Gold and Iron Oxide Nanoparticle Assemblies on Turnip Yellow Mosaic Virus for In-Solution Photothermal Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Virus Extraction and Purification
2.2. Synthesis of Citrate-Coated IONPs
2.3. Synthesis of Citrate-Coated AuNPs and Functionalization
2.4. NPs Grafting onto TYMV
2.5. Characterization of Nano-Biohybrid Materials
2.6. Photothermal Experiments
3. Results and Discussions
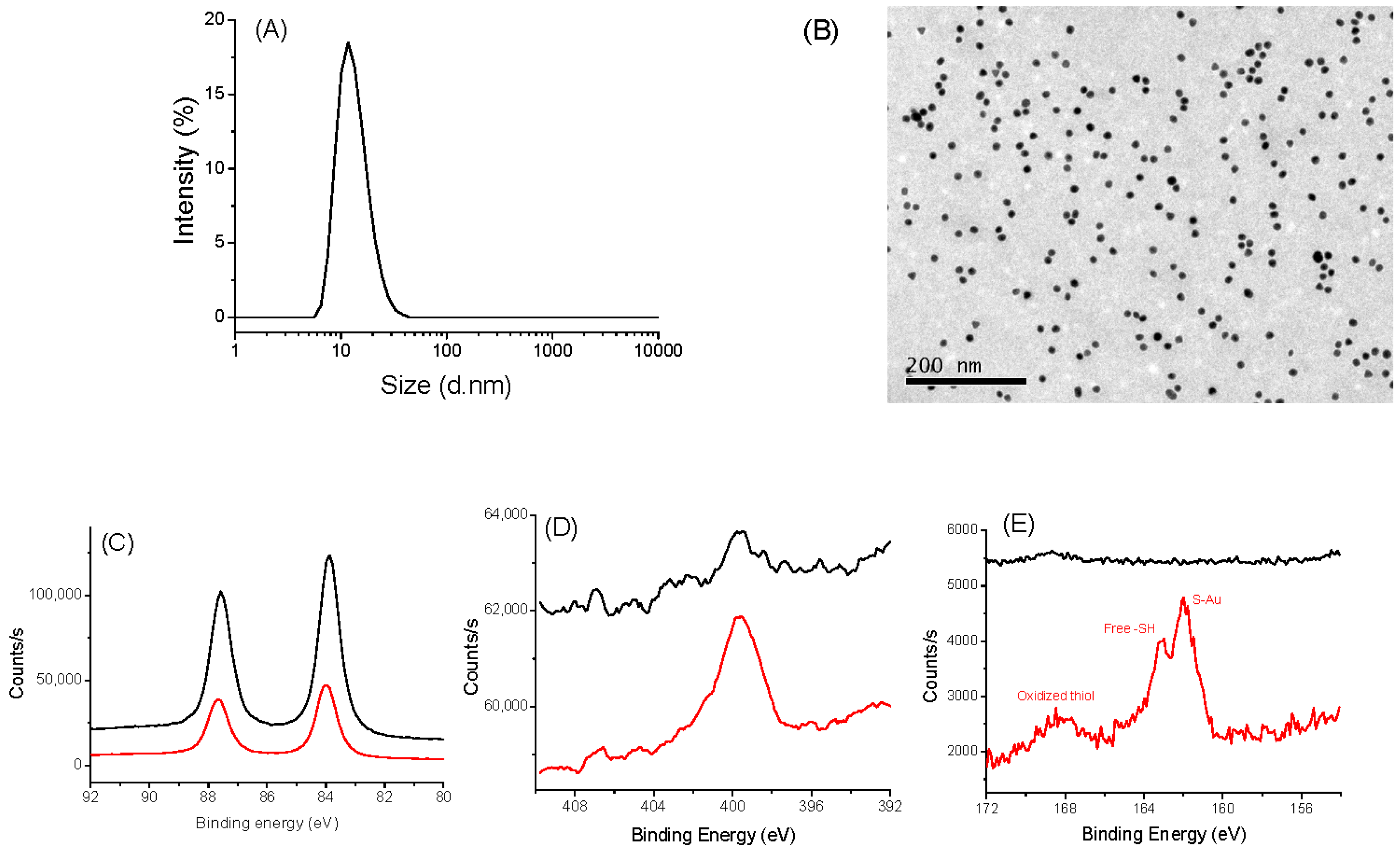
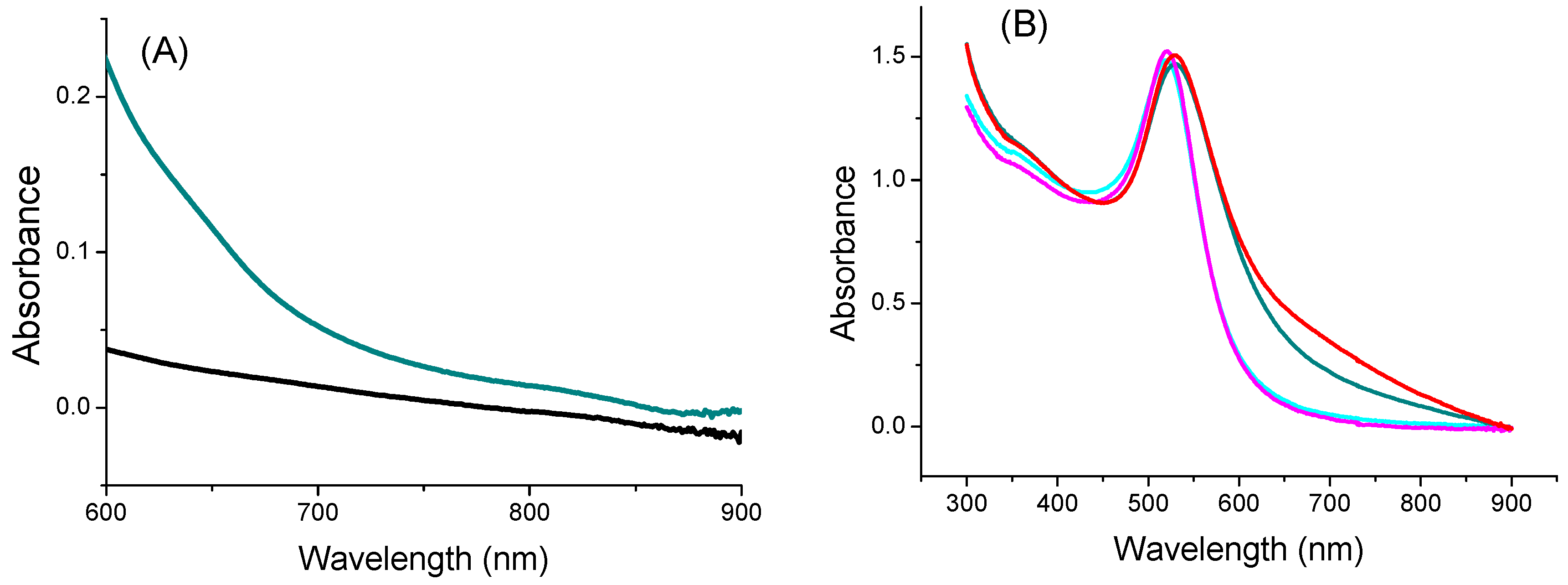
3.1. Characterization of IONPs and AuNPs
3.2. Characterization of the Nano-Biohybrids
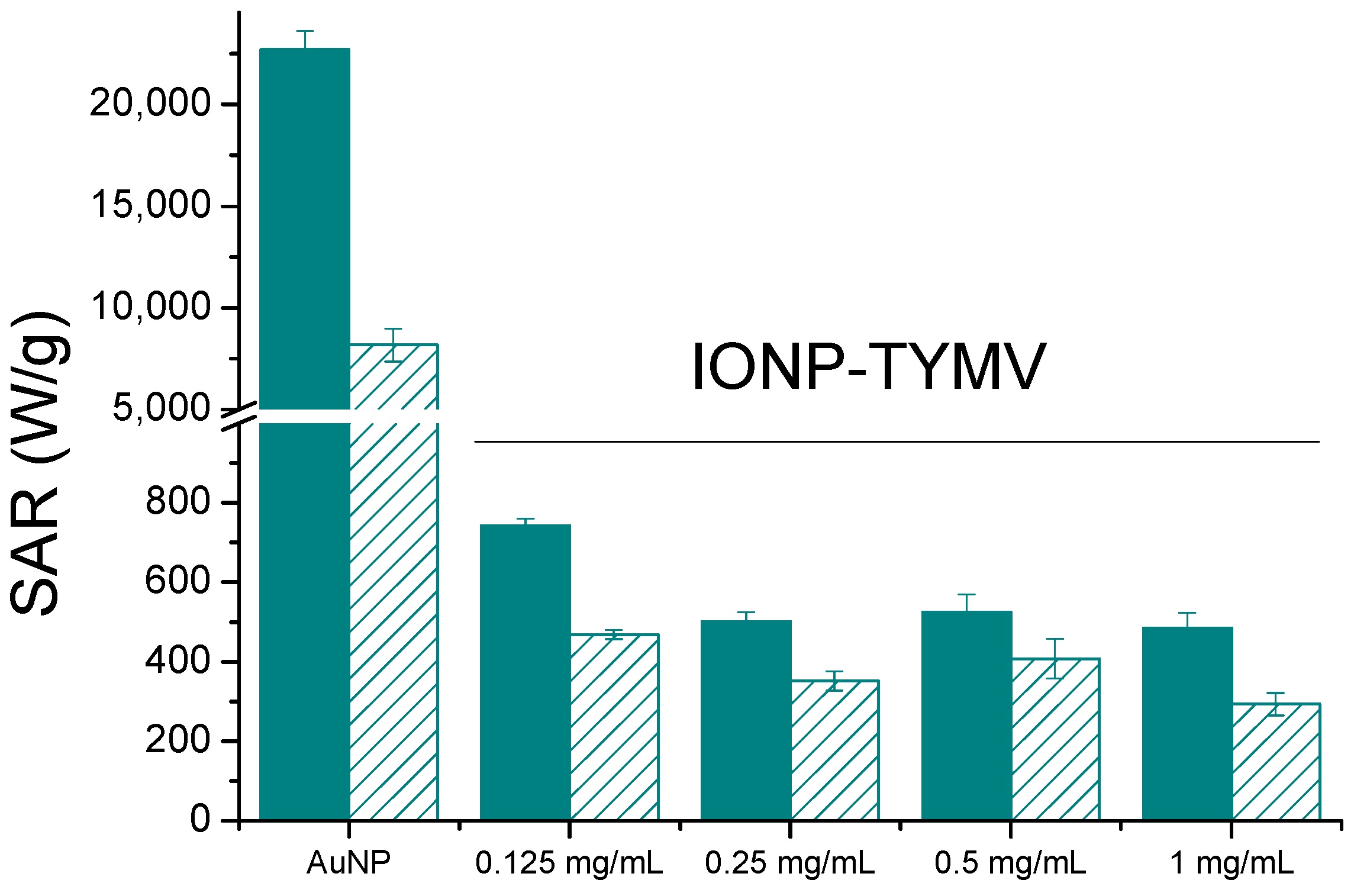
3.3. Evaluation of Photothermal Properties
| Particles | [Fe] (mg/mL) | Laser Power Density (W/cm2) | ΔT (°C) along 10 min of Exposition | SAR (W/gFe) | Ref. |
|---|---|---|---|---|---|
| Spherical free 10 nm-sized maghemite particles | 0.125 | 0.41 | 3.5 | 568 | * |
| 1.00 | 0.41 | 15 | 293 | * | |
| 10 nm-sized maghemite particles assembled on TYMV | 0.125 | 0.41 | 6.7 | 742 | * |
| 1.00 | 0.2 | 7.4 | 184 | * | |
| 1.00 | 0.41 | 16.2 | 585 | * | |
| 1.00 | 0.6 | 18.9 | 523 | * | |
| 10 nm-sized maghemite grafted to HSA | 0.14 | 1.0 | 9.2 | 1870 | [60] |
| Iron oxide nanoflowers, 25 nm in size | 1.4 | 0.3 | 2.5 | ~180 | [56] |
| 1.4 | 1.0 | 8 | ~550 |
| Particles | Laser Power Density (W/cm2) | ΔT (°C) along 5 min of Exposition | [Au] mg/mL | SAR (kW/gAu) | η (%) | Ref. |
|---|---|---|---|---|---|---|
| Spherical free 10 nm-sized Au particles | 0.41 | 5 | 0.019 | 8.2 ± 0.4 | Nd | * |
| 10 nm-sized Au particles assembled on TYMV | 0.41 | 8 | 0.019 | 22.7 ± 0.9 | 88 | * |
| Spherical free 20 nm-sized Au particles | 0.41 | 4 | 0.024 | 12.6 ± 1.7 | Nd | * |
| 20 nm-sized Au particles assembled on TYMV | 0.41 | 12 | 0.024 | 57.4 ± 3.4 | 66 | * |
| Nanoraspberry of 41 nm | 0.30 | 15 | 0.1 | 5 | 66 | [16] |
| Nanorod 40 × 15 nm | 0.3 | 12 | 0.1 | 4 | 65 | |
| Nanorod 55 × 15 nm | 0.3 | 20 | 0.1 | 8.5 | 65 | |
| Nanocluster | 1.2 | 40 | 0.02 | nd | 83.7 | [59] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deb, P.K.; Odetallah, H.a.M.A.; Al-Jaidi, B.; Akkinepalli, R.R.; Al-Aboudi, A.; Tekade, R.K. Chapter 11—Biomaterials and Nanoparticles for Hyperthermia Therapy. In Biomaterials and Bionanotechnology; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 375–413. [Google Scholar] [CrossRef]
