Preparation and Properties of Magnetically Responsive Graphene/Boron Nitride/Iron Oxide Filler Composite Epoxy Resin Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation and Characterisation of Magnetically Responsive Fillers
2.2.1. Preparation of Magnetically Responsive Fillers
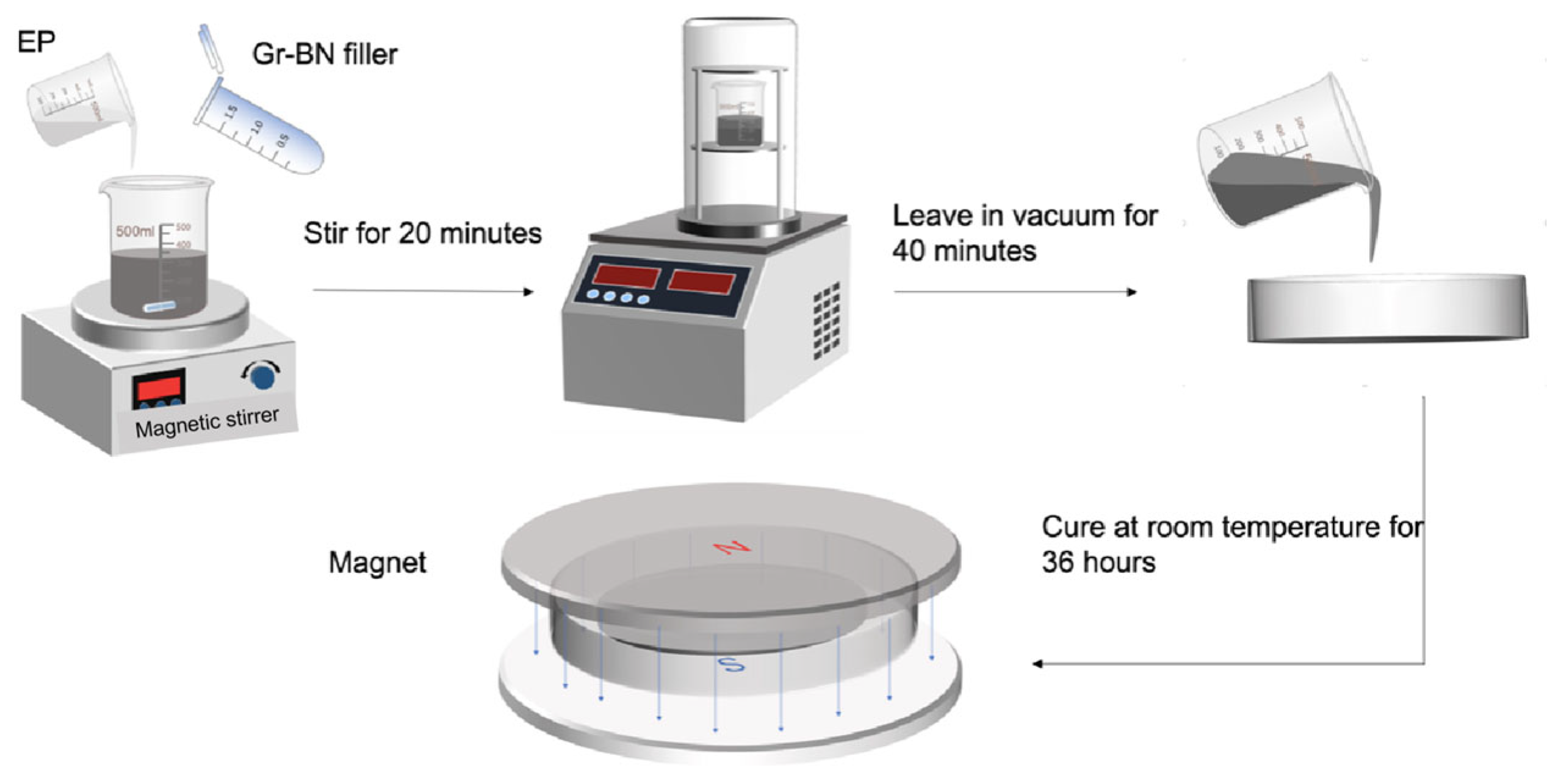
2.2.2. Preparation of Magnetically Responsive Filler Composite Epoxy Materials
2.3. Characterisation and Testing of Materials
2.3.1. Characterisation of Fillers
2.3.2. Performance Testing of Epoxy Composites
3. Results and Discussion
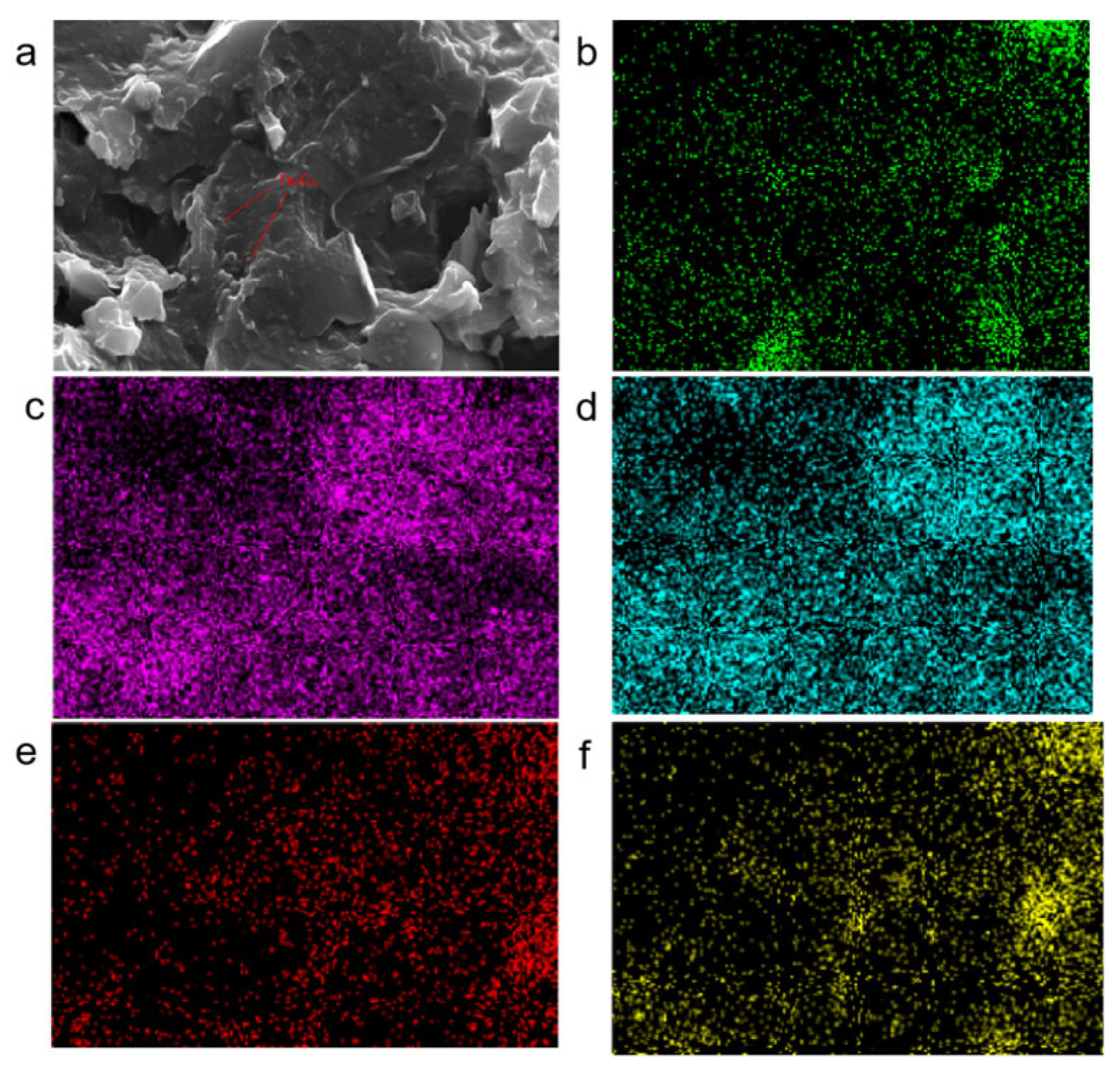
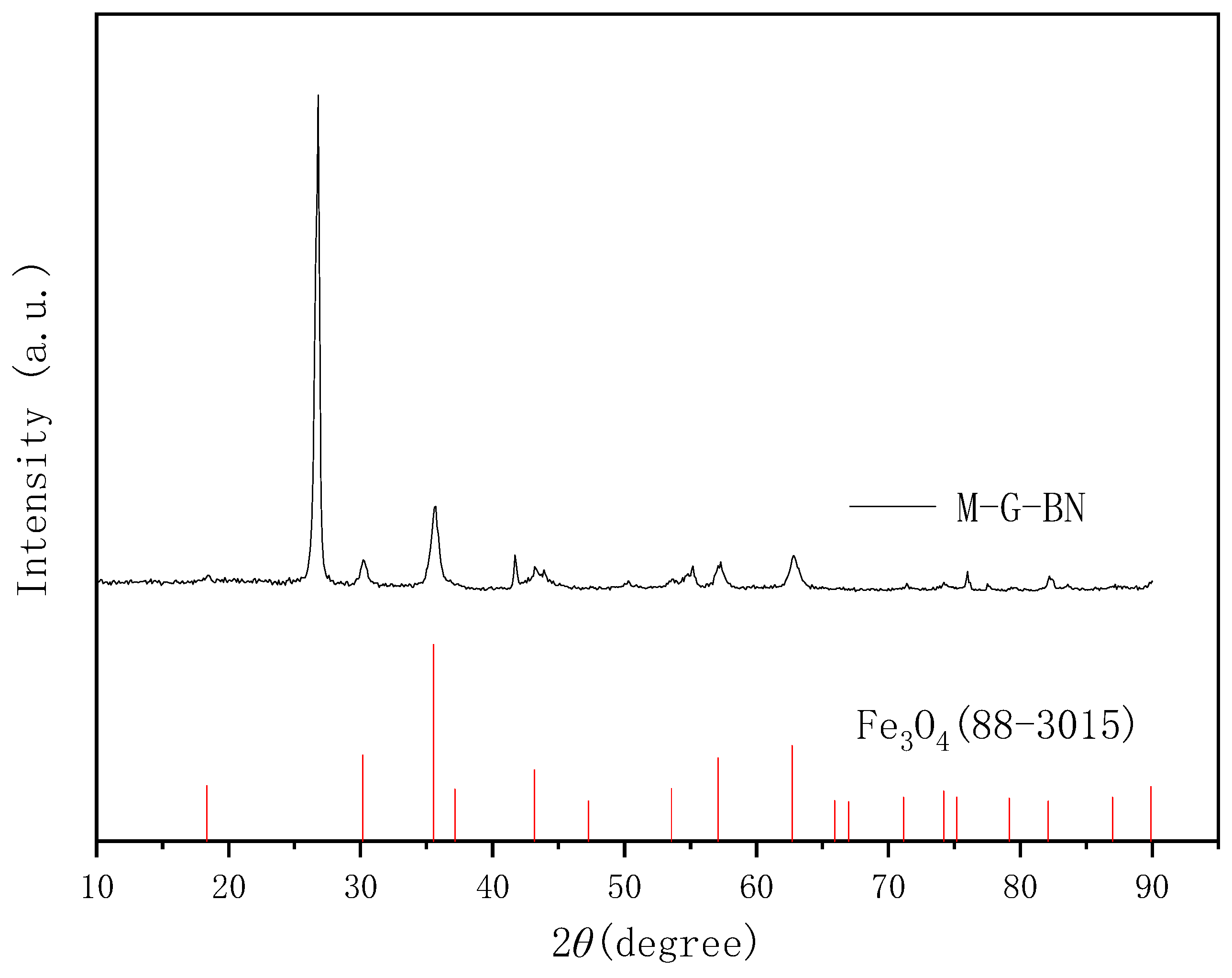
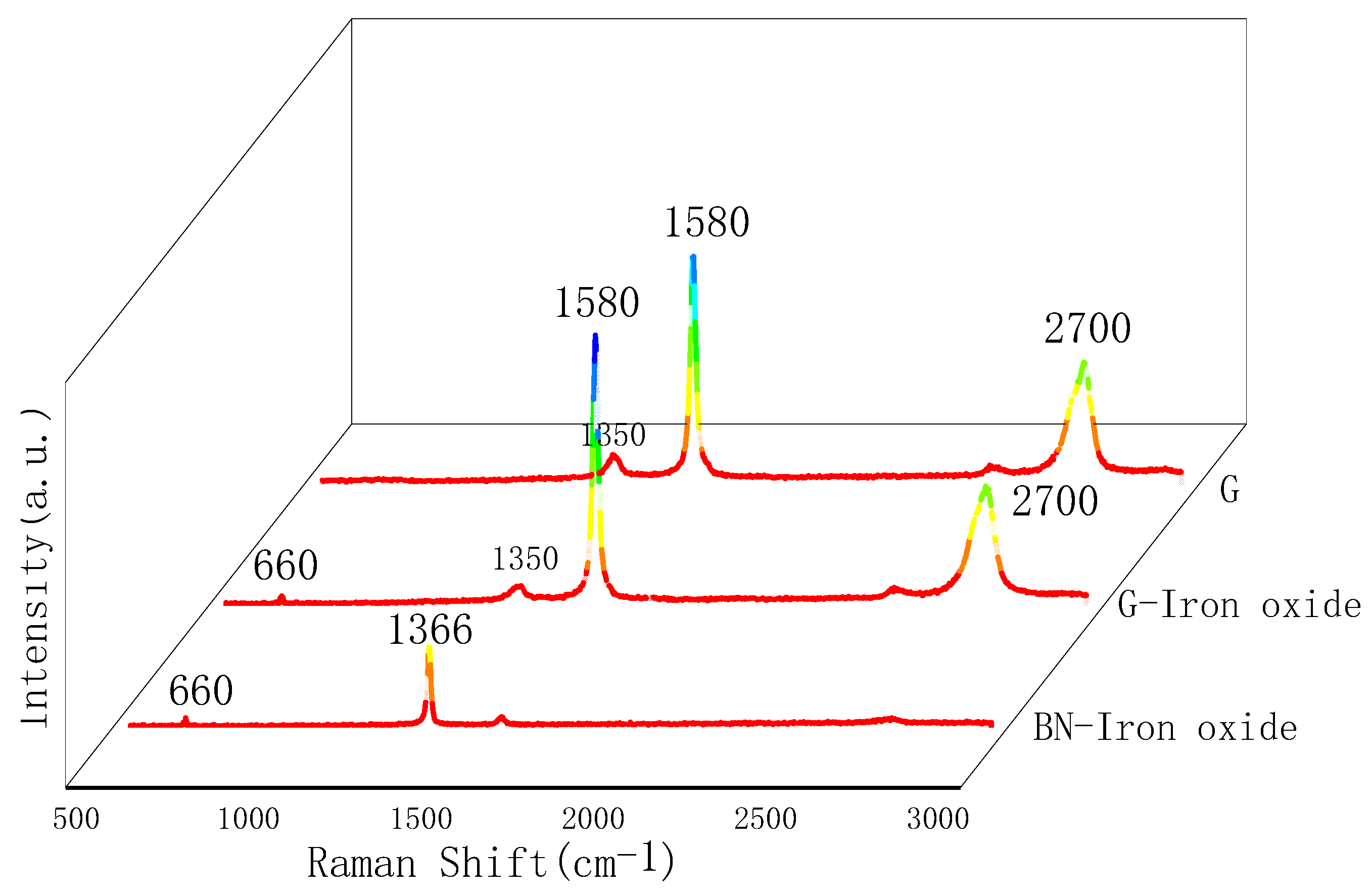
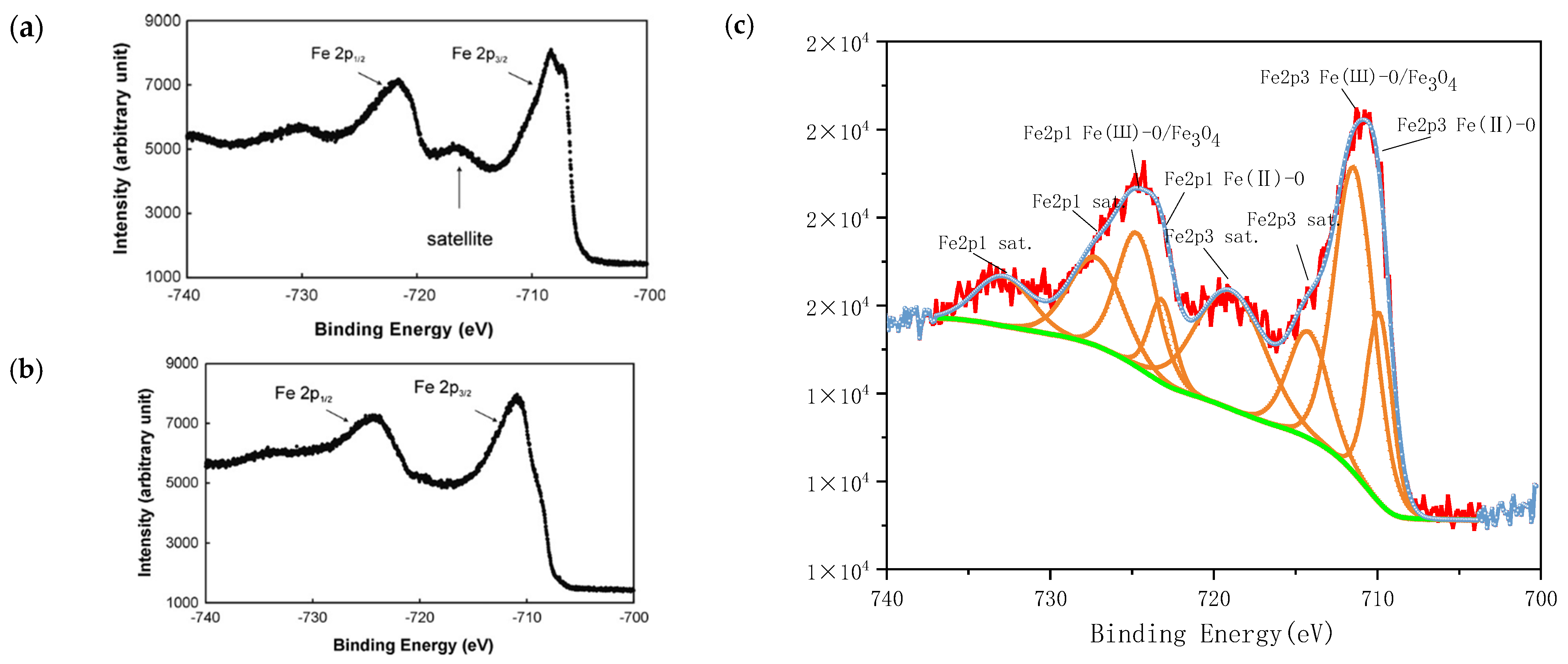
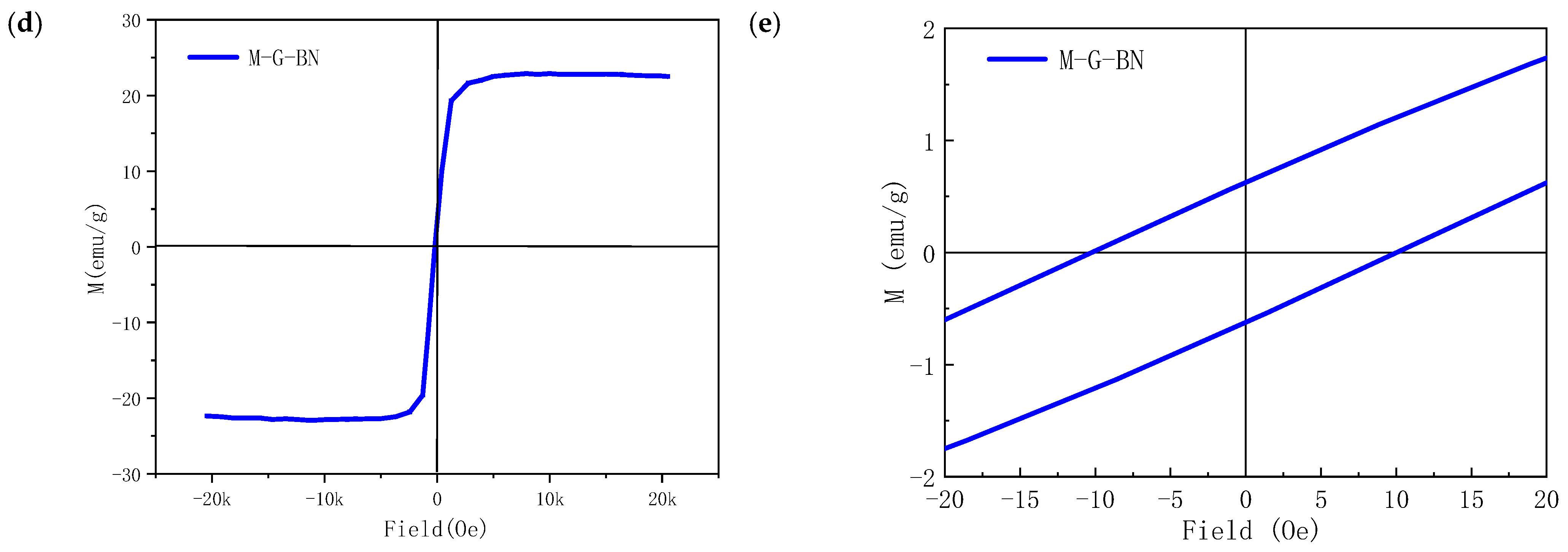
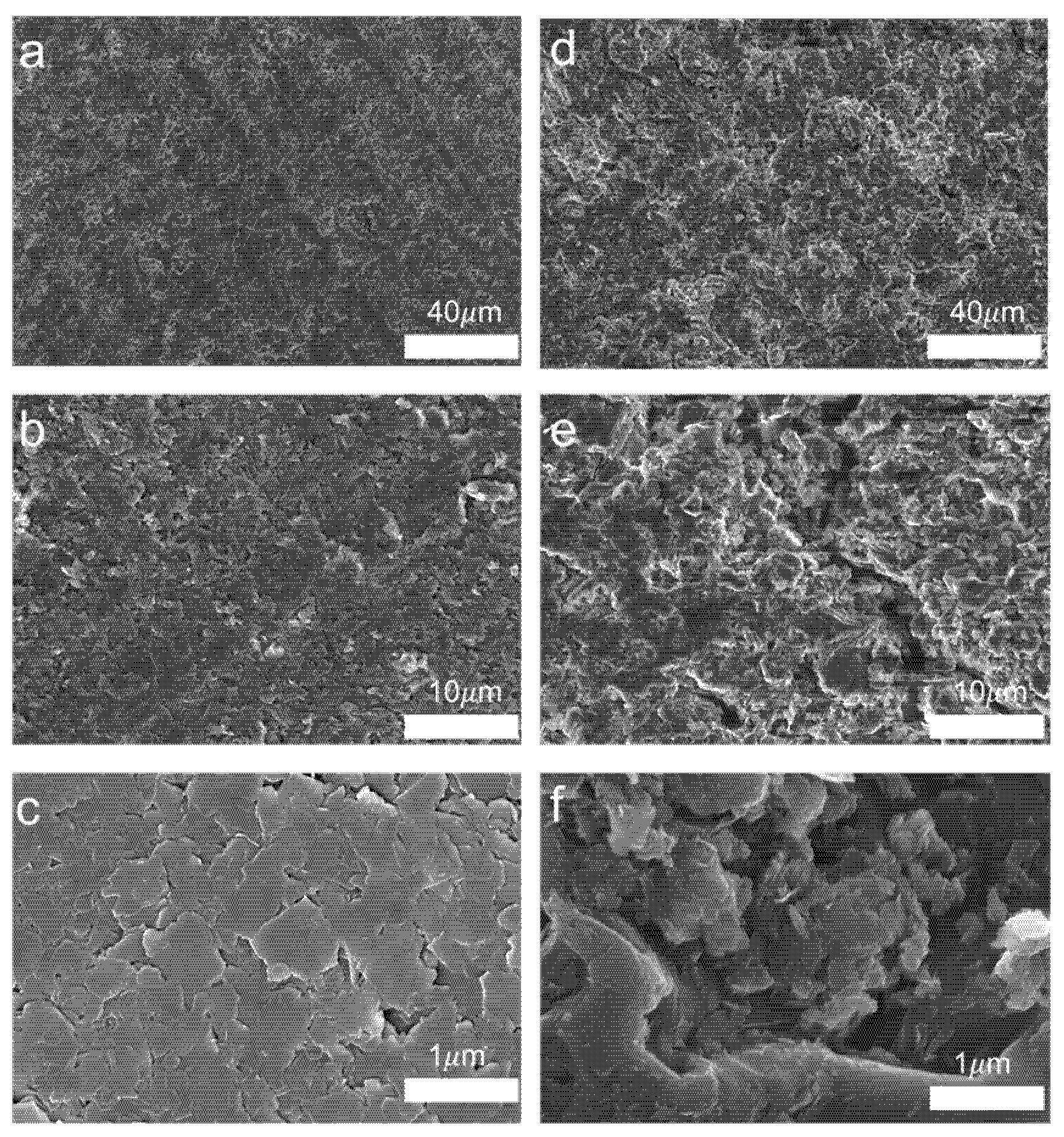
3.1. Characterisation of Magnetically Responsive Packings
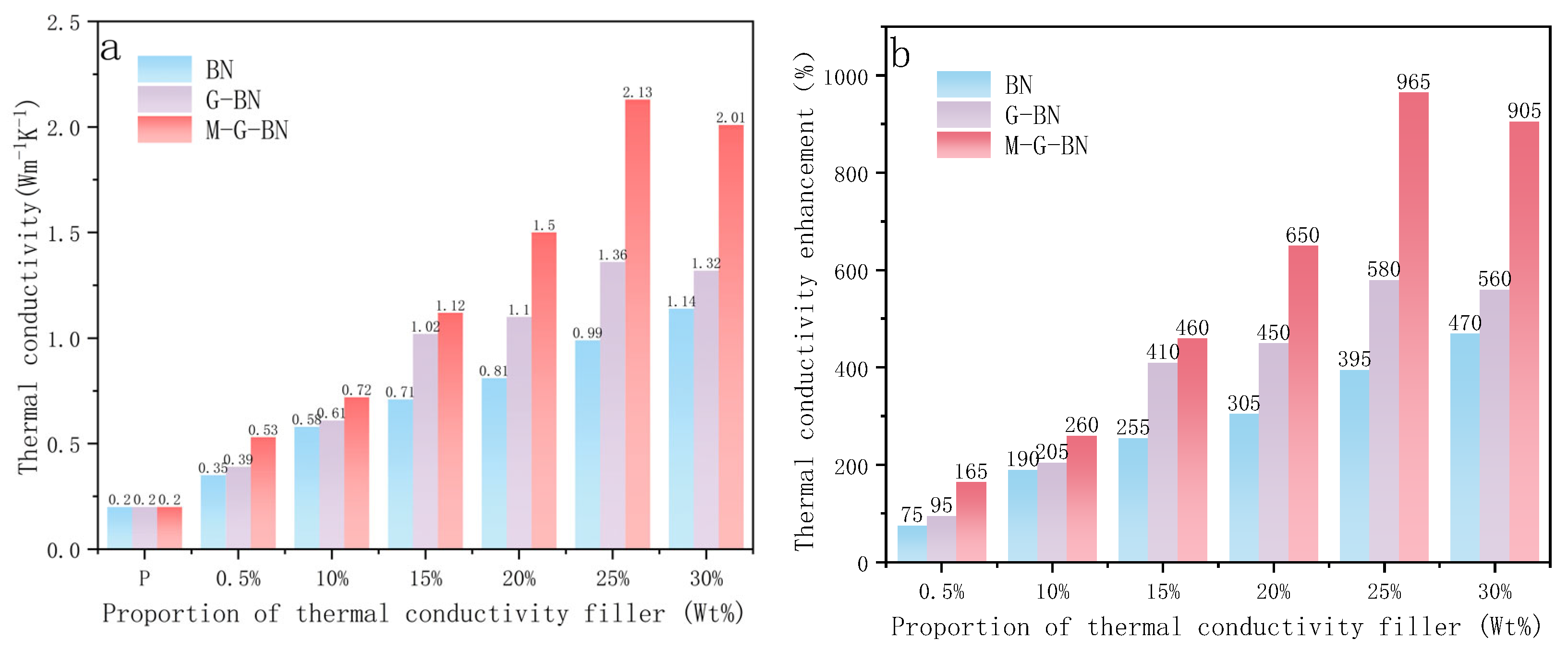
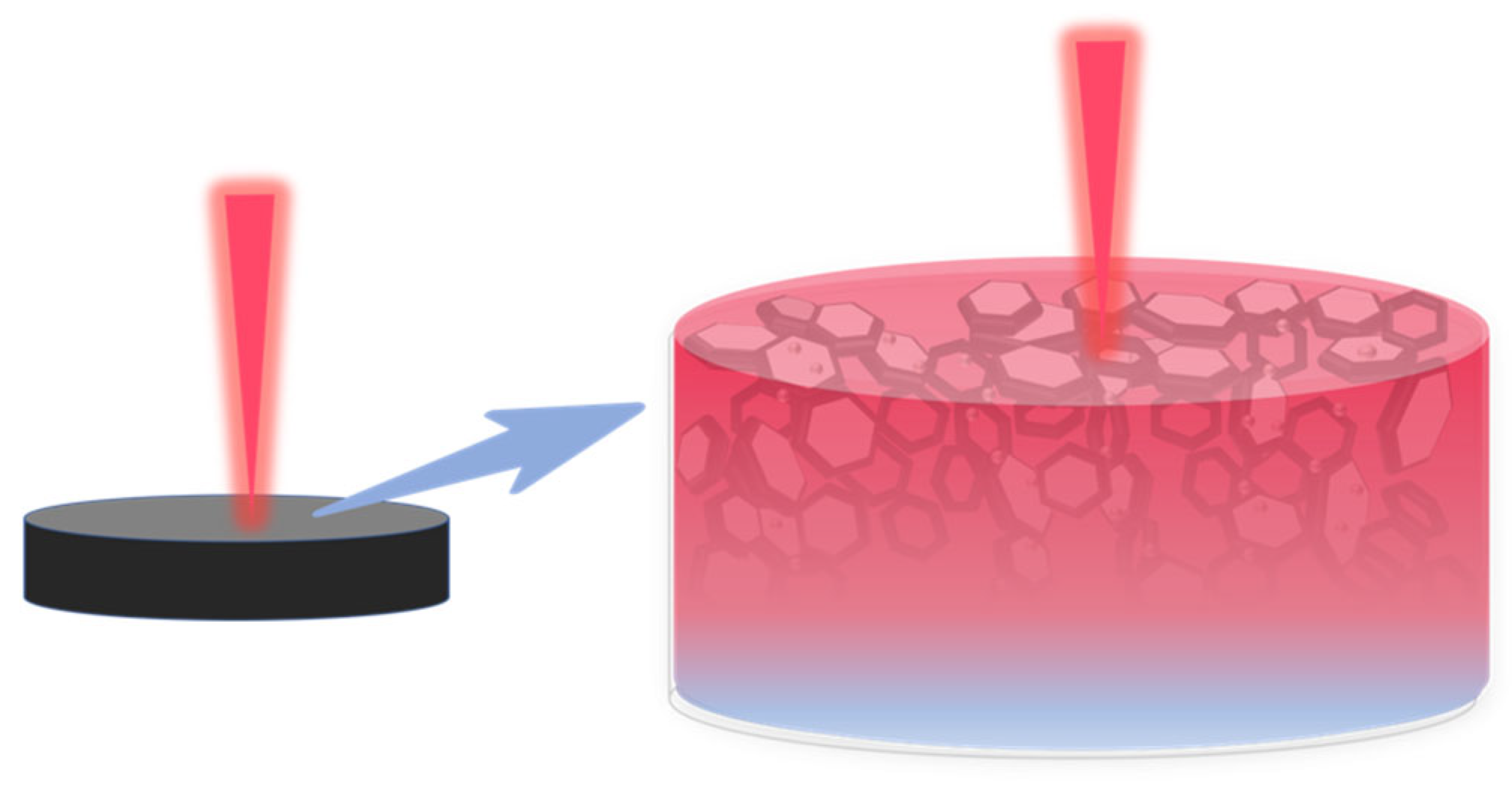
3.2. Thermal Conductivity Test
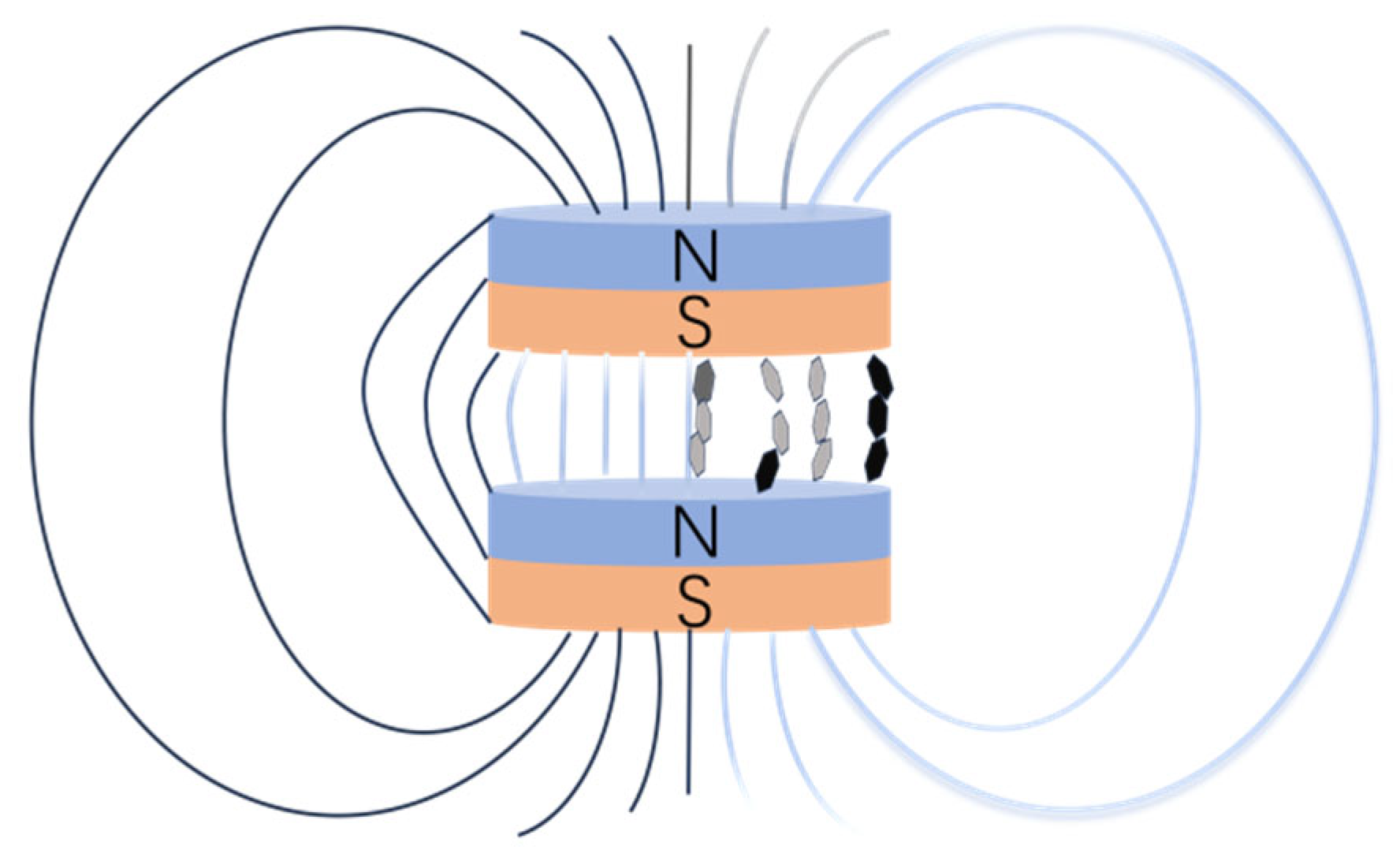
3.3. Mechanism of Magnetic Response Fillers to Enhance Thermal Conductivity of Epoxy Resin
3.4. Thermal Stability of Magnetic Responsive Filler Composite Epoxy Resin Materials
3.5. Ablation Resistance of Magnetic Responsive Filler Composite Epoxy Resin Materials Performance
