Interstitial Ag+ Engineering Enables Superior Resistive Switching in Quasi-2D Halide Perovskites
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural and Morphological Characterization
2.2. Charge Carrier Dynamics
2.3. Density Functional Theory Analysis
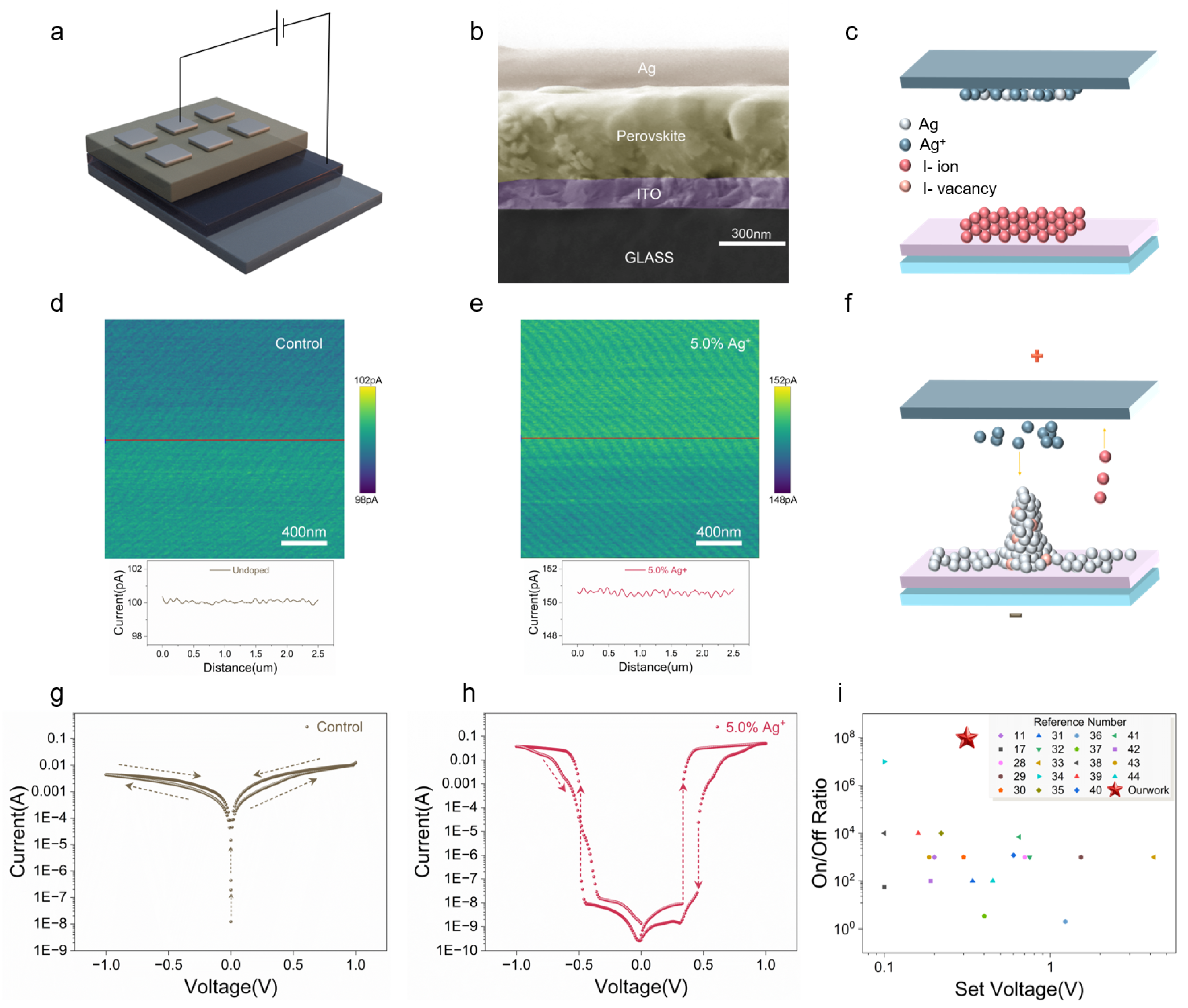
2.4. Memristor Device Performance Optimization
3. Conclusions
4. Materials and Methods
4.1. Materials Preparation
4.2. Device Fabrication
4.3. Materials Characterization
4.4. DFT and Quantum Transport Calculations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hinton, G.E. Learning multiple layers of representation. Trends Cogn. Sci. 2007, 11, 428–434. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, X.; Strachan, J.P.; Yang, Y.; Lu, W.D. Dynamical memristors for higher-complexity neuromorphic computing. Nat. Rev. Mater. 2022, 7, 575–591. [Google Scholar] [CrossRef]
- Aguirre, F.; Sebastian, A.; Le Gallo, M.; Song, W.; Wang, T.; Yang, J.J.; Lu, W.; Chang, M.F.; Ielmini, D.; Yang, Y.; et al. Hardware implementation of memristor-based artificial neural networks. Nat. Commun. 2024, 15, 1974. [Google Scholar] [CrossRef]
- Wang, K.L.; Alzate, J.G.; Khalili Amiri, P. Low-power non-volatile spintronic memory: STT-RAM and beyond. J. Phys. D Appl. Phys. 2013, 46, 074003. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, S.; Savel’ev, S.E.; Jiang, H.; Midya, R.; Lin, P.; Hu, M.; Ge, N.; Strachan, J.P.; Li, Z.; et al. Memristors with diffusive dynamics as synaptic emulators for neuromorphic computing. Nat. Mater. 2017, 16, 101–108. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Frost, J.M.; McMahon, A.P.; Sakai, V.G.; Kockelmann, W.; Law, C.; Li, X.; Foglia, F.; Walsh, A.; O’Regan, B.C.; et al. The dynamics of methylammonium ions in hybrid organic–inorganic perovskite solar cells. Nat. Commun. 2015, 6, 7124. [Google Scholar] [CrossRef]
- Amat, A.; Mosconi, E.; Ronca, E.; Quarti, C.; Umari, P.; Nazeeruddin, M.K.; Grätzel, M.; De Angelis, F. Cation-Induced Band-Gap Tuning in Organohalide Perovskites: Interplay of Spin–Orbit Coupling and Octahedra Tilting. Nano Lett. 2014, 14, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Dunlap-Shohl, W.A.; Zhou, Y.; Padture, N.P.; Mitzi, D.B. Synthetic Approaches for Halide Perovskite Thin Films. Chem. Rev. 2019, 119, 3193–3295. [Google Scholar] [CrossRef] [PubMed]
- Knapic, D.; Minenkov, A.; Atanasova, E.; Zrinski, I.; Hassel, A.W.; Mardare, A.I. Interfacial Resistive Switching of Niobium–Titanium Anodic Memristors with Self-Rectifying Capabilities. Nanomaterials 2024, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lu, L.; Zhang, J.; Yun, Y.; Jiang, S.; Tian, Y.; Guo, Z.; Zhao, S.; Wei, W.; Li, W.; et al. In Situ Unveiling of the Resistive Switching Mechanism of Halide Perovskite-Based Memristors. J. Phys. Chem. Lett. 2024, 15, 2453–2461. [Google Scholar] [CrossRef]
