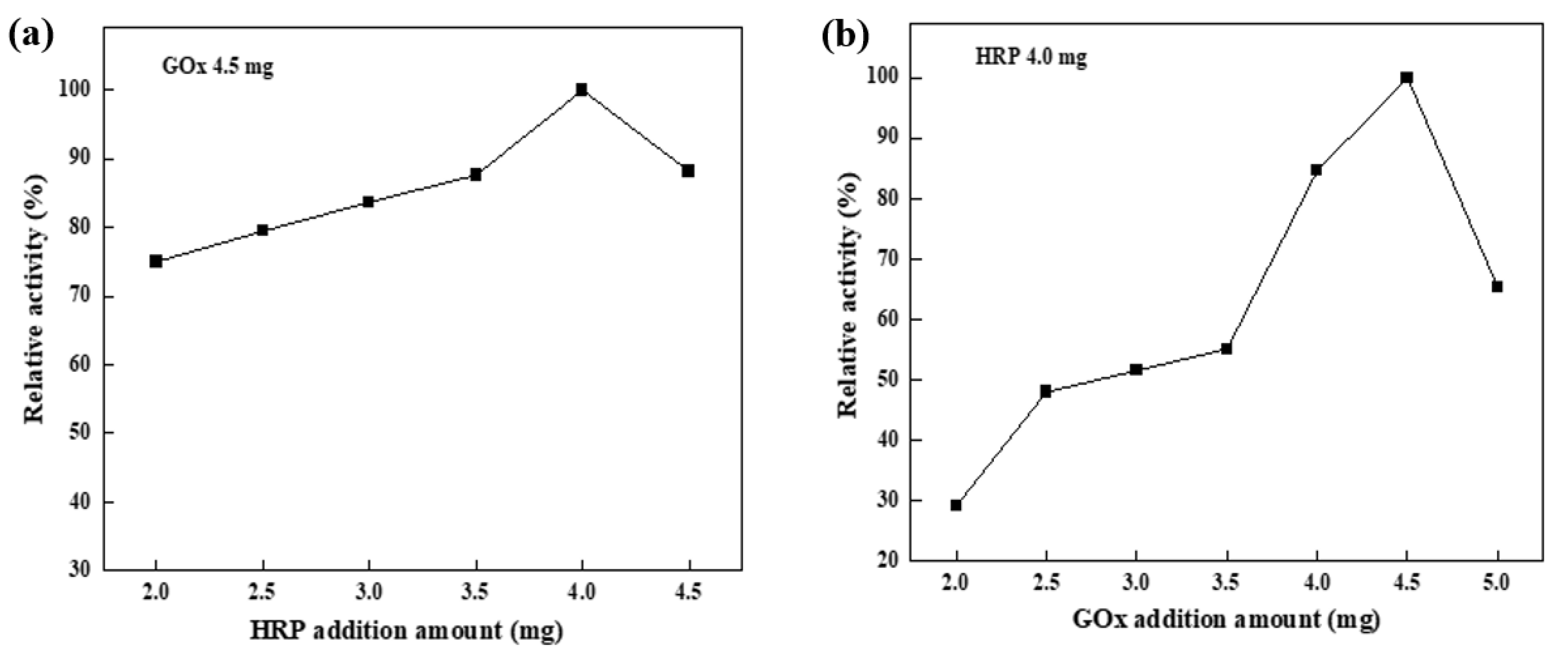
3.3.1. Effect of Dual Enzyme Addition on HRP Enzyme Activity
3.3.2. Effect of Glucose Concentration on the Properties of Biocatalytic Membranes
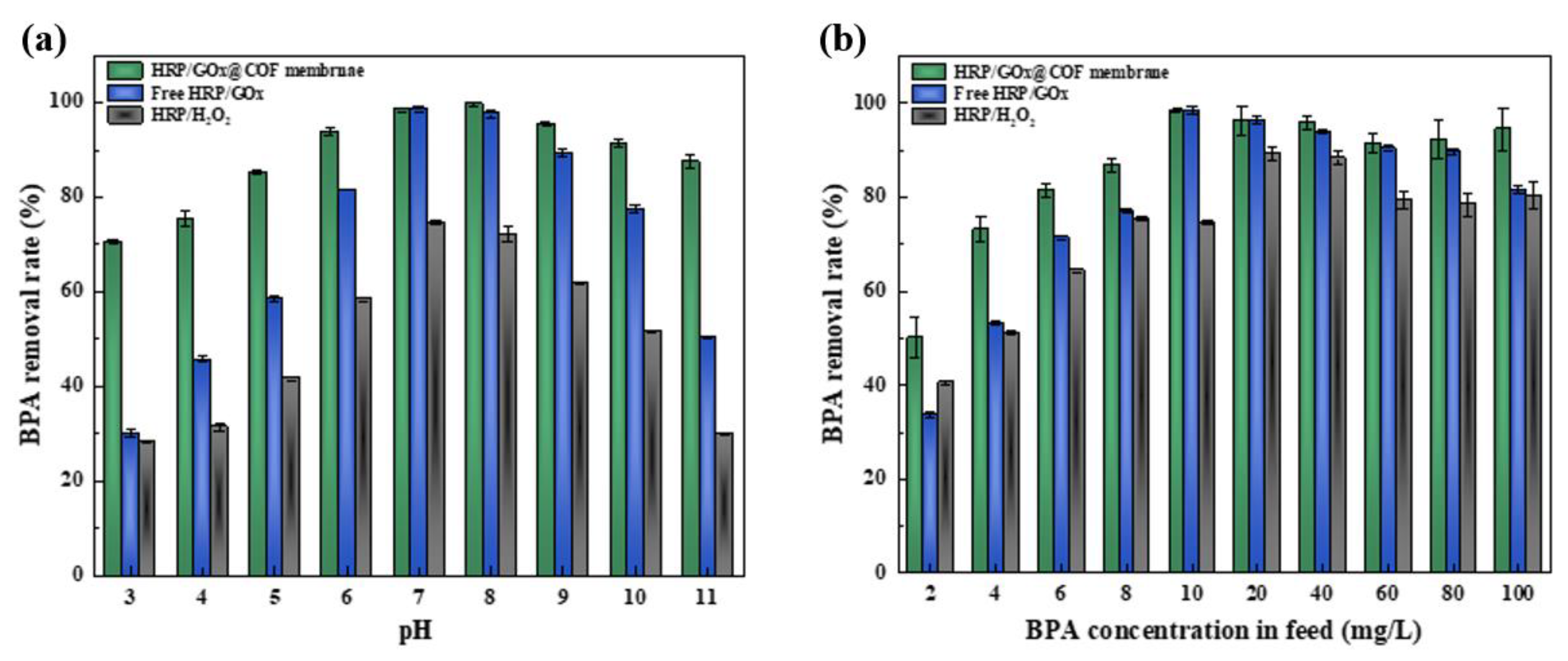
3.3.3. Removal of BPA by Biocatalytic Membranes Under Various Conditions
3.3.4. Removal of Different MPs by Biocatalytic Membranes
3.3.5. Removal of Dyes by Biocatalytic Membranes
3.3.6. Comparison of Hydroxyl Radicals in Dual-Enzyme and Single-Enzyme Systems
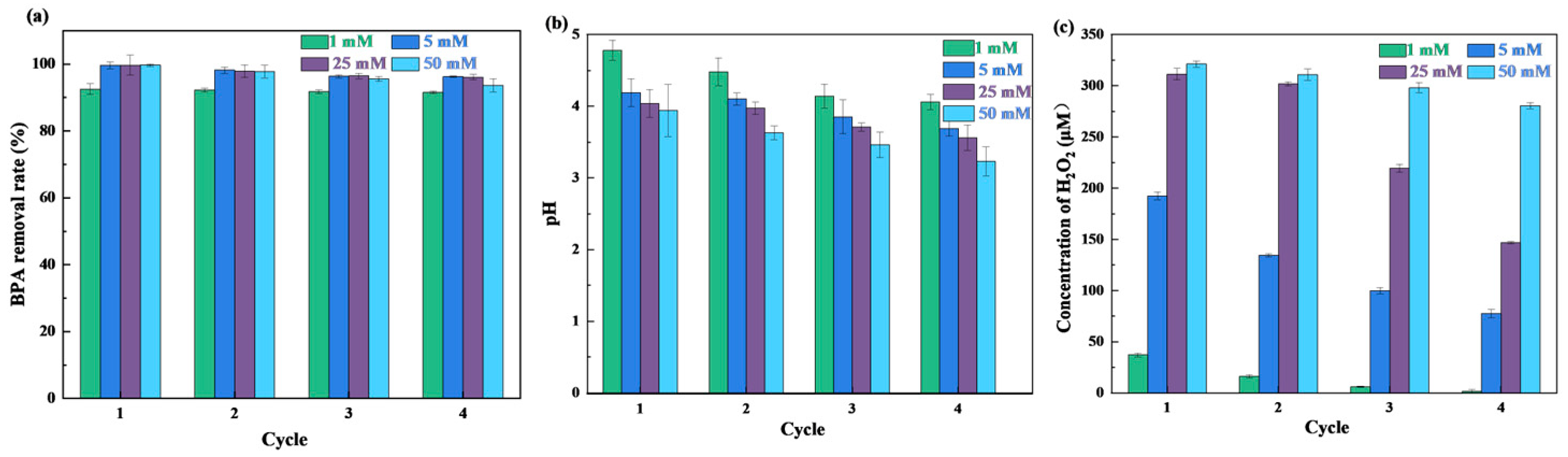
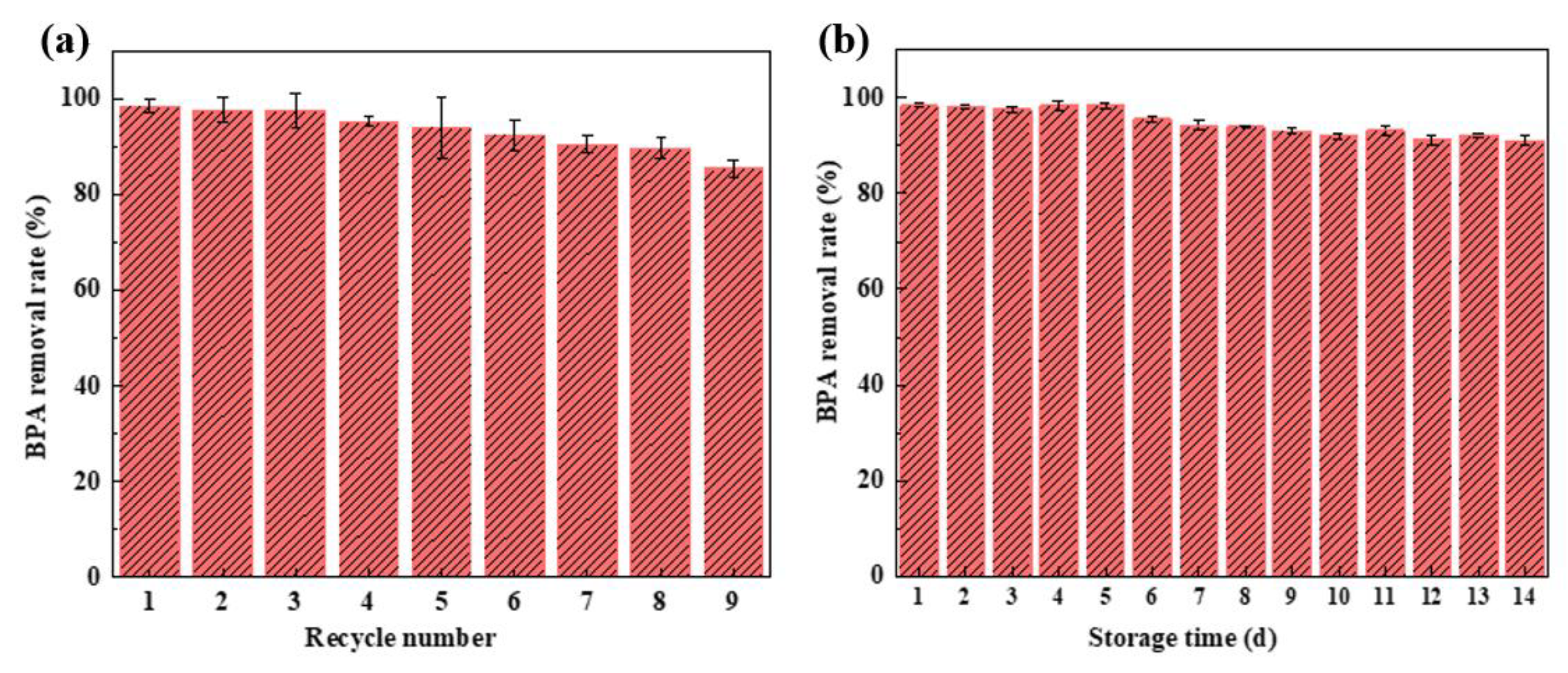
3.3.7. Reusability and Storage Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Luo, J.; Wan, Y. Regenerable biocatalytic nanofiltration membrane for aquatic micropollutants removal. J. Membr. Sci. 2018, 549, 120–128. [Google Scholar] [CrossRef]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Dudás, J.; Mazzei, R.; Drioli, E.; Giorno, L. Description of the diffusive–convective mass transport in a hollow-fiber biphasic biocatalytic membrane reactor. J. Membr. Sci. 2015, 482, 144–157. [Google Scholar] [CrossRef]
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. Occurrence, fate and removal of pharmaceutically active compounds (PhACs) in water and wastewater treatment plants-A review. J. Water Process Eng. 2019, 32, 100927. [Google Scholar] [CrossRef]
- Rees Clayton Erin, M.; Todd, M.; Dowd Jennifer, B.; Aiello Allison, E. The Impact of Bisphenol A and Triclosan on Immune Parameters in the U.S. Population, NHANES 2003–2006. Environ. Health Perspect. 2011, 119, 390–396. [Google Scholar] [CrossRef]
- Doherty, L.F.; Bromer, J.G.; Zhou, Y.; Aldad, T.S.; Taylor, H.S. In Utero Exposure to Diethylstilbestrol (DES) or Bisphenol-A (BPA) Increases EZH2 Expression in the Mammary Gland: An Epigenetic Mechanism Linking Endocrine Disruptors to Breast Cancer. Horm. Cancer 2010, 1, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Teppala, S.; Madhavan, S.; Shankar, A. Bisphenol A and Metabolic Syndrome: Results from NHANES. Int. J. Endocrinol. 2012, 2012, 598180. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S.; Sabanayagam, C. Bisphenol A and Peripheral Arterial Disease: Results from the NHANES. Environ. Health Perspect. 2012, 120, 1297–1300. [Google Scholar] [CrossRef]
