Comparative Study of Red and Grey Selenium Nanoparticles on Organ-Specific Selenium Deposition and Growth Performance in Japanese Quails
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Selenium Nanoparticle Preparation and Characterization
2.3. Experimental Design
- o
- C0 (Control): Basal diet without selenium nanoparticle (SeNP) supplementation.
- o
- T1: Basal diet supplemented with 0.05 mg/kg red SeNPs.
- o
- T2: Basal diet supplemented with 0.5 mg/kg red SeNPs.
- o
- T3: Basal diet supplemented with 0.05 mg/kg grey SeNPs.
- o
- T4: Basal diet supplemented with 0.5 mg/kg grey SeNPs.
2.4. Growth Performance
2.5. Tissue Sampling
2.6. Statistical Analyses
3. Results
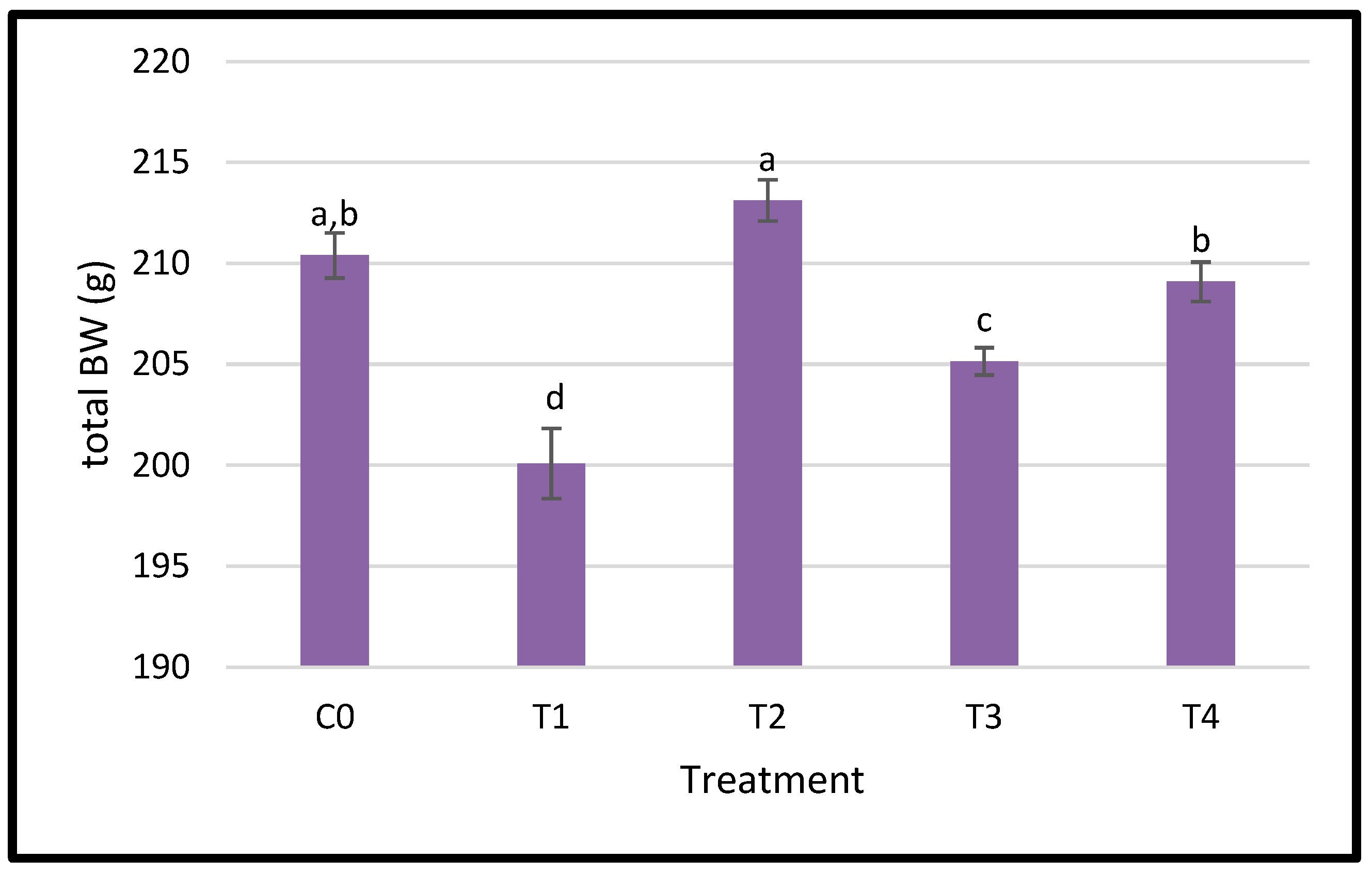
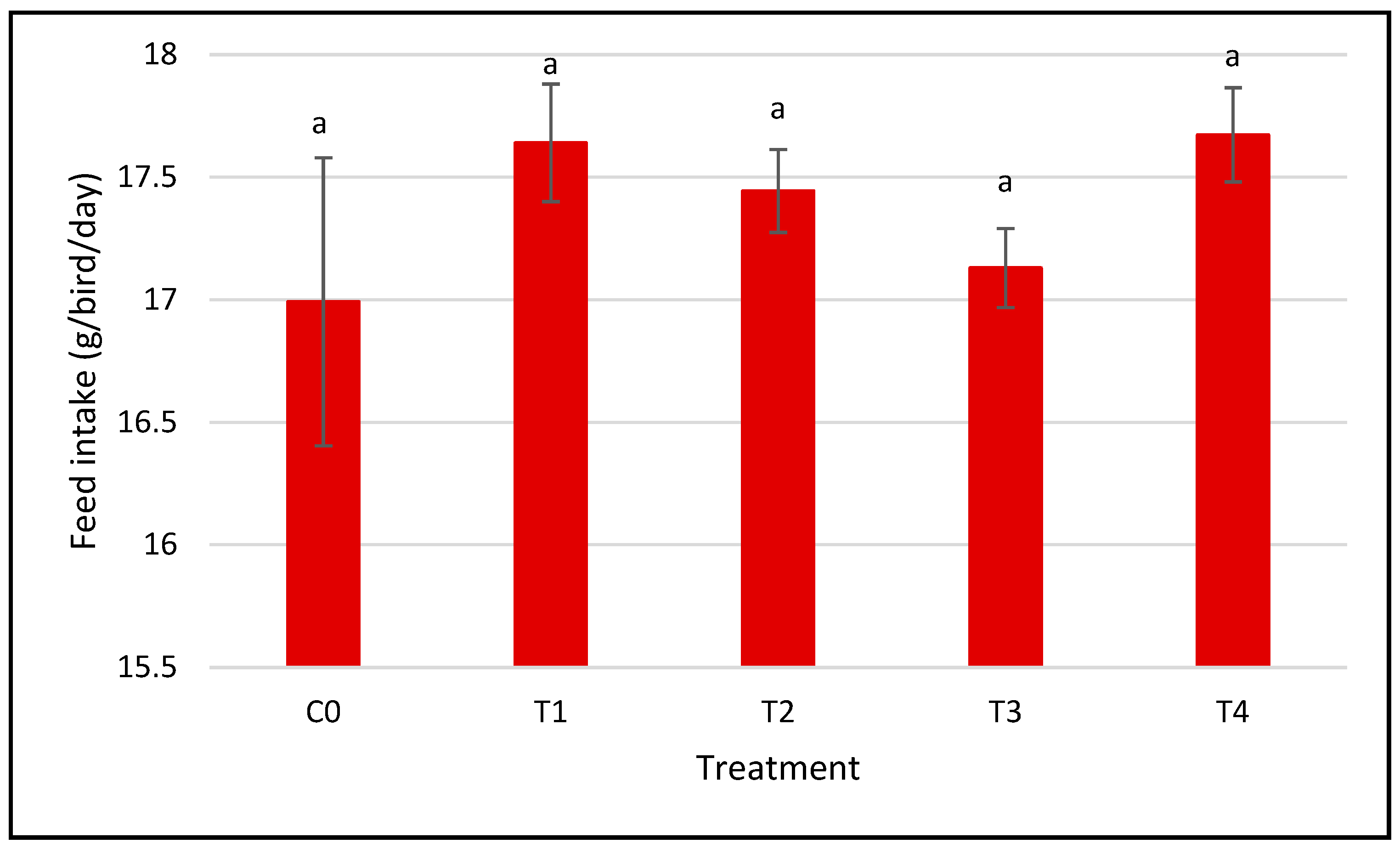
3.1. Growth Performance
3.2. Organ’s Indices
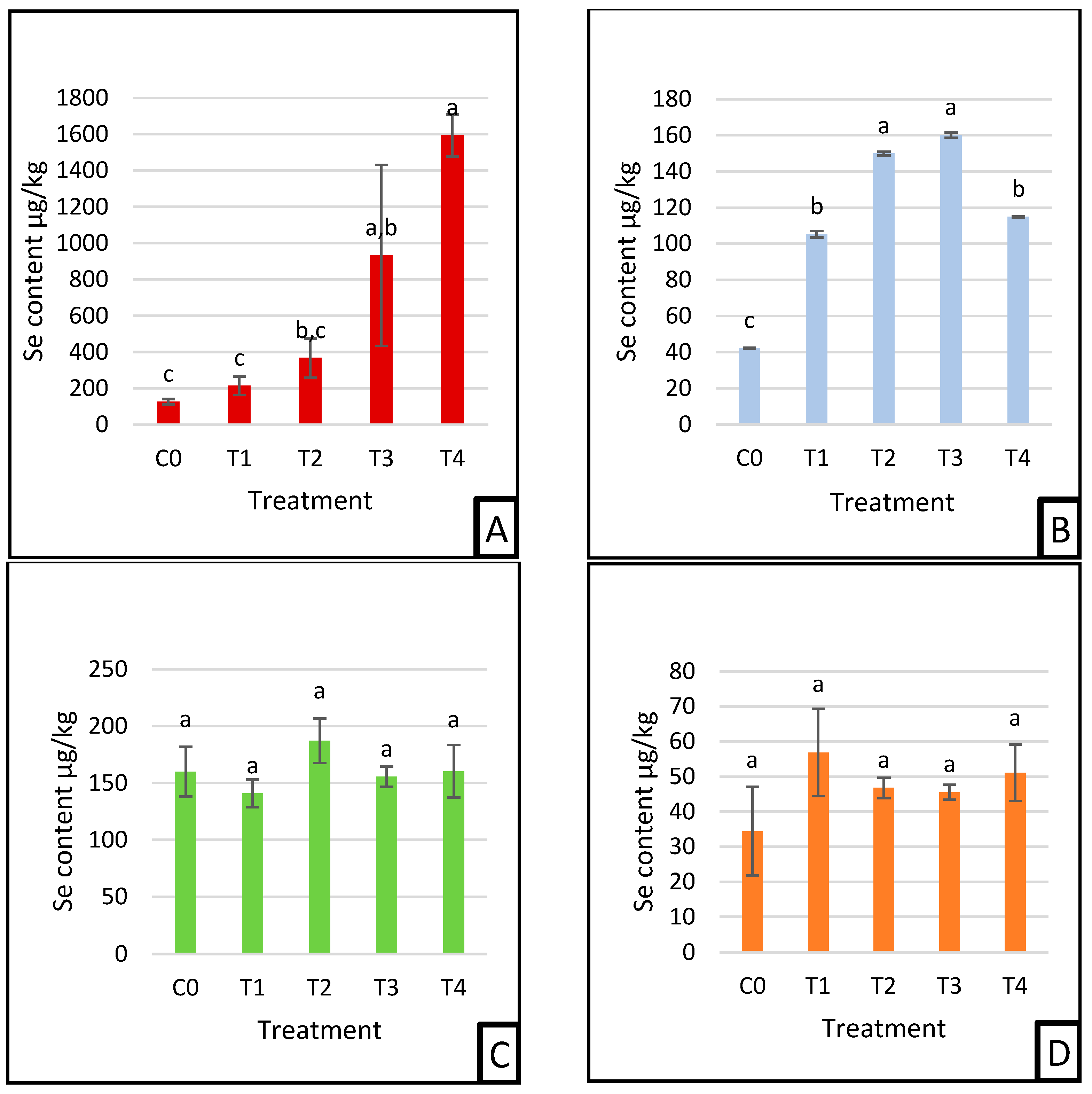
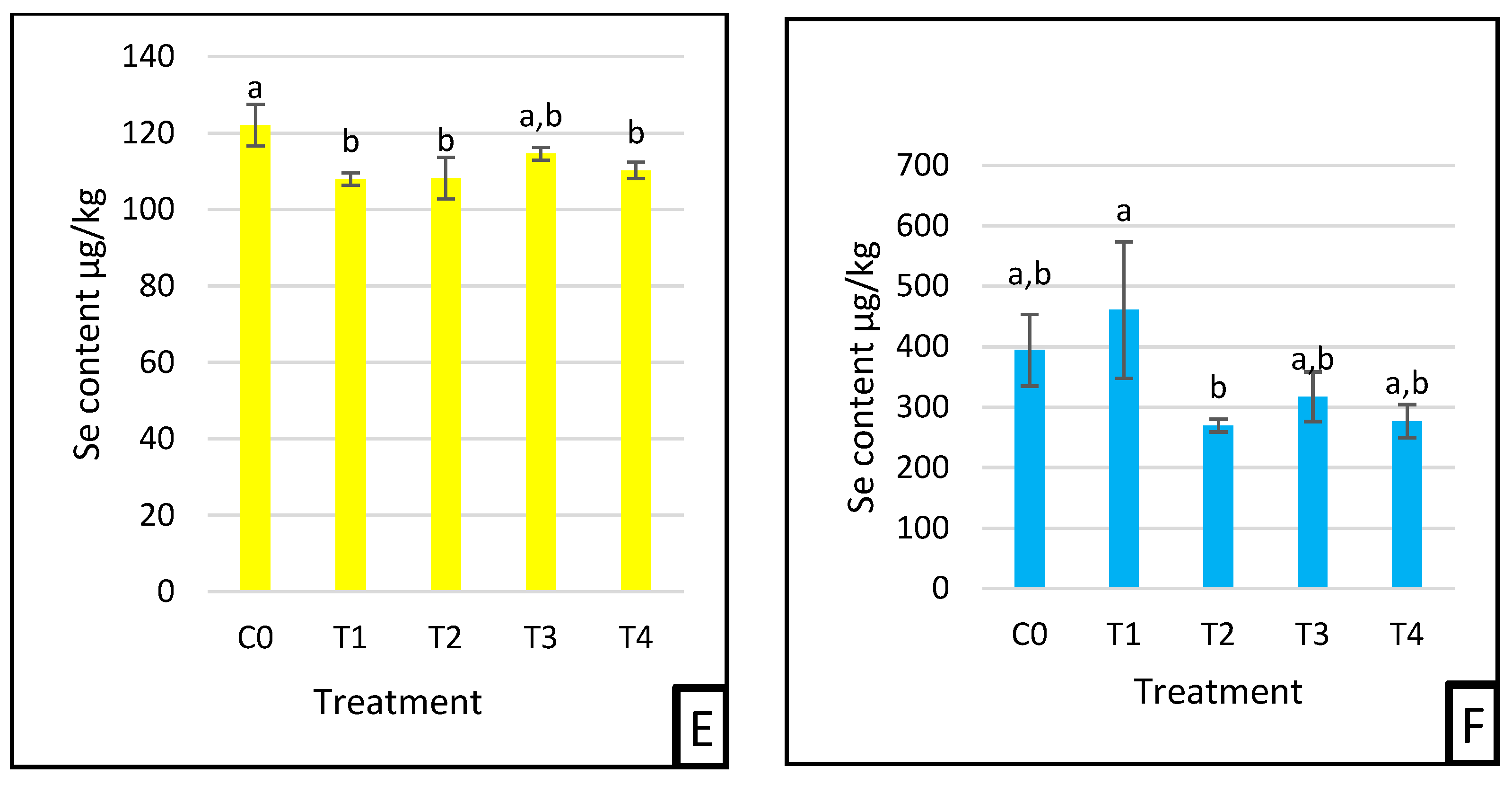
3.3. Selenium Deposition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SeNP | Selenium nanoparticle |
| Se | Selenium |
| BCF | Blood cellular fraction |
| BW | Body weight |
| AFS | Atomic Fluorescence Spectroscopy |
| HNO3 | Nitric Acid |
| HCl | Hydrochloric Acid |
| GPx | Glutathione peroxidase |
References
- Xu, J.-X.; Cao, C.-Y.; Sun, Y.-C.; Wang, L.-L.; Li, N.; Xu, S.-W.; Li, J.-L. Effects on Liver Hydrogen Peroxide Metabolism Induced by Dietary Selenium Deficiency or Excess in Chickens. Biol. Trace Elem. Res. 2014, 159, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Li, S. Effects of Selenium Deficiency on Testis Development and Autophagy in Chicks. Ital. J. Anim. Sci. 2020, 19, 753–761. [Google Scholar] [CrossRef]
- Mohapatra, P.; Swain, R.K.; Mishra, S.K.; Behera, T.; Swain, P.; Mishra, S.S.; Behura, N.C.; Sabat, S.C.; Sethy, K.; Dhama, K.; et al. Effects of Dietary Nano-Selenium on Tissue Selenium Deposition, Antioxidant Status and Immune Functions in Layer Chicks. Int. J. Pharmacol. 2014, 10, 160–167. [Google Scholar] [CrossRef]
- Elkhateeb, F.S.O.; Ghazalah, A.A.; Lohakare, J.; Abdel-Wareth, A.A.A. Selenium Nanoparticle Inclusion in Broiler Diets for Enhancing Sustainable Production and Health. Sci. Rep. 2024, 14, 18557. [Google Scholar] [CrossRef] [PubMed]