- Ahmed, B.; Paulina, K.W.; Diana, A.A.-B. Hyperthermia: Cancer Treatment and Beyond. In Cancer Treatment; Letícia, R., Ed.; IntechOpen: Rijeka, Croatia, 2013; Chapter 12. [Google Scholar] [CrossRef]
- Kaur, P.; Aliru, M.L.; Chadha, A.S.; Asea, A.; Krishnan, S. Hyperthermia using nanoparticles—Promises and pitfalls. Int. J. Hyperth. 2016, 32, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Martínez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Yougbaré, S.; Mutalik, C.; Krisnawati, D.I.; Kristanto, H.; Jazidie, A.; Nuh, M.; Cheng, T.-M.; Kuo, T.-R. Nanomaterials for the Photothermal Killing of Bacteria. Nanomaterials 2020, 10, 1123. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.K.; Kim, J.C.; Shin, Y.; Han, S.M.; Won, W.R.; Her, J.; Park, J.Y.; Oh, K.T. Principles and applications of nanomaterial-based hyperthermia in cancer therapy. Arch. Pharmacal Res. 2020, 43, 46–57. [Google Scholar] [CrossRef]
- Norouzi, H.; Khoshgard, K.; Akbarzadeh, F. In vitro outlook of gold nanoparticles in photo-thermal therapy: A literature review. Lasers Med. Sci. 2018, 33, 917–926. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lee, H.-L.; Chiou, J.-F.; Lo, L.-W. Recent Advances in Gold Nanomaterials for Photothermal Therapy. J. Nanotheranostics 2022, 3, 117–131. [Google Scholar] [CrossRef]
- Sharifi, M.; Attar, F.; Saboury, A.A.; Akhtari, K.; Hooshmand, N.; Hasan, A.; El-Sayed, M.A.; Falahati, M. Plasmonic gold nanoparticles: Optical manipulation, imaging, drug delivery and therapy. J. Control. Release 2019, 311–312, 170–189. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C.M.; Xia, Y. Gold Nanocages: Synthesis, Properties, and Applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef]
- Xu, Q.; Wan, J.; Bie, N.; Song, X.; Yang, X.; Yong, T.; Zhao, Y.; Yang, X.; Gan, L. A Biomimetic Gold Nanocages-Based Nanoplatform for Efficient Tumor Ablation and Reduced Inflammation. Theranostics 2018, 8, 5362–5378. [Google Scholar] [CrossRef]