- Xing, L.; Xu, Y.; Xiao, Y.; Shang, L.; Liu, R.; Wei, X.; Jiang, J.; Hao, W. Embryotoxic and Teratogenic Effects of the Combination of Bisphenol A and Genistein on In Vitro Cultured Postimplantation Rat Embryos. Toxicol. Sci. 2010, 115, 577–588. [Google Scholar] [CrossRef]
- Zdarta, J.; Jesionowski, T.; Meyer, A.S.; Pinelo, M. Removal of tetracycline in enzymatic membrane reactor: Enzymatic conversion as the predominant mechanism over adsorption and membrane rejection. J. Environ. Chem. Eng. 2022, 10, 106973. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, G.; Kumar, A.; Dhiman, P.; Mola, G.T.; Shan, A.; Si, C. Microplastic pollutants in water: A comprehensive review on their remediation by adsorption using various adsorbents. Chemosphere 2024, 352, 141365. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Bagheri, M.; Aidan, A. Membrane bioreactor for wastewater treatment: A review. Case Stud. Chem. Environ. Eng. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Najafabadipour, N.; Mojtabavi, S.; Jafari-Nodoushan, H.; Samadi, N.; Faramarzi, M.A. High efficiency of osmotically stable laccase for biotransformation and micro-detoxification of levofloxacin in the urea-containing solution: Catalytic performance and mechanism. Colloids Surf. B Biointerfaces 2021, 207, 112022. [Google Scholar] [CrossRef]
- Sun, K.; Huang, Q.; Li, S. Transformation and toxicity evaluation of tetracycline in humic acid solution by laccase coupled with 1-hydroxybenzotriazole. J. Hazard. Mater. 2017, 331, 182–188. [Google Scholar] [CrossRef]
- Zdarta, J.; Nguyen, L.N.; Jankowska, K.; Jesionowski, T.; Nghiem, L.D. A contemporary review of enzymatic applications in the remediation of emerging estrogenic compounds. Crit. Rev. Environ. Sci. Tec. 2022, 52, 2661–2690. [Google Scholar] [CrossRef]