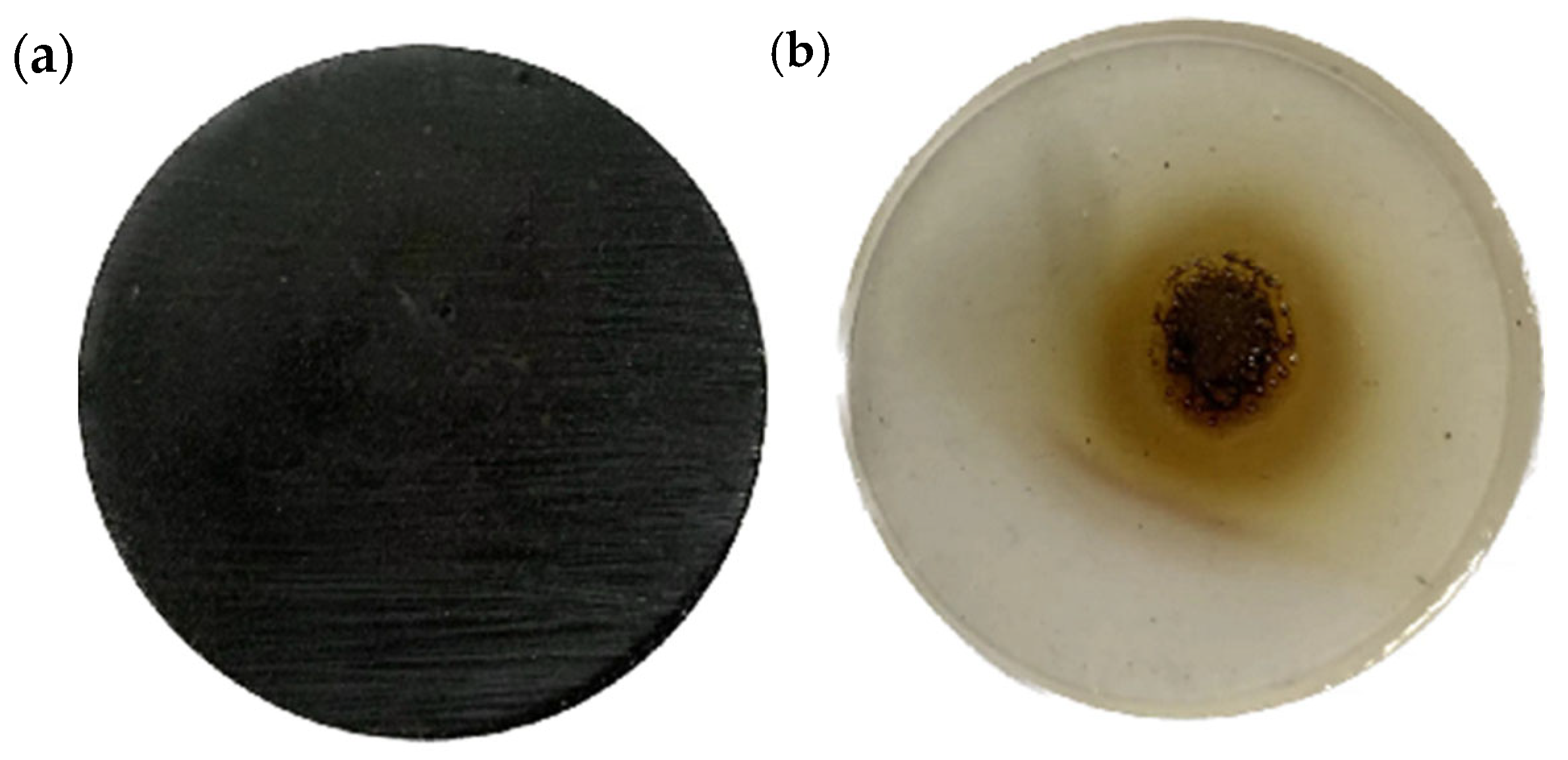
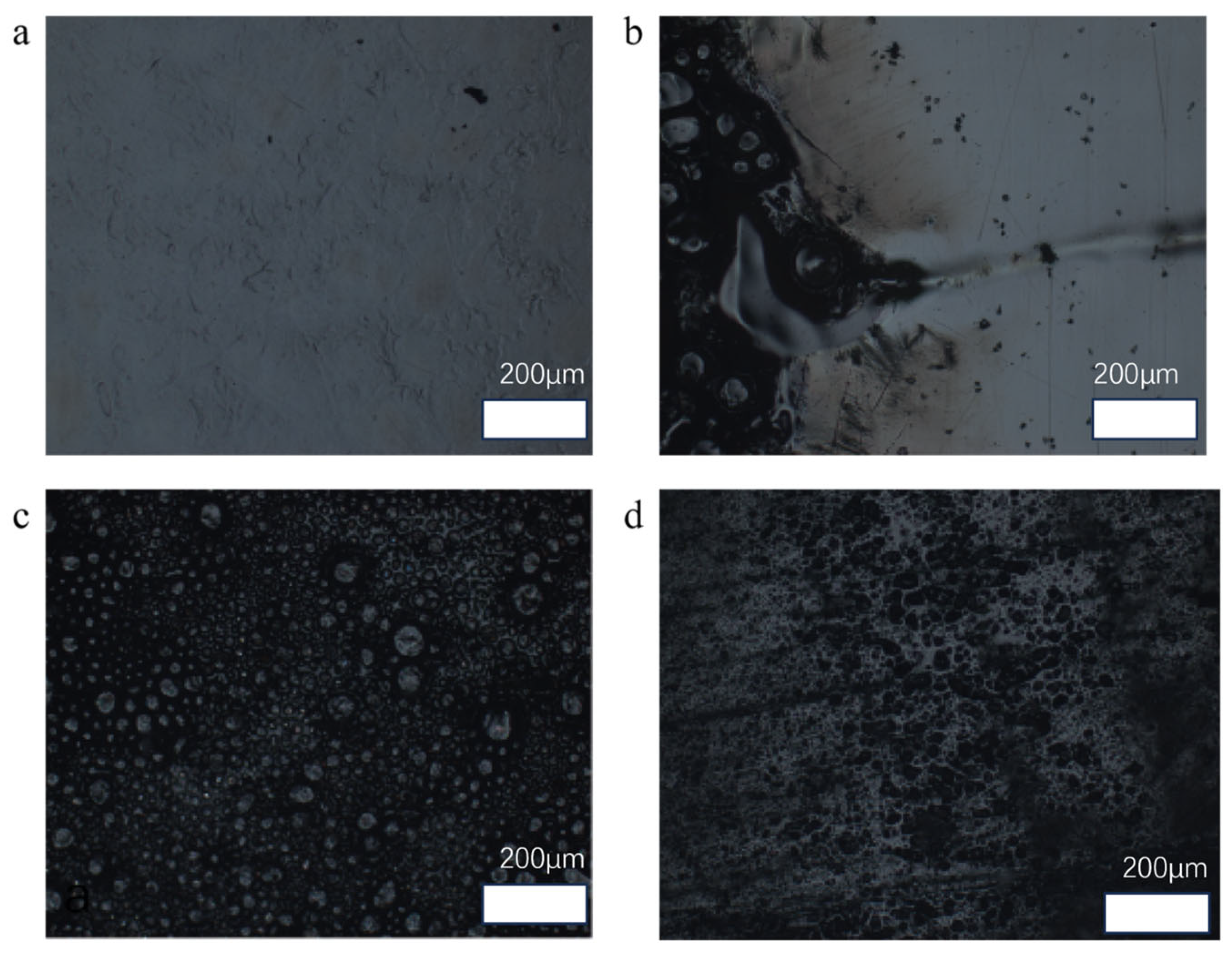
3.5.1. Ablation Morphology of Magnetically Responsive Filler Composite Epoxy Resin Materials
3.5.2. Anti-Burnout Mechanism of Magnetic Response Filler Composite Epoxy Resin Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, L.; Chen, Z.; Ren, J.; Yang, L.; Li, Y.; Wang, Z.; Ning, W.; Jia, S. Synchronously improved thermal conductivity and dielectric constant for epoxy composites by introducing functionalized silicon carbide nanoparticles and boron nitride microspheres. J. Colloid Interface Sci. 2022, 627, 205–214. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, J.; Guo, M.; Chen, D.; Chen, G. Study on Thermal Conductivity of P-Phenylenediamine Modified Graphene/Epoxy Composites. Polymers 2022, 14, 3660. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, A.M.; Muralishwara, K.; Hindi, J.; Acharya, S.; Naik, N.; Gurumurthy, B.M.; Hiremath, P.; Kasipandian, K.; Kasip, K. Effect of Seawater Absorption on Mechanical and Flexural Properties of Pineapple Leaf Fiber Reinforced Epoxy Nanoclay Composites. ES Mater. Manuf. 2023, 22, 1067. [Google Scholar] [CrossRef]