- Reda, F.M.; Alagawany, M.; Salah, A.S.; Mahmoud, M.A.; Azzam, M.M.; Di Cerbo, A.; El-Saadony, M.T.; Elnesr, S.S. Biological Selenium Nanoparticles in Quail Nutrition: Biosynthesis and Its Impact on Performance, Carcass, Blood Chemistry, and Cecal Microbiota. Biol. Trace Elem. Res. 2024, 202, 4191–4202. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Q.; Yang, J.; Yao, H.; Fan, R.; Cao, C.; Liu, C.; Zhang, S.; Lei, X.; Xu, S. The Proteomic Profiling of Multiple Tissue Damage in Chickens for a Selenium Deficiency Biomarker Discovery. Food Funct. 2020, 11, 1312–1321. [Google Scholar] [CrossRef]
- Kobayashi, R.; Hasegawa, M.; Kawaguchi, C.; Ishikawa, N.; Tomiwa, K.; Shima, M.; Nogami, K. Thyroid Function in Patients with Selenium Deficiency Exhibits High Free T4 to T3 Ratio. Clin. Pediatr. Endocrinol. 2021, 30, 19–26. [Google Scholar] [CrossRef]
- Wang, L.; Yin, J.; Liao, C.; Cheng, R.; Chen, F.; Yu, H.; Zhang, X. Selenium Deficiency-Induced High Concentration of Reactive Oxygen Species Restricts Hypertrophic Growth of Skeletal Muscle in Juvenile Zebrafish by Suppressing TORC1-Mediated Protein Synthesis. Br. J. Nutr. 2023, 130, 1841–1851. [Google Scholar] [CrossRef]
- Sadeghian, S.; Kojouri, G.A.; Mohebbi, A. Nanoparticles of Selenium as Species with Stronger Physiological Effects in Sheep in Comparison with Sodium Selenite. Biol. Trace Elem. Res. 2012, 146, 302–308. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Alagawany, M.; Hashem, N.M.; Farag, M.R.; Alghamdi, E.S.; Hassan, F.U.; Bilal, R.M.; Elnesr, S.S.; Dawood, M.A.O.; Nagadi, S.A.; et al. Nanominerals: Fabrication Methods, Benefits and Hazards, and Their Applications in Ruminants with Special Reference to Selenium and Zinc Nanoparticles. Animals 2021, 11, 1916. [Google Scholar] [CrossRef]
- Long, S.; Li, Z.; Dong, X.; Yan, X.; Liu, H.; Tan, B.; Zhang, S.; Pan, S.; Li, T.; Suo, X.; et al. The Effect of Oxidized Fish Oil on the Spleen Index, Antioxidant Activity, Histology and Transcriptome in Juvenile Hybrid Grouper (♀ Epinephelus Fuscoguttatus × ♂ Epinephelus lanceolatus). Front. Mar. Sci. 2021, 8, 779305. [Google Scholar] [CrossRef]