- Ba, S.; Vinoth Kumar, V. Recent developments in the use of tyrosinase and laccase in environmental applications. Crit. Rev. Biotechnol. 2017, 37, 819–832. [Google Scholar] [CrossRef]
- Das, R.; Li, G.; Mai, B.; An, T. Spore cells from BPA degrading bacteria Bacillus sp. GZB displaying high laccase activity and stability for BPA degradation. Sci. Total Environ. 2018, 640–641, 798–806. [Google Scholar] [CrossRef]
- Welinder, K.G. Covalent structure of the glycoprotein horseradish peroxidase (EC 1.11.1.7). FEBS Lett. 1976, 72, 19–23. [Google Scholar] [CrossRef]
- Zhang, L.; Mi, J.; Hu, G.; Zhang, C.; Qi, H. Facile fabrication of a high-efficient and biocompatibility biocatalyst for bisphenol A removal. Int. J. Biol. Macromol. 2020, 150, 948–954. [Google Scholar] [CrossRef]
- Xie, P.; Zhang, W.; Wu, W.; Shen, Z.; Wang, M.; Lai, Y.; Chen, Y.; Jia, Z. Phenoxyl mediators improve enzymatic degradation of organic pollutants: Effect and mechanism. Int. J. Biol. Macromol. 2022, 215, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Tharmavaram, M.; Pandey, G.; Bhatt, P.; Prajapati, P.; Rawtani, D.; Sooraj, K.P.; Ranjan, M. Chitosan functionalized Halloysite Nanotubes as a receptive surface for laccase and copper to perform degradation of chlorpyrifos in aqueous environment. Int. J. Biol. Macromol. 2021, 191, 1046–1055. [Google Scholar] [CrossRef]
- Conceição, J.C.S.; Machado, R.G.; Alvarenga, A.D.; Maia, D.L.d.S.; Mesquita, P.R.R.; Mercante, L.A.; Correa, D.S.; Silva, E.O. Bio-nano hybrid material for mitigating recalcitrant phenolic compounds. Chem. Eng. Process 2025, 208, 110121. [Google Scholar] [CrossRef]
- Luo, J.; Song, S.; Zhang, H.; Zhang, H.; Zhang, J.; Wan, Y. Biocatalytic membrane: Go far beyond enzyme immobilization. Eng. Life Sci. 2020, 20, 441–450. [Google Scholar] [CrossRef]
- Luo, J.; Meyer, A.S.; Mateiu, R.V.; Kalyani, D.; Pinelo, M. Functionalization of a Membrane Sublayer Using Reverse Filtration of Enzymes and Dopamine Coating. ACS Appl. Mater. Interfaces 2014, 6, 22894–22904. [Google Scholar] [CrossRef]