- Tsai, M.-F.; Chang, S.-H.G.; Cheng, F.-Y.; Shanmugam, V.; Cheng, Y.-S.; Su, C.-H.; Yeh, C.-S. Au Nanorod Design as Light-Absorber in the First and Second Biological Near-Infrared Windows for in Vivo Photothermal Therapy. ACS Nano 2013, 7, 5330–5342. [Google Scholar] [CrossRef]
- Cai, K.; Zhang, W.; Zhang, J.; Li, H.; Han, H.; Zhai, T. Design of Gold Hollow Nanorods with Controllable Aspect Ratio for Multimodal Imaging and Combined Chemo-Photothermal Therapy in the Second Near-Infrared Window. ACS Appl. Mater. Interfaces 2018, 10, 36703–36710. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Wang, A.; Fei, J.; Zhao, J.; Li, J. Polypyrrole-stabilized gold nanorods with enhanced photothermal effect towards two-photon photothermal therapy. J. Mater. Chem. B 2015, 3, 4539–4545. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.-Y.; Chen, C.-T.; Yeh, C.-S. Comparative efficiencies of photothermal destruction of malignant cells using antibody-coated silica@Au nanoshells, hollow Au/Ag nanospheres and Au nanorods. Nanotechnology 2009, 20, 425104. [Google Scholar] [CrossRef]
- Plan Sangnier, A.; Aufaure, R.; Cheong, S.; Motte, L.; Palpant, B.; Tilley, R.D.; Guenin, E.; Wilhelm, C.; Lalatonne, Y. Raspberry-like small multicore gold nanostructures for efficient photothermal conversion in the first and second near-infrared windows. Chem. Commun. 2019, 55, 4055–4058. [Google Scholar] [CrossRef]
- Chung, U.S.; Kim, J.-H.; Kim, B.; Kim, E.; Jang, W.-D.; Koh, W.-G. Dendrimer porphyrin-coated gold nanoshells for the synergistic combination of photodynamic and photothermal therapy. Chem. Commun. 2016, 52, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.M.; Tam, J.O.; Murthy, A.; Ingram, D.R.; Ma, L.L.; Travis, K.; Johnston, K.P.; Sokolov, K.V. Controlled Assembly of Biodegradable Plasmonic Nanoclusters for Near-Infrared Imaging and Therapeutic Applications. ACS Nano 2010, 4, 2178–2184. [Google Scholar] [CrossRef]
- Pratap, D.; Vikas; Gautam, R.; Shaw, A.K.; Soni, S. Photothermal properties of stable aggregates of gold nanorods. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 635, 128054. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Lim, S.C.; Lim, K.; Lee, E.S.; Oh, K.T.; Choi, H.