- Giménez, R.; Serrano, B.; San-Miguel, V.; Cabanelas, J.C. Recent Advances in MXene/Epoxy Composites: Trends and Prospects. Polymers 2022, 14, 1170. [Google Scholar] [CrossRef]
- Kangishwar, S.; Radhika, N.; Sheik, A.A.; Chavali, A.; Hariharan, S. A comprehensive review on polymer matrix composites: Material selection, fabrication, and application. Polym. Bull. 2023, 80, 47–87. [Google Scholar] [CrossRef]
- Ruan, K.; Zhong, X.; Shi, X.; Dang, J.; Gu, J. Liquid crystal epoxy resins with high intrinsic thermal conductivities and their composites: A mini-review. Mater. Today Phys. 2021, 20, 100456. [Google Scholar] [CrossRef]
- Yang, X.; Zhong, X.; Zhang, J.; Gu, J. Intrinsic high thermal conductive liquid crystal epoxy film simultaneously combining with excellent intrinsic self-healing performance. J. Mater. Sci. Technol. 2021, 68, 209–215. [Google Scholar] [CrossRef]
- Ben Hamida, M.B.; Almeshaal, M.A.; Hajlaoui, K.; Rothan, Y.A. A three-dimensional thermal management study for cooling a square Light Edding Diode. Case Stud. Therm. Eng. 2021, 27, 101223. [Google Scholar] [CrossRef]
- Anithambigai, P.; Shanmugan, S.; Mutharasu, D.; Zahner, T.; Lacey, D. Study on thermal performance of high power LED employing aluminium filled epoxy composite as thermal interface material. Microelectron. J. 2014, 45, 1726–1733. [Google Scholar] [CrossRef]