- Luo, J.; Zeuner, B.; Morthensen, S.T.; Meyer, A.S.; Pinelo, M. Separation of phenolic acids from monosaccharides by low-pressure nanofiltration integrated with laccase pre-treatments. J. Membr. Sci. 2015, 482, 83–91. [Google Scholar] [CrossRef]
- Barrios-Estrada, C.; de Jesús Rostro-Alanis, M.; Muñoz-Gutiérrez, B.D.; Iqbal, H.M.N.; Kannan, S.; Parra-Saldívar, R. Emergent contaminants: Endocrine disruptors and their laccase-assisted degradation—A review. Sci. Total Environ. 2018, 612, 1516–1531. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Uliana, A.; Zhang, Y.; Tian, M.; Van der Bruggen, B. The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. J. Mater. Chem. A 2018, 6, 3773–3792. [Google Scholar] [CrossRef]
- Talekar, S.; Tak, Y.; Joshi, A.; Ahn, K.; Yeon, K.-M.; Kim, J. Magnetic hollow fibers of covalent organic frameworks (COF) for pollutant degradation and adsorptive removal. Environ. Res. 2024, 259, 119519. [Google Scholar] [CrossRef]
- Tang, Y.; Li, W.; Muhammad, Y.; Jiang, S.; Huang, M.; Zhang, H.; Zhao, Z.; Zhao, Z. Fabrication of hollow covalent-organic framework microspheres via emulsion-interfacial strategy to enhance laccase immobilization for tetracycline degradation. Chem. Eng. J. 2021, 421, 129743. [Google Scholar] [CrossRef]
- Xu, S.; Liang, J.; Mohammad, M.I.B.; Lv, D.; Cao, Y.; Qi, J.; Liang, K.; Ma, J. Biocatalytic metal–organic framework membrane towards efficient aquatic micropollutants removal. Chem. Eng. J. 2021, 426, 131861. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Luo, J.; Wan, Y. Enzymatic Cascade Catalysis in a Nanofiltration Membrane: Engineering the Microenvironment by Synergism of Separation and Reaction. ACS Appl. Mater. Interfaces 2019, 11, 22419–22428. [Google Scholar] [CrossRef]
- Haase, F.; Lotsch, B.V. Solving the COF trilemma: Towards crystalline, stable and functional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 8469–8500. [Google Scholar] [CrossRef]
- Xing, C.; Mei, P.; Mu, Z.; Li, B.; Feng, X.; Zhang, Y.; Wang, B. Enhancing Enzyme Activity by the Modulation of Covalent Interactions in the Confined Channels of Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2022, 61, e202201378. [Google Scholar] [CrossRef]