- Khazraei, S.K.; Tabeidian, S.A.; Habibian, M. Selenium Nanoparticles Are More Efficient than Sodium Selenite in Reducing the Toxicity of Aflatoxin B1 in Japanese Quail. Vet. Med. Sci. 2022, 8, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Loeschner, K.; Hadrup, N.; Hansen, M.; Pereira, S.A.; Gammelgaard, B.; Møller, L.H.; Mortensen, A.; Lam, H.R.; Larsen, E.H. Absorption, Distribution, Metabolism and Excretion of Selenium Following Oral Administration of Elemental Selenium Nanoparticles or Selenite in Rats. Metallomics 2014, 6, 330–337. [Google Scholar] [CrossRef]
- Ringuet, M.T.; Hunne, B.; Lenz, M.; Bravo, D.M.; Furness, J.B. Analysis of Bioavailability and Induction of Glutathione Peroxidase by Dietary Nanoelemental, Organic and Inorganic Selenium. Nutrients 2021, 13, 1073. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Mahan, D.C. Comparative Effects of High Dietary Levels of Organic and Inorganic Selenium on Selenium Toxicity of Growing-Finishing Pigs. J. Anim. Sci. 2001, 79, 942–948. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Toxicity of Repeated Oral Intake of Organic Selenium, Inorganic Selenium, and Selenium Nanoparticles: A Review. J. Trace Elem. Med. Biol. 2023, 79, 127235. [Google Scholar] [CrossRef]
- Ruiz, L.F.G.F.; Piedrahita, J.C.P.; Vázquez, N.G.C. Selenosis due to Brazil nut. Med. Int. Mex. 2024, 40, 374–378. [Google Scholar]
- Zhang, J.; Wang, H.; Yan, X.; Zhang, L. Comparison of Short-Term Toxicity between Nano-Se and Selenite in Mice. Life Sci. 2005, 76, 1099–1109. [Google Scholar] [CrossRef]
- Takahashi, K.; Suzuki, N.; Ogra, Y. Bioavailability Comparison of Nine Bioselenocompounds In Vitro and In Vivo. Int. J. Mol. Sci. 2017, 18, 506. [Google Scholar] [CrossRef]
- Cheng, W.-H. Revisiting Selenium Toxicity. J. Nutr. 2021, 151, 747–748. [Google Scholar] [CrossRef]