- Wu, F.C.; Su, Z.M.; Hsu, Y.C.; Chou, W.Y.; Lai, W.C.; Tsai, C.C.; Cheng, H.L. Microstructure-modulated conductive filaments in Ruddlesden-Popper perovskite-based memristors and their application in artificial synapses. Mater. Today Phys. 2025, 53, 101708. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Y.; Guo, J.; Qin, X.; Zhang, Q.; Tian, C.; Xu, P.; Li, Y.; Tian, W.; Zheng, X.; et al. Phase Regulation and Defect Passivation Enabled by Phosphoryl Chloride Molecules for Efficient Quasi-2D Perovskite Light-Emitting Diodes. Nano-Micro Lett. 2023, 15, 119. [Google Scholar] [CrossRef]
- Caiazzo, A.; Janssen, R.A.J. High Efficiency Quasi-2D Ruddlesden–Popper Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2202830. [Google Scholar] [CrossRef]
- Kim, S.J.; Im, I.H.; Baek, J.H.; Choi, S.; Park, S.H.; Lee, D.E.; Kim, J.Y.; Kim, S.Y.; Park, N.G.; Lee, D.; et al. Linearly programmable two-dimensional halide perovskite memristor arrays for neuromorphic computing. Nat. Nanotechnol. 2025, 20, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Mathew, P.; Cho, J.; Kamat, P.V. Ramifications of Ion Migration in 2D Lead Halide Perovskites. ACS Energy Lett. 2024, 9, 1103–1114. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, L.; Xue, C.; Liu, P.; Du, X.; Wen, K.; Zhang, H.; Xu, L.; Xiang, C.; Lin, C.; et al. Efficient and bright warm-white electroluminescence from lead-free metal halides. Nat. Commun. 2021, 12, 1421. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gao, Q.; Huang, J.; Feng, X.; Geng, X.; Li, H.; Wang, G.; Liang, B.; Chen, X.; Su, Y.; et al. Influence of Lithium Doping on Volcanic-like Perovskite Memristors and Artificial Synaptic Simulation for Neurocomputing. ACS Appl. Nano Mater. 2023, 6, 7975–7983. [Google Scholar] [CrossRef]
- Pérez-Martínez, J.C.; Berruet, M.; Gonzales, C.; Salehpour, S.; Bahari, A.; Arredondo, B.; Guerrero, A. Role of Metal Contacts on Halide Perovskite Memristors. Adv. Funct. Mater. 2023, 33, 2305211. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Kan, M.; Li, Y.; Wang, T.; Zhao, Y. Efficient α-CsPbI3 Photovoltaics with Surface Terminated Organic Cations. Joule 2018, 2, 2065–2075. [Google Scholar] [CrossRef]
- Rahil, M.; Ansari, R.M.; Prakash, C.; Islam, S.S.; Dixit, A.; Ahmad, S. Ruddlesden–Popper 2D perovskites of type (C6H9C2H4NH3)2(CH3NH3)n−1PbnI3n+1 (n = 1–4) for optoelectronic applications. Sci. Rep. 2022, 12, 2176. [Google Scholar] [CrossRef]