- Kumar, P.; Chaudhary, D.; Varshney, P.; Varshney, U.; Yahya, S.M.; Rafat, Y. Critical review on battery thermal management and role of nanomaterial in heat transfer enhancement for electrical vehicle application. J. Energy Storage 2020, 32, 102003. [Google Scholar] [CrossRef]
- Ling, Y.; Qiu, B.; Wang, L.; Zhang, H.; Yang, Y.; Zhang, X.; Liang, M.; Chen, Y.; Zou, H. Phase morphology modulation of silicone-modified epoxy resins and effects on thermal, mechanical and ablative properties. Prog. Org. Coat. 2024, 196, 108689. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Song, W.; Li, C.; Fan, W.; Bian, C.; Zhang, C.; Jing, X. The aryl-boron phenolic resins with super ablation properties for resin-transfer moulding process of three-dimensional fabric. Polym. Degrad. Stab. 2023, 208, 110252. [Google Scholar] [CrossRef]
- Hao, L.; Li, Z.; Sun, F.; Ding, K.; Zhou, X.-N.; Song, Z.-X.; Shi, Z.-Q.; Yang, J.-F.; Wang, B. High-performance epoxy composites reinforced with three-dimensional Al2O3 ceramic framework. Compos. Part A Appl. Sci. Manuf. 2019, 127, 105648. [Google Scholar] [CrossRef]