- Yuan, S.; Mason, A.M.; Carter, P.; Vithayathil, M.; Kar, S.; Burgess, S.; Larsson, S.C. Selenium and Cancer Risk: Wide-Angled Mendelian Randomization Analysis. Int. J. Cancer 2022, 150, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Qattan, S.Y.A.; Attia, Y.A.; El-Saadony, M.T.; Elnesr, S.S.; Mahmoud, M.A.; Madkour, M.; Abd El-Hack, M.E.; Reda, F.M. Use of Chemical Nano-Selenium as an Antibacterial and Antifungal Agent in Quail Diets and Its Effect on Growth, Carcasses, Antioxidant, Immunity and Caecal Microbes. Animals 2021, 11, 3027. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wu, Y.; Zhang, F.; Zheng, S.; Wang, L.; Bai, J.; Yang, Y. Preparation of Ribes nigrum L. Polysaccharides-Stabilized Selenium Nanoparticles for Enhancement of the Anti-Glycation and α-Glucosidase Inhibitory Activities. Int. J. Biol. Macromol. 2023, 253, 127122. [Google Scholar] [CrossRef]
- Hosseintabar-Ghasemabad, B.; Kvan, O.V.; Sheida, E.V.; Bykov, A.V.; Zigo, F.; Seidavi, A.; Elghandour, M.M.M.Y.; Cipriano-Salazar, M.; Lackner, M.; Salem, A.Z.M. Nano Selenium in Broiler Feeding: Physiological Roles and Nutritional Effects. AMB Express 2024, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Gangadoo, S.; Dinev, I.; Willson, N.-L.; Moore, R.J.; Chapman, J.; Stanley, D. Nanoparticles of Selenium as High Bioavailable and Non-Toxic Supplement Alternatives for Broiler Chickens. Env. Sci. Pollut. Res. Int. 2020, 27, 16159–16166. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-Selenium and Its Nanomedicine Applications: A Critical Review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef]
- Khandsuren, B.; Prokisch, J. Preparation of Red and Grey Elemental Selenium for Food Fortification. Acta Aliment. 2021, 50, 289–298. [Google Scholar] [CrossRef]
- Li, K.; Li, J.; Zhang, S.; Zhang, J.; Xu, Q.; Xu, Z.; Guo, Y. Amorphous Structure and Crystal Stability Determine the Bioavailability of Selenium Nanoparticles. J. Hazard. Mater. 2024, 465, 133287. [Google Scholar] [CrossRef]
- Biswas, A.; Mohan, J.; Sastry, K.V.H. Effect of Higher Levels of Dietary Selenium on Production Performance and Immune Responses in Growing Japanese Quail. Br. Poult. Sci. 2006, 47, 511–515. [Google Scholar] [CrossRef]