-G.; Youn, Y.S. Gold nanocluster-loaded hybrid albumin nanoparticles with fluorescence-based optical visualization and photothermal conversion for tumor detection/ablation. J. Control. Release 2019, 304, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, F.; Zhu, Y.; Wang, W.; Hu, J.; Liu, J.; Dai, Z.; Wang, K.; Wei, Y.; Bai, J.; et al. Salt-induced aggregation of gold nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Nanoscale 2016, 8, 4452–4457. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M.A. Iron Oxide Nanoparticles in Photothermal Therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef]
- Cabana, S.; Curcio, A.; Michel, A.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Mediated Photothermal Therapy in the Second Biological Window: A Comparative Study between Magnetite/Maghemite Nanospheres and Nanoflowers. Nanomaterials 2020, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Shao, Y.; Peng, J.; Dai, X.; Li, H.; Wu, Q.; Shi, D. Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials 2013, 34, 4078–4088. [Google Scholar] [CrossRef]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar] [CrossRef]
- Capek, I. Viral nanoparticles, noble metal decorated viruses and their nanoconjugates. Adv. Colloid Interface Sci. 2015, 222, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Culver, J.N.; Brown, A.D.; Zang, F.; Gnerlich, M.; Gerasopoulos, K.; Ghodssi, R. Plant virus directed fabrication of nanoscale materials and devices. Virology 2015, 479–480, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Q. Fabrication of Nanoarchitectures Templated by Virus-Based Nanoparticles: Strategies and Applications. Small 2014, 10, 230–245. [Google Scholar] [CrossRef]
- Liu, Z.; Qiao, J.; Niu, Z.; Wang, Q. Natural supramolecular building blocks: From virus coat proteins to viral nanoparticles. Chem. Soc. Rev. 2012, 41, 6178–6194. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, E.; Dehshahri, A.; Dehghanian, A.R.; Afsharifar, A.; Behjatnia, A.A.; Izadpanah, K.; Ahmadi, F. Enhanced anti-tumor efficacy and reduced cardiotoxicity of doxorubicin delivered in a novel plant virus nanoparticle. Colloids Surf. B Biointerfaces 2019, 174, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Franke, C.E.; Czapar, A.E.; Patel, R.B.; Steinmetz, N.F. Tobacco Mosaic Virus-Delivered Cisplatin Restores Efficacy in Platinum-Resistant Ovarian Cancer Cells. Mol. Pharm. 2018, 15, 2922–2931. [Google Scholar] [CrossRef]