- Liang, J.; Ruan, J.; Njegic, B.; Rawal, A.; Scott, J.; Xu, J.; Boyer, C.; Liang, K. Insight into Bioactivity of In-situ Trapped Enzyme-Covalent-Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202303001. [Google Scholar] [CrossRef]
- Wang, Q.; Xing, C.; Feng, M.; Yang, Y.; Pang, D.; Feng, X.; Zhang, Y.; Wang, B. Enzyme-Assisted Confined Synthesis of Metal Nanoparticles in Covalent Organic Frameworks for Efficient Enzyme-Metal Cascade Catalysis. Angew. Chem. Int. Ed. 2025, 64, e202509105. [Google Scholar] [CrossRef]
- Feriante, C.; Evans, A.M.; Jhulki, S.; Castano, I.; Strauss, M.J.; Barlow, S.; Dichtel, W.R.; Marder, S.R. New Mechanistic Insights into the Formation of Imine-Linked Two-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 18637–18644. [Google Scholar] [CrossRef]
- Ma, W.; Zheng, Q.; He, Y.; Li, G.; Guo, W.; Lin, Z.; Zhang, L. Size-Controllable Synthesis of Uniform Spherical Covalent Organic Frameworks at Room Temperature for Highly Efficient and Selective Enrichment of Hydrophobic Peptides. J. Am. Chem. Soc. 2019, 141, 18271–18277. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Perman, J.; Earl, L.D.; Abney, C.W.; Cheng, Y.; Wei, H.; Nguyen, N.; Wojtas, L.; Ma, S. Postsynthetically Modified Covalent Organic Frameworks for Efficient and Effective Mercury Removal. J. Am. Chem. Soc. 2017, 139, 2786–2793. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, C.; Mu, Z.; Niu, Z.; Feng, X.; Zhang, Y.; Wang, B. Harnessing Self-Repairing and Crystallization Processes for Effective Enzyme Encapsulation in Covalent Organic Frameworks. J. Am. Chem. Soc. 2023, 145, 13469–13475. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, X.; Hua, R.; Zhang, R.; Yao, Y.; Zhao, B.; Liu, T.; Zheng, J.; Lu, G. Remarkably catalytic activity in reduction of 4-nitrophenol and methylene blue by Fe3O4@COF supported noble metal nanoparticles. Appl. Catal. B Environ. 2020, 260, 118142. [Google Scholar] [CrossRef]