- Shpatz Dayan, A.; Cohen, B.E.; Aharon, S.; Tenailleau, C.; Wierzbowska, M.; Etgar, L. Enhancing Stability and Photostability of CsPbI3 by Reducing Its Dimensionality. Chem. Mater. 2018, 30, 8017–8024. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, J.; Ni, Y.; Yang, J.; Wang, Y.; Jiu, T.; Yuan, M.; Chen, J. Reduced-Dimensional α-CsPbX3 Perovskites for Efficient and Stable Photovoltaics. Joule 2018, 2, 1356–1368. [Google Scholar] [CrossRef]
- Cai, W.; Ali, M.U.; Liu, P.; He, M.; Zhao, C.; Chen, Z.; Zang, Y.; Tang, M.C.; Meng, H.; Fu, H.; et al. Unravelling Alkali-Metal-Assisted Domain Distribution of Quasi-2D Perovskites for Cascade Energy Transfer toward Efficient Blue Light-Emitting Diodes. Adv. Sci. 2022, 9, 2200393. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Yu, J.; Qin, Z.; Wang, J.; Sun, J.; Chan, C.C.S.; Ding, S.; Wang, K.; Chen, R.; Wong, K.S.; et al. High-Performance Blue Perovskite Light-Emitting Diodes Enabled by Efficient Energy Transfer between Coupled Quasi-2D Perovskite Layers. Adv. Mater. 2021, 33, 2005570. [Google Scholar] [CrossRef]
- Li, B.; Huang, X.; Wu, X.; Zuo, Q.; Cao, Y.; Zhu, Q.; Li, Y.; Xu, Y.; Zheng, G.; Chen, D.; et al. High-Performing Quasi-2D Perovskite Photodetectors with Efficient Charge Transport Network Built from Vertically Orientated and Evenly Distributed 3D-Like Phases. Adv. Funct. Mater. 2023, 33, 2300216. [Google Scholar] [CrossRef]
- Im, I.H.; Baek, J.H.; Kim, S.J.; Kim, J.; Park, S.H.; Kim, J.Y.; Yang, J.J.; Jang, H.W. Halide Perovskites-Based Diffusive Memristors for Artificial Mechano-Nociceptive System. Adv. Mater. 2024, 36, 2307334. [Google Scholar] [CrossRef]
- Targhi, F.F.; Jalili, Y.S.; Kanjouri, F. MAPbI3 and FAPbI3 perovskites as solar cells: Case study on structural, electrical and optical properties. Results Phys. 2018, 10, 616–627. [Google Scholar] [CrossRef]
- Dutta, S.; Panchanan, S.; Yoo, J.H.; Kumar, S.; Yoo, H.C.; Seok, S.I.; Dastgeer, G.; Yoon, D.H. Synaptic Behavior of Iodine-Enriched Copper-Based Perovskite Memristors Developed Through a Sustainable Solution Approach. Adv. Funct. Mater. 2024, 34, 2410810. [Google Scholar] [CrossRef]
- Cheng, X.F.; Qian, W.H.; Wang, J.; Yu, C.; He, J.H.; Li, H.; Xu, Q.F.; Chen, D.Y.; Li, N.J.; Lu, J.M. Environmentally Robust Memristor Enabled by Lead-Free Double Perovskite for High-Performance Information Storage. Small 2019, 15, 1905731. [Google Scholar] [CrossRef]
- Zhai, S.; Gong, J.; Feng, Y.; Que, Z.; Mao, W.; He, X.; Xie, Y.; Li, X.; Chu, L. Multilevel resistive switching in stable all-inorganic n-i-p double perovskite memristor. iScience 2023, 26, 106461. [Google Scholar] [CrossRef]