- Shukla, S.; Roe, A.J.; Liu, R.; Veliz, F.A.; Commandeur, U.; Wald, D.N.; Steinmetz, N.F. Affinity of plant viral nanoparticle potato virus X (PVX) towards malignant B cells enables cancer drug delivery. Biomater. Sci. 2020, 8, 3935–3943. [Google Scholar] [CrossRef]
- Canady, M.A.; Larson, S.B.; Day, J.; McPherson, A. Crystal structure of turnip yellow mosaic virus. Nat. Struct. Biol. 1996, 3, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Barnhill, H.N.; Reuther, R.; Ferguson, P.L.; Dreher, T.; Wang, Q. Turnip Yellow Mosaic Virus as a Chemoaddressable Bionanoparticle. Bioconjugate Chem. 2007, 18, 852–859. [Google Scholar] [CrossRef]
- Zeng, Q.; Saha, S.; Lee, L.A.; Barnhill, H.; Oxsher, J.; Dreher, T.; Wang, Q. Chemoselective Modification of Turnip Yellow Mosaic Virus by Cu(I) Catalyzed Azide−Alkyne 1,3-Dipolar Cycloaddition Reaction and Its Application in Cell Binding. Bioconjugate Chem. 2011, 22, 58–66. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Jupin, I.; Decorse, P.; Lau-Truong, S.; Ammar, S.; Ha-Duong, N.-T. Assembly of gold nanoparticles using turnip yellow mosaic virus as an in-solution SERS sensor. RSC Adv. 2019, 9, 32296–32307. [Google Scholar] [CrossRef]
- Prod’homme, D.; Le Panse, S.; Drugeon, G.; Jupin, I. Detection and Subcellular Localization of the Turnip Yellow Mosaic Virus 66K Replication Protein in Infected Cells. Virology 2001, 281, 88–101. [Google Scholar] [CrossRef]
- Fiévet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Hanini, A.; Schmitt, A.; Kacem, K.; Chau, F.; Ammar, S.; Gavard, J. Evaluation of iron oxide nanoparticle biocompatibility. Int. J. Nanomed. 2011, 6, 787–794. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Girard, A.; Gehan, H.; Crut, A.; Mermet, A.; Saviot, L.; Margueritat, J. Mechanical Coupling in Gold Nanoparticles Supermolecules Revealed by Plasmon-Enhanced Ultralow Frequency Raman Spectroscopy. Nano Lett. 2016, 16, 3843–3849. [Google Scholar] [CrossRef]
- Moreira Da Silva, C.; Ortiz-Peña, N.; Boubekeur-Lecaque, L.; Dušek, J.; Moravec, T.; Alloyeau, D.; Ha-Duong, N.-T. In Situ Insights into the Nucleation and Growth Mechanisms of Gold Nanoparticles on Tobacco Mosaic Virus. Nano Lett. 2023, 23, 5281–5287. [Google Scholar] [CrossRef]