- Fan, M.; Wang, W.D.; Zhu, Y.; Sun, X.; Zhang, F.; Dong, Z. Palladium clusters confined in triazinyl-functionalized COFs with enhanced catalytic activity. Appl. Catal. B Environ. 2019, 257, 117942. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, Q.; Dong, S.; Wang, E. In situ electrochemical scanning tunnelling microscopy investigation of structure for horseradish peroxidase and its electricatalytic property. Bioelectrochem. Bioenerg. 1996, 39, 267–274. [Google Scholar] [CrossRef]
- Zhuang, W.; Huang, J.; Liu, X.; Ge, L.; Niu, H.; Wang, Z.; Wu, J.; Yang, P.; Chen, Y.; Ying, H. Co-localization of glucose oxidase and catalase enabled by a self-assembly approach: Matching between molecular dimensions and hierarchical pore sizes. Food Chem. 2019, 275, 197–205. [Google Scholar] [CrossRef]
- Lyu, Q.; Hsueh, N.; Chai, C.L.L. The Chemistry of Bioinspired Catechol(amine)-Based Coatings. ACS Biomater. Sci. Eng. 2019, 5, 2708–2724. [Google Scholar] [CrossRef]
- Gajhede, M.; Schuller, D.J.; Henriksen, A.; Smith, A.T.; Poulos, T.L. Crystal structure of horseradish peroxidase C at 2.15 Å resolution. Nat. Struct. Biol. 1997, 4, 1032–1038. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Mazumdar, S. Structural and Conformational Stability of Horseradish Peroxidase: Effect of Temperature and pH. Biochemistry 2000, 39, 263–270. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Li, M.; Qin, S.; Zhao, Z.; Lin, B.; Ding, Y.; Xiang, Y.; Li, C. Horseradish peroxidase (HRP) and glucose oxidase (GOX) based dual-enzyme system: Sustainable release of H2O2 and its effect on the desirable ping pong bibi degradation mechanism. Environ. Res. 2023, 229, 115979. [Google Scholar] [CrossRef]
- Reihmann, M.; Ritter, H. Synthesis of Phenol Polymers Using Peroxidases. In Enzyme-Catalyzed Synthesis of Polymers; Kobayashi, S., Ritter, H., Kaplan, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–49. [Google Scholar]
- Razzaghi, M.; Karimi, A.; Aghdasinia, H.; Joghataei, M.-T. Oxidase-Peroxidase sequential polymerization for removal of a dye from contaminated water by horseradish peroxidase (HRP)/glucose oxidase (GOx)/polyurethane hybrid catalyst. Korean J. Chem. Eng. 2017, 34, 2870–2878. [Google Scholar] [CrossRef]