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- Kumar, R.; Nayak, S.K.; Sahoo, S.; Panda, B.P.; Mohanty, S. Study on thermal conductive epoxy adhesive based on adopting hexagonal boron nitride/graphite hybrids. J. Mater. Sci. Mater. Electron. 2018, 29, 16932–16938. [Google Scholar] [CrossRef]
- Dwivedi, A.; Khan, M.M.; Pali, H.S. A comprehensive review of thermal enhancement techniques in microchannel heat exchangers and heat sinks. J. Therm. Anal. Calorim. 2023, 148, 13189–13231. [Google Scholar] [CrossRef]
- Fang, H.; Bai, S.; Wong, C.P. Microstructure engineering of graphene towards highly thermal conductive composites. Compos. Part A Appl. Sci. Manuf. 2018, 112, 216–238. [Google Scholar] [CrossRef]
- Sádovská, G.; Honcová, P.; Morávková, J.; Jirka, I.; Vorokhta, M.; Pilař, R.; Rathouský, J.; Kaucký, D.; Mikysková, E.; Sazama, P. The thermal stability of carbon materials in the air: Quantitative structural investigation of thermal stability of carbon materials in air. Carbon 2023, 206, 211–225. [Google Scholar] [CrossRef]
- Kuanyshbekov, T.; Guseinov, N.; Kurbanova, B.; Nemkaeva, R.; Akatan, K.; Tulegenova, M.; Aitzhanov, M.; Zhasasynov, E.; Thomas, S. Local Natural Graphite as a Promising Raw Material for the Production of Thermally Reduced Graphene-Like Films. ES Mater. Manuf. 2024, 23, 1000. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Chen, X.; Zhu, Y.; Wang, H.; Yuan, S.; Xu, F.; Cui, Y.; Bao, D.; Shen, X.; et al. A high-performance thermal conductive and outstanding electrical insulating composite based on robust neuron-like microstructure. Chem. Eng. J. 2021, 426, 131280. [Google Scholar] [CrossRef]
- Ruan, K.; Shi, X.; Guo, Y.; Gu, J. Interfacial thermal resistance in thermally conductive polymer composites: A review. Compos. Commun. 2020, 22, 100518. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Z.; Ma, X.; Sun, Y.; Abdul, W.; Dong, M.; Guo, J. Positive Magnetoresistance of Polyaniline/Epoxy Composites at Room Temperature. ES Gen. 2024, 6, 1325. [Google Scholar] [CrossRef]
- Sabagh, S.; Azar, A.A.; Bahramian, A.R. High temperature ablation and thermo-physical properties improvement of carbon fibre reinforced composite using graphene oxide nanopowder. Compos. Part A 2017, 101, 326–333. [Google Scholar] [CrossRef]
- Ahmad, S.; Ali, S.; Salman, M.; Baluch, A.H. A comparative study on the effect of carbon-based and ceramic additives on the properties of fibre reinforced polymer matrix composites for high temperature applications. Ceram. Int. 2021, 47, 33956–33971. [Google Scholar] [CrossRef]