- Khuyen, H.T.; Huong, T.T.; Van, N.D.; Huong, N.T.; Vu, N.; Lien, P.T.; Nam, P.H.; Nghia, V.X. Synthesis of Multifunctional Eu(III) Complex Doped Fe3O4/Au Nanocomposite for Dual Photo-Magnetic Hyperthermia and Fluorescence Bioimaging. Molecules 2023, 28, 749. [Google Scholar] [CrossRef] [PubMed]
- Radu, T.; Iacovita, C.; Benea, D.; Turcu, R. X-Ray Photoelectron Spectroscopic Characterization of Iron Oxide Nanoparticles. Appl. Surf. Sci. 2017, 405, 337–343. [Google Scholar] [CrossRef]
- Atrei, A.; Lesiak-Orlowska, B.; Tóth, J. Magnetite nanoparticles functionalized with citrate: A surface science study by XPS and ToF-SIMS. Appl. Surf. Sci. 2022, 602, 154366. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS study of supported gold catalysts: The role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Betelu, S.; Tijunelyte, I.; Boubekeur-Lecaque, L.; Ignatiadis, I.; Ibrahim, J.; Gaboreau, S.; Berho, C.; Toury, T.; Guenin, E.; Lidgi-Guigui, N.; et al. Evidence of the Grafting Mechanisms of Diazonium Salts on Gold Nanostructures. J. Phys. Chem. C 2016, 120, 18158–18166. [Google Scholar] [CrossRef]
- Sokolov, P.; Demidov, V.; Vedeneeva, L.; Kasyanenko, N. Adsorption of 1,10-Phenanthroline-2,9-dithiol on Gold and Silicon Surfaces. J. Phys. Chem. C 2015, 119, 24358–24363. [Google Scholar] [CrossRef]
- Creczynski-Pasa, T.B.; Millone, M.A.D.; Munford, M.L.; de Lima, V.R.; Vieira, T.O.; Benitez, G.A.; Pasa, A.A.; Salvarezza, R.C.; Vela, M.E. Self-assembled dithiothreitol on Au surfaces for biological applications: Phospholipid bilayer formation. Phys. Chem. Chem. Phys. 2009, 11, 1077–1084. [Google Scholar] [CrossRef]
- Fukuto, M.; Nguyen, Q.L.; Vasilyev, O.; Mank, N.; Washington-Hughes, C.L.; Kuzmenko, I.; Checco, A.; Mao, Y.; Wang, Q.; Yang, L. Crystallization, structural diversity and anisotropy effects in 2D arrays of icosahedral viruses. Soft Matter 2013, 9, 9633–9642. [Google Scholar] [CrossRef]
- Richardson, H.H.; Carlson, M.T.; Tandler, P.J.; Hernandez, P.; Govorov, A.O. Experimental and Theoretical Studies of Light-to-Heat Conversion and Collective Heating Effects in Metal Nanoparticle Solutions. Nano Lett. 2009, 9, 1139–1146. [Google Scholar] [CrossRef]