- National Research Council Subcommittee on Poultry Nutrition. Nutrient Requirements of Poultry: 1994; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Abdel-Moneim, A.-M.E.; Sabic, E.M.; Abu-Taleb, A.M.; Ibrahim, N.S. Growth Performance, Hemato-Biochemical Indices, Thyroid Activity, Antioxidant Status, and Immune Response of Growing Japanese Quail Fed Diet with Full-Fat Canola Seeds. Trop. Anim. Health Prod. 2020, 52, 1853–1862. [Google Scholar] [CrossRef]
- Marković, R.; Ćirić, J.; Starčević, M.; Šefer, D.; Baltić, M.Ž. Effects of Selenium Source and Level in Diet on Glutathione Peroxidase Activity, Tissue Selenium Distribution, and Growth Performance in Poultry. Anim. Health. Res. Rev. 2018, 19, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Tsekhmistrenko, O.; Bityutskii, V.; Tsekhmistrenko, S.; Kharchyshyn, V.; Tymoshok, N.; Spivak, M. Efficiency of Application of Inorganic and Nanopreparations of Selenium and Probiotics for Growing Young Quails. Theor. Appl. Vet. Med. 2020, 8, 206–212. [Google Scholar] [CrossRef]
- Kaewsatuan, P.; Morawong, T.; Lu, P.; Kamkaew, A.; Molee, A.; Molee, W. In Ovo Feeding of L-Arginine and Selenium Nanoparticles Influences Post-Hatch Growth, Muscle Development, Antioxidant Status, and Meat Quality in Slow-Growing Chickens. J. Anim. Sci. 2024, 102, skae290. [Google Scholar] [CrossRef]
- Ren, Z.; Okyere, S.K.; Zhang, M.; Zhang, X.; He, H.; Hu, Y. Glycine Nano-Selenium Enhances Immunoglobulin and Cytokine Production in Mice Immunized with H9N2 Avian Influenza Virus Vaccine. Int. J. Mol. Sci. 2022, 23, 7914. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2022/1459 of 2 September 2022 Amending Implementing Regulation (EU) 2019/804 as Regards the Terms of Authorisation of the Organic Form of Selenium Produced by Saccharomyces Cerevisiae CNCM I-3060 as Feed Additive for All Animal Species (Text with EEA Relevance). Off. J. Eur. Union 2022, 229, 22–25. [Google Scholar]
- Bhattacharjee, A.; Basu, A.; Bhattacharya, S. Selenium Nanoparticles Are Less Toxic than Inorganic and Organic Selenium to Mice in Vivo. Nucleus 2019, 62, 259–268. [Google Scholar] [CrossRef]