- Zeng, F.; Guo, Y.; Hu, W.; Tan, Y.; Zhang, X.; Feng, J.; Tang, X. Opportunity of the Lead-Free All-Inorganic Cs3Cu2I5 Perovskite Film for Memristor and Neuromorphic Computing Applications. ACS Appl. Mater. Interfaces 2020, 12, 23094–23101. [Google Scholar] [CrossRef]
- Patil, H.; Kim, H.; Kadam, K.D.; Rehman, S.; Patil, S.A.; Aziz, J.; Dongale, T.D.; Ali Sheikh, Z.; Khalid Rahmani, M.; Khan, M.F.; et al. Flexible Organic–Inorganic Halide Perovskite-Based Diffusive Memristor for Artificial Nociceptors. ACS Appl. Mater. Interfaces 2023, 15, 13238–13248. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Lin, Z.; Su, J.; Wang, J.; Zhang, J.; Liu, S.; Chang, J.; Hao, Y. Two-Dimensional (C6H5C2H4NH3)2PbI4 Perovskite Single Crystal Resistive Switching Memory Devices. IEEE Electron Device Lett. 2021, 42, 327–330. [Google Scholar] [CrossRef]
- Pérez-Martínez, J.C.; Martín-Martín, D.; Arredondo, B.; Romero, B. Unraveling Conductive Filament Formation in High Performance Halide Perovskite Memristor. Adv. Electron. Mater. 2024, 10, 2400067. [Google Scholar] [CrossRef]
- Tang, L.; Wang, J.; Huang, Y.; Wang, H.; Wang, C.; Yang, Y. A low power flexible halide perovskite-based threshold switching memristor as an artificial nociceptor. J. Mater. Chem. C 2024, 12, 3622–3631. [Google Scholar] [CrossRef]
- Gao, J.; Meng, L.; Li, H.; Luo, F.; Wang, F.; Zhang, X. Simulations of biological associative learning behaviors using Cs2SnI6 perovskite memristors. Chem. Eng. J. 2025, 514, 163362. [Google Scholar] [CrossRef]
- Lassoued, M.S.; Wang, K.; Ahmad, F.; Sun, B.; Zheng, Y.Z. Exploring the potential of two-dimensional bismuth–copper hybrid double perovskites for memristor applications. Inorg. Chem. Front. 2025. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Y.; Li, Z.; Liu, X.; Cao, G.; Yao, J. Resistive Switching in Nonperovskite-Phase CsPbI3 Film-Based Memory Devices. ACS Appl. Mater. Interfaces 2020, 12, 9409–9420. [Google Scholar] [CrossRef]
- Ye, H.; Sun, B.; Wang, Z.; Liu, Z.; Zhang, X.; Tan, X.; Shi, T.; Tang, Z.; Liao, G. High performance flexible memristors based on a lead free AgBiI4 perovskite with an ultralow operating voltage. J. Mater. Chem. C 2020, 8, 14155–14163. [Google Scholar] [CrossRef]
- Wang, Y.; Ran, Q.; Chen, T.; Zhang, W.; Zhang, K. Lead-Free Halide Perovskite-Based Flexible Memristor for an Artificial Mechano-nociceptive System. J. Phys. Chem. Lett. 2025, 16, 3177–3184. [Google Scholar] [CrossRef]
- Patel, M.; Kumbhar, D.D.; Gosai, J.; Sekhar, M.R.; Mallajosyula, A.T.; Solanki, A. Hybrid Perovskite-Based Flexible and Stable Memristor by Complete Solution Process for Neuromorphic Computing. Adv. Electron. Mater. 2023, 9, 2200908. [Google Scholar] [CrossRef]