- Zhao, H.; Joseph, J.; Zhang, H.; Karoui, H.; Kalyanaraman, B. Synthesis and biochemical applications of a solid cyclic nitrone spin trap: A relatively superior trap for detecting superoxide anions and glutathiyl radicals. Free Radic. Biol. Med. 2001, 31, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Yamazaki, I. The mechanism of oxyperoxidase formation from ferryl peroxidase and hydrogen peroxide. J. Biol. Chem. 1987, 262, 2576–2581. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-X.; Schopfer, P. Hydroxyl-radical production in physiological reactions. Eur. J. Biochem. 1999, 260, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Acosta, M.; del Rio, J.A.; García-Cánovas, F. Inactivation of peroxidase by hydrogen peroxide and its protection by a reductant agent. Biochim. Biophys. Acta BBA-Protein Struct. Mol. Enzymol. 1990, 1038, 85–89. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Liu, G.; Zheng, X.; Zhou, L.; Ma, L.; He, Y.; Yue, X.; Jiang, Y. Covalent Organic Framework-Based Nanomembrane with Co-Immobilized Dual Enzymes for Micropollutant Removal. Nanomaterials 2025, 15, 1431. https://doi.org/10.3390/nano15181431
Zhao J, Liu G, Zheng X, Zhou L, Ma L, He Y, Yue X, Jiang Y. Covalent Organic Framework-Based Nanomembrane with Co-Immobilized Dual Enzymes for Micropollutant Removal. Nanomaterials. 2025; 15(18):1431. https://doi.org/10.3390/nano15181431
Chicago/Turabian StyleZhao, Junda, Guanhua Liu, Xiaobing Zheng, Liya Zhou, Li Ma, Ying He, Xiaoyang Yue, and Yanjun Jiang. 2025. "Covalent Organic Framework-Based Nanomembrane with Co-Immobilized Dual Enzymes for Micropollutant Removal" Nanomaterials 15, no. 18: 1431. https://doi.org/10.3390/nano15181431
APA StyleZhao, J., Liu, G., Zheng, X., Zhou, L., Ma, L., He, Y., Yue, X., & Jiang, Y. (2025). Covalent Organic Framework-Based Nanomembrane with Co-Immobilized Dual Enzymes for Micropollutant Removal. Nanomaterials, 15(18), 1431. https://doi.org/10.3390/nano15181431