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative Study of the Antimicrobial Activity of Selenium Nanoparticles with Different Surface Chemistry and Structure. Front. Bioeng. Biotechnol. 2021, 8, 624621. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y. Influence of Dietary Nano Elemental Selenium on Growth Performance, Tissue Selenium Distribution, Meat Quality, and Glutathione Peroxidase Activity in Guangxi Yellow Chicken. Poult. Sci. 2011, 90, 680–686. [Google Scholar] [CrossRef]
- Dehkordi, A.J.; Mohebbi, A.; Aslani, M.; Ghoreyshi, S. Evaluation of Nanoselenium (Nano-Se) Effect on Hematological and Serum Biochemical Parameters of Rat in Experimentally Lead Poisoning. Hum. Exp. Toxicol. 2017, 36, 421–427. [Google Scholar] [CrossRef]
- Kazaz, S.; Samaha, M.; Hafez, M.; Shobokshy, S.; Wirtu, G. Dietary Supplementation of Nano-Selenium Improves Reproductive Performance, Sexual Behavior and Deposition of Selenium in the Testis and Ovary of Japanese Quail. J. Adv. Vet. Anim. Res. 2020, 7, 597. [Google Scholar] [CrossRef]
- McFarland, L.Z.; Winget, C.M.; Wilson, W.O.; Johnson, C.M. Role of Selenium in Neural Physiology of Avian Species: 1. The Distribution of Selenium in Tissues of Chickens, Turkeys and Coturnix. Poult. Sci. 1970, 49, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Christen, W.G.; Glynn, R.J.; Gaziano, J.M.; Darke, A.K.; Crowley, J.J.; Goodman, P.J.; Lippman, S.M.; Lad, T.E.; Bearden, J.D.; Goodman, G.E.; et al. Age-Related Cataract in Men in the Selenium and Vitamin E Cancer Prevention Trial Eye Endpoints Study: A Randomized Clinical Trial. JAMA Ophthalmol. 2015, 133, 17–24. [Google Scholar] [CrossRef]
- Chandrinos, A.; Tzamouranis, D.; Kakoura, S. Vitamin E and Supplements Offer Eye Neuroprotection—Myth or Reality? Ophthalmol. Res. Int. J. 2023, 18, 16–24. [Google Scholar] [CrossRef]
- Gawor, A.; Ruszczynska, A.; Czauderna, M.; Bulska, E. Determination of Selenium Species in Muscle, Heart, and Liver Tissues of Lambs Using Mass Spectrometry Methods. Animals 2020, 10, 808. [Google Scholar] [CrossRef]