- Shen, S.; Wang, S.; Zheng, R.; Zhu, X.; Jiang, X.; Fu, D.; Yang, W. Magnetic nanoparticle clusters for photothermal therapy with near-infrared irradiation. Biomaterials 2015, 39, 67–74. [Google Scholar] [CrossRef]
- Nemec, S.; Kralj, S.; Wilhelm, C.; Abou-Hassan, A.; Rols, M.-P.; Kolosnjaj-Tabi, J. Comparison of Iron Oxide Nanoparticles in Photothermia and Magnetic Hyperthermia: Effects of Clustering and Silica Encapsulation on Nanoparticles’ Heating Yield. Appl. Sci. 2020, 10, 7322. [Google Scholar] [CrossRef]
- Chang, D.; Lim, M.; Goos, J.A.C.M.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 931. [Google Scholar] [CrossRef]
- Espinosa, A.; Kolosnjaj-Tabi, J.; Abou-Hassan, A.; Plan Sangnier, A.; Curcio, A.; Silva, A.K.A.; Di Corato, R.; Neveu, S.; Pellegrino, T.; Liz-Marzán, L.M.; et al. Magnetic (Hyper)Thermia or Photothermia? Progressive Comparison of Iron Oxide and Gold Nanoparticles Heating in Water, in Cells, and In Vivo. Adv. Funct. Mater. 2018, 28, 1803660. [Google Scholar] [CrossRef]
- Southern, P.; Pankhurst, Q.A. Commentary on the clinical and preclinical dosage limits of interstitially administered magnetic fluids for therapeutic hyperthermia based on current practice and efficacy models. Int. J. Hyperth. 2018, 34, 671–686. [Google Scholar] [CrossRef]
- Ovejero, J.G.; Morales, I.; de la Presa, P.; Mille, N.; Carrey, J.; Garcia, M.A.; Hernando, A.; Herrasti, P. Hybrid nanoparticles for magnetic and plasmonic hyperthermia. Phys. Chem. Chem. Phys. 2018, 20, 24065–24073. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gong, M.; Fan, Y.; Feng, J.; Han, L.; Xin, H.L.; Cao, M.; Zhang, Q.; Zhang, D.; Lei, D.; et al. Collective Plasmon Coupling in Gold Nanoparticle Clusters for Highly Efficient Photothermal Therapy. ACS Nano 2022, 16, 910–920. [Google Scholar] [CrossRef]
- Hai, J.; Piraux, H.; Mazarío, E.; Volatron, J.; Ha-Duong, N.T.; Decorse, P.; Lomas, J.S.; Verbeke, P.; Ammar, S.; Wilhelm, C.; et al. Maghemite nanoparticles coated with human serum albumin: Combining targeting by the iron-acquisition pathway and potential in photothermal therapies. J. Mater. Chem. B 2017, 5, 3154–3162. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shao, L.; Ming, T.; Sun, Z.; Zhao, C.; Yang, B.; Wang, J. Understanding the Photothermal Conversion Efficiency of Gold Nanocrystals. Small 2010, 6, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Smith, D.A.; Pinchuk, A. Size-Dependent Photothermal Conversion Efficiencies of Plasmonically Heated Gold Nanoparticles. J. Phys. Chem. C 2013, 117, 27073–27080. [Google Scholar] [CrossRef]
- Nikolaou, V.; Charalambidis, G.; Coutsolelos, A.G. Photocatalytic hydrogen production of porphyrin nanostructures: Spheres vs. fibrils, a case study. Chem. Commun. 2021, 57, 4055–4058. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.-J.; Du, X.; Wen, D. Photothermal conversion characteristics of gold nanoparticle dispersions. Sol. Energy 2014, 100, 141–147. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H. A friendly Java program for material analysis using diffraction. CPD Newsl. 1999, 21, 14–15. [Google Scholar]
- Dong, Y.L.; Ren, C.; Zhang, Z.Y.; Chen, X. Protein adsorption on citrate modified magnetic nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 2598–2606. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.A.; Darwish, S.; Pham, H.N.; Ammar, S.; Ha-Duong, N.-T. Gold and Iron Oxide Nanoparticle Assemblies on Turnip Yellow Mosaic Virus for In-Solution Photothermal Experiments. Nanomaterials 2023, 13, 2509. https://doi.org/10.3390/nano13182509
Nguyen HA, Darwish S, Pham HN, Ammar S, Ha-Duong N-T. Gold and Iron Oxide Nanoparticle Assemblies on Turnip Yellow Mosaic Virus for In-Solution Photothermal Experiments. Nanomaterials. 2023; 13(18):2509. https://doi.org/10.3390/nano13182509
Chicago/Turabian StyleNguyen, Ha Anh, Sendos Darwish, Hong Nam Pham, Souad Ammar, and Nguyet-Thanh Ha-Duong. 2023. "Gold and Iron Oxide Nanoparticle Assemblies on Turnip Yellow Mosaic Virus for In-Solution Photothermal Experiments" Nanomaterials 13, no. 18: 2509. https://doi.org/10.3390/nano13182509
APA StyleNguyen, H. A., Darwish, S., Pham, H. N., Ammar, S., & Ha-Duong, N.-T. (2023). Gold and Iron Oxide Nanoparticle Assemblies on Turnip Yellow Mosaic Virus for In-Solution Photothermal Experiments. Nanomaterials, 13(18), 2509. https://doi.org/10.3390/nano13182509