- Feng, J.; Fan, Y.; Wang, Y.; Song, Q.; Liu, Y.; Chen, Y.; Li, D.; Huang, W. Stable Halide Perovskite Memristor Utilizing Innovative Silver/Bismuth Electrode as an Alternative to Gold. Adv. Funct. Mater. 2025, 35, 2420547. [Google Scholar] [CrossRef]
- Pendyala, N.K.; Gonzales, C.; Guerrero, A. Decoupling Volatile and Nonvolatile Response in Reliable Halide Perovskite Memristors. Small Struct. 2025, 6, 2400380. [Google Scholar] [CrossRef]
- Guo, Z.; Xiong, R.; Zhu, Y.; Wang, Z.; Zhou, J.; Liu, Y.; Luo, D.; Wang, Y.; Wang, H. High-performance and humidity robust multilevel lead-free all-inorganic Cs3Cu2Br5 perovskite-based memristors. Appl. Phys. Lett. 2023, 122, 053502. [Google Scholar] [CrossRef]
- Soler, J.M.; Artacho, E.; Gale, J.D.; García, A.; Junquera, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA method for ab initio order-N materials simulation. J. Phys. Condens. Matter 2002, 14, 2745. [Google Scholar] [CrossRef]
- García, A.; Papior, N.; Akhtar, A.; Artacho, E.; Blum, V.; Bosoni, E.; Brandimarte, P.; Brandbyge, M.; Cerdá, J.I.; Corsetti, F.; et al. Siesta: Recent developments and applications. J. Chem. Phys. 2020, 152, 204108. [Google Scholar] [CrossRef]
- Brandbyge, M.; Mozos, J.L.; Ordejón, P.; Taylor, J.; Stokbro, K. Density-functional method for nonequilibrium electron transport. Phys. Rev. B 2002, 65, 165401. [Google Scholar] [CrossRef]
- Papior, N.; Lorente, N.; Frederiksen, T.; García, A.; Brandbyge, M. Improvements on non-equilibrium and transport Green function techniques: The next-generation transiesta. Comput. Phys. Commun. 2017, 212, 8–24. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, H.; Wang, Z.; Li, Q.; Lin, J.; Lu, D.; Huang, Y.; Gao, W.; Wang, H.; Bi, C. Interstitial Ag+ Engineering Enables Superior Resistive Switching in Quasi-2D Halide Perovskites. Nanomaterials 2025, 15, 1267. https://doi.org/10.3390/nano15161267
Qin H, Wang Z, Li Q, Lin J, Lu D, Huang Y, Gao W, Wang H, Bi C. Interstitial Ag+ Engineering Enables Superior Resistive Switching in Quasi-2D Halide Perovskites. Nanomaterials. 2025; 15(16):1267. https://doi.org/10.3390/nano15161267
Chicago/Turabian StyleQin, Haiyang, Zijia Wang, Qinrao Li, Jianxin Lin, Dongzhu Lu, Yicong Huang, Wenke Gao, Huachuan Wang, and Chenghao Bi. 2025. "Interstitial Ag+ Engineering Enables Superior Resistive Switching in Quasi-2D Halide Perovskites" Nanomaterials 15, no. 16: 1267. https://doi.org/10.3390/nano15161267
APA StyleQin, H., Wang, Z., Li, Q., Lin, J., Lu, D., Huang, Y., Gao, W., Wang, H., & Bi, C. (2025). Interstitial Ag+ Engineering Enables Superior Resistive Switching in Quasi-2D Halide Perovskites. Nanomaterials, 15(16), 1267. https://doi.org/10.3390/nano15161267