- Ashouri, S.; Keyvanshokooh, S.; Salati, A.P.; Johari, S.A.; Pasha-Zanoosi, H. Effects of Different Levels of Dietary Selenium Nanoparticles on Growth Performance, Muscle Composition, Blood Biochemical Profiles and Antioxidant Status of Common Carp (Cyprinus carpio). Aquaculture 2015, 446, 25–29. [Google Scholar] [CrossRef]
- Dlouhá, G.; Ševčíková, S.; Dokoupilová, A.; Zita, L.; Heindl, J.; Skřivan, M. Effect of Dietary Selenium Sources on Growth Performance, Breast Muscle Selenium, Glutathione Peroxidase Activity and Oxidative Stability in Broilers. Czech J. Anim. Sci. 2008, 53, 265–269. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Gu, Q.; Li, W. Effects of Different Dietary Selenium Sources (Selenium Nanoparticle and Selenomethionine) on Growth Performance, Muscle Composition and Glutathione Peroxidase Enzyme Activity of Crucian Carp (Carassius auratus gibelio). Aquaculture 2009, 291, 78–81. [Google Scholar] [CrossRef]
- Mohammadi, E.; Janmohammadi, H.; Olyayee, M.; Helan, J.A.; Kalanaky, S. Nano Selenium Improves Humoral Immunity, Growth Performance and Breast-Muscle Selenium Concentration of Broiler Chickens. Anim. Prod. Sci. 2020, 60, 1902–1910. [Google Scholar] [CrossRef]
- Mahmoud, R.; Salama, B.; Safhi, F.A.; Pet, I.; Pet, E.; Ateya, A. Assessing the Impacts of Different Levels of Nano-Selenium on Growth Performance, Serum Metabolites, and Gene Expression in Heat-Stressed Growing Quails. Vet. Sci. 2024, 11, 228. [Google Scholar] [CrossRef]
- Kralik, Z.; Kralik, G.; Grčević, M.; Suchý, P.; Straková, E. Effects of Increased Content of Organic Selenium in Feed on the Selenium Content and Fatty Acid Profile in Broiler Breast Muscle. Acta Vet. Brno 2012, 81, 31–35. [Google Scholar] [CrossRef]
- Wan, X.; Ju, G.; Xu, L.; Yang, H.; Wang, Z. Selenomethionine Improves Antioxidant Capacity of Breast Muscle in Geese Via Stimulating Glutathione System and Thiol Pool. Biol. Trace Elem. Res. 2020, 198, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, E.; Demiris, N.; Kominakis, A.; Pappas, A.C. Meta-Analysis of Selenium Accumulation and Expression of Antioxidant Enzymes in Chicken Tissues. Animal 2014, 8, 542–554. [Google Scholar] [CrossRef] [PubMed]