- Rahimian-Koloor, S.M.; Shokrieh, M.M. Investigating the Effect of the Curing-Induced Residual Stress on the Mechanical Behaviour of Carbon Nanotube/Epoxy Nanocomposites by Molecular Dynamics Simulation. Eng. Sci. 2023, 22, 817. [Google Scholar] [CrossRef]
- Thibeau, R.J.; Brown, C.W.; Heidersbach, R.H. Raman Spectra of Possible Corrosion Products of Iron. Appl. Spectrosc. 1987, 6, 532–535. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Chen, P.; Yan, K.; Leng, J.; Zhang, F.; Jiao, L. Synergistic smoke suppression effect of epoxy cross-linked structure and ferrocene on epoxy-based intumescent flame-retardant coating. Plast. Rubber Compos. 2018, 47, 258–265. [Google Scholar] [CrossRef]
- Li, H.; Wang, N.; Han, X.; Yuan, H.; Xie, J. Mechanism Identification and Kinetics Analysis of Thermal Degradation for Carbon Fiber/Epoxy Resin. Polymers 2021, 13, 569. [Google Scholar] [CrossRef]
- Yum, S.H.; Lee, W.I.; Kim, S.Y. A comparison study of polymer-matrix nanocomposites as sacrificial thermal protective materials. Mater. Today Commun. 2023, 34, 105381. [Google Scholar] [CrossRef]
- Reyes, A.N.; Saleh, Y.; Gustavsson, J.; Jolowsky, C.N.; Kumar, R.; Treadwell, L.J.; Sweat, R.D. Supersonic hot jet ablative testing and analysis of boron nitride nanotube hybrid composites. Compos. Part B Eng. 2024, 284, 111684. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L. Pyrolysis and combustion characteristics and reaction kinetics of carbon fiber/epoxy composites. AIP Adv. 2019, 9, 125110. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Zhang, D.; He, H.; Luo, C.; Zhou, M. Preparation and Properties of Magnetically Responsive Graphene/Boron Nitride/Iron Oxide Filler Composite Epoxy Resin Materials. Nanomaterials 2025, 15, 936. https://doi.org/10.3390/nano15120936
Yu Y, Zhang D, He H, Luo C, Zhou M. Preparation and Properties of Magnetically Responsive Graphene/Boron Nitride/Iron Oxide Filler Composite Epoxy Resin Materials. Nanomaterials. 2025; 15(12):936. https://doi.org/10.3390/nano15120936
Chicago/Turabian StyleYu, Yiheng, Duo Zhang, Hui He, Chaogui Luo, and Ming Zhou. 2025. "Preparation and Properties of Magnetically Responsive Graphene/Boron Nitride/Iron Oxide Filler Composite Epoxy Resin Materials" Nanomaterials 15, no. 12: 936. https://doi.org/10.3390/nano15120936
APA StyleYu, Y., Zhang, D., He, H., Luo, C., & Zhou, M. (2025). Preparation and Properties of Magnetically Responsive Graphene/Boron Nitride/Iron Oxide Filler Composite Epoxy Resin Materials. Nanomaterials, 15(12), 936. https://doi.org/10.3390/nano15120936