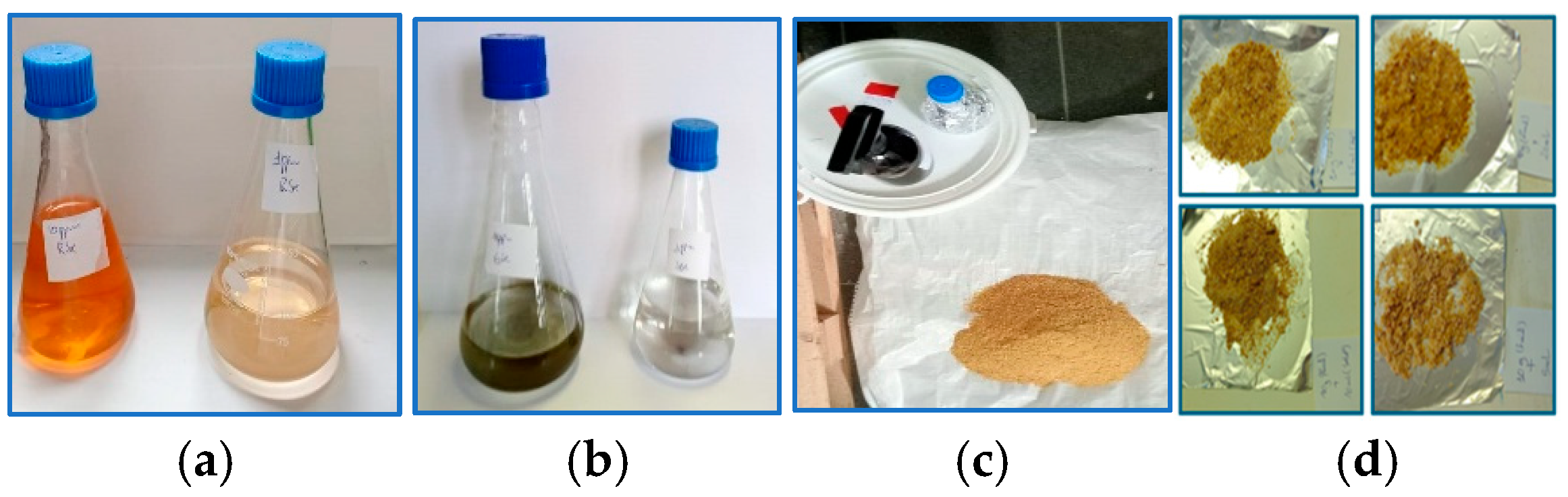

| Feed Ingredients | Inclusion Rate, % |
|---|---|
| Soybean meal (46% CP) | 34.88 |
| Corn | 30.37 |
| Wheat | 20.00 |
| Sunflower oil | 6.79 |
| Limestone | 5.64 |
| MCP | 1.29 |
| Salt | 0.38 |
| DL-Methionine | 0.15 |
| Vitamin and mineral premix a | 0.50 |
| Nutrient content, % | |
| Metabolizable energy MJ/kg | 12.13 |
| Crude protein | 20.0 |
| Calcium | 2.50 |
| Available Phosphorus | 0.35 |
| Sodium | 0.15 |
| Methionine | 0.45 |
| Methionine + cysteine | 0.75 |
| Lysine | 1.08 |
| Threonine | 0.74 |
| Leucine | 1.59 |
| Isoleucine | 0.86 |
| Arginine | 1.33 |
| Tryptophan | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferroudj, A.; Muthu, A.; Sári, D.; Törős, G.; Beni, Á.; Czeglédi, L.; Knop, R.; El-Ramady, H.; Prokisch, J. Comparative Study of Red and Grey Selenium Nanoparticles on Organ-Specific Selenium Deposition and Growth Performance in Japanese Quails. Nanomaterials 2025, 15, 801. https://doi.org/10.3390/nano15110801
Ferroudj A, Muthu A, Sári D, Törős G, Beni Á, Czeglédi L, Knop R, El-Ramady H, Prokisch J. Comparative Study of Red and Grey Selenium Nanoparticles on Organ-Specific Selenium Deposition and Growth Performance in Japanese Quails. Nanomaterials. 2025; 15(11):801. https://doi.org/10.3390/nano15110801
Chicago/Turabian StyleFerroudj, Aya, Arjun Muthu, Daniella Sári, Gréta Törős, Áron Beni, Levente Czeglédi, Renáta Knop, Hassan El-Ramady, and József Prokisch. 2025. "Comparative Study of Red and Grey Selenium Nanoparticles on Organ-Specific Selenium Deposition and Growth Performance in Japanese Quails" Nanomaterials 15, no. 11: 801. https://doi.org/10.3390/nano15110801
APA StyleFerroudj, A., Muthu, A., Sári, D., Törős, G., Beni, Á., Czeglédi, L., Knop, R., El-Ramady, H., & Prokisch, J. (2025). Comparative Study of Red and Grey Selenium Nanoparticles on Organ-Specific Selenium Deposition and Growth Performance in Japanese Quails. Nanomaterials, 15(11), 801. https://doi.org/10.3390/nano15110801










