Lamivudine and Zidovudine-Loaded Nanostructures: Green Chemistry Preparation for Pediatric Oral Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lipid-Based Nanostructures
2.3. Characterization of Lipid-Based Nanostructures
2.3.1. Particle Size and Size Distributions
2.3.2. Zeta Potential
2.3.3. pH Measurements
2.3.4. Lamivudine and Zidovudine Content and Incorporation Efficiency
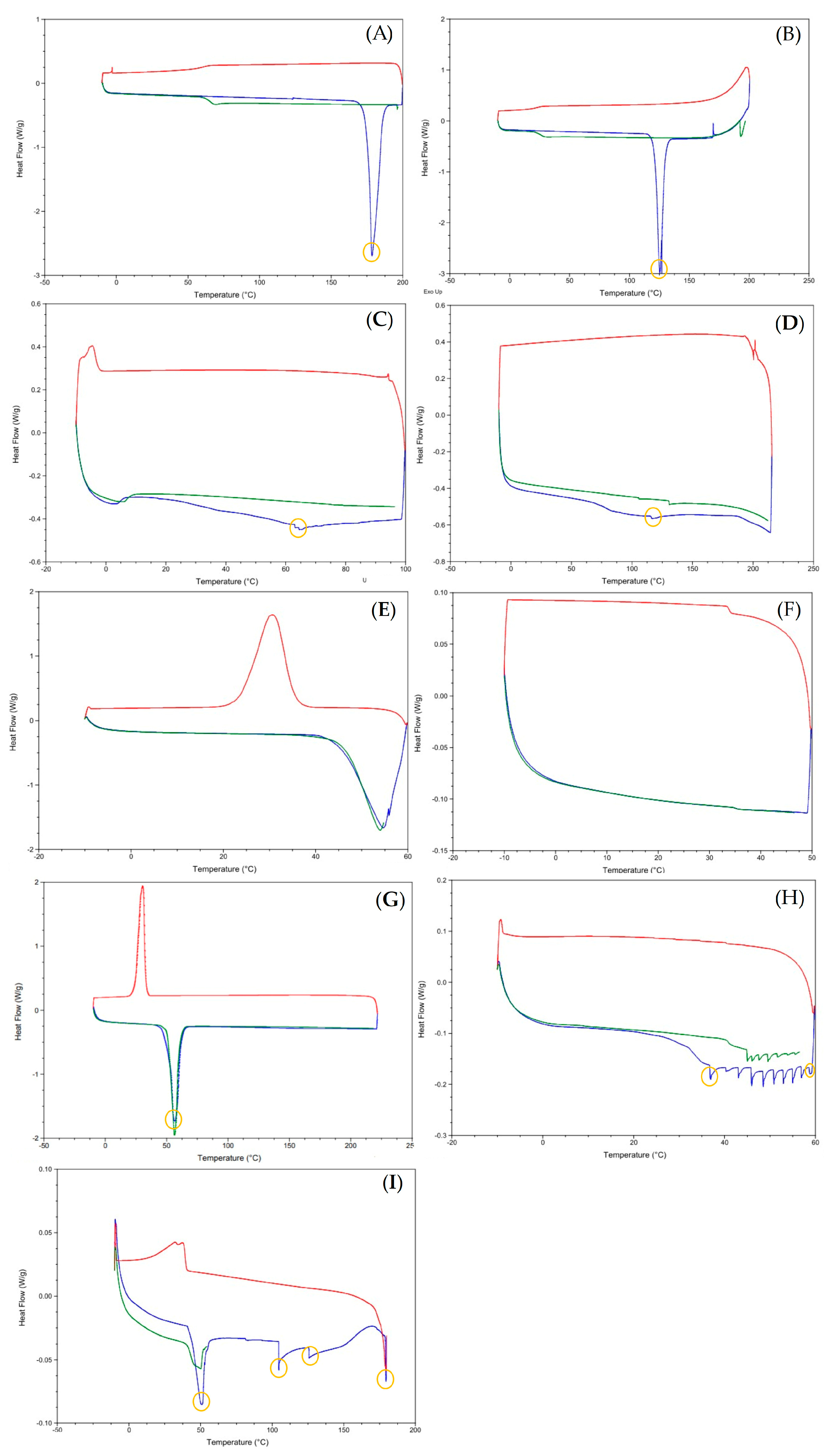
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. Small Angle X-ray Scattering Measurements (SAXS)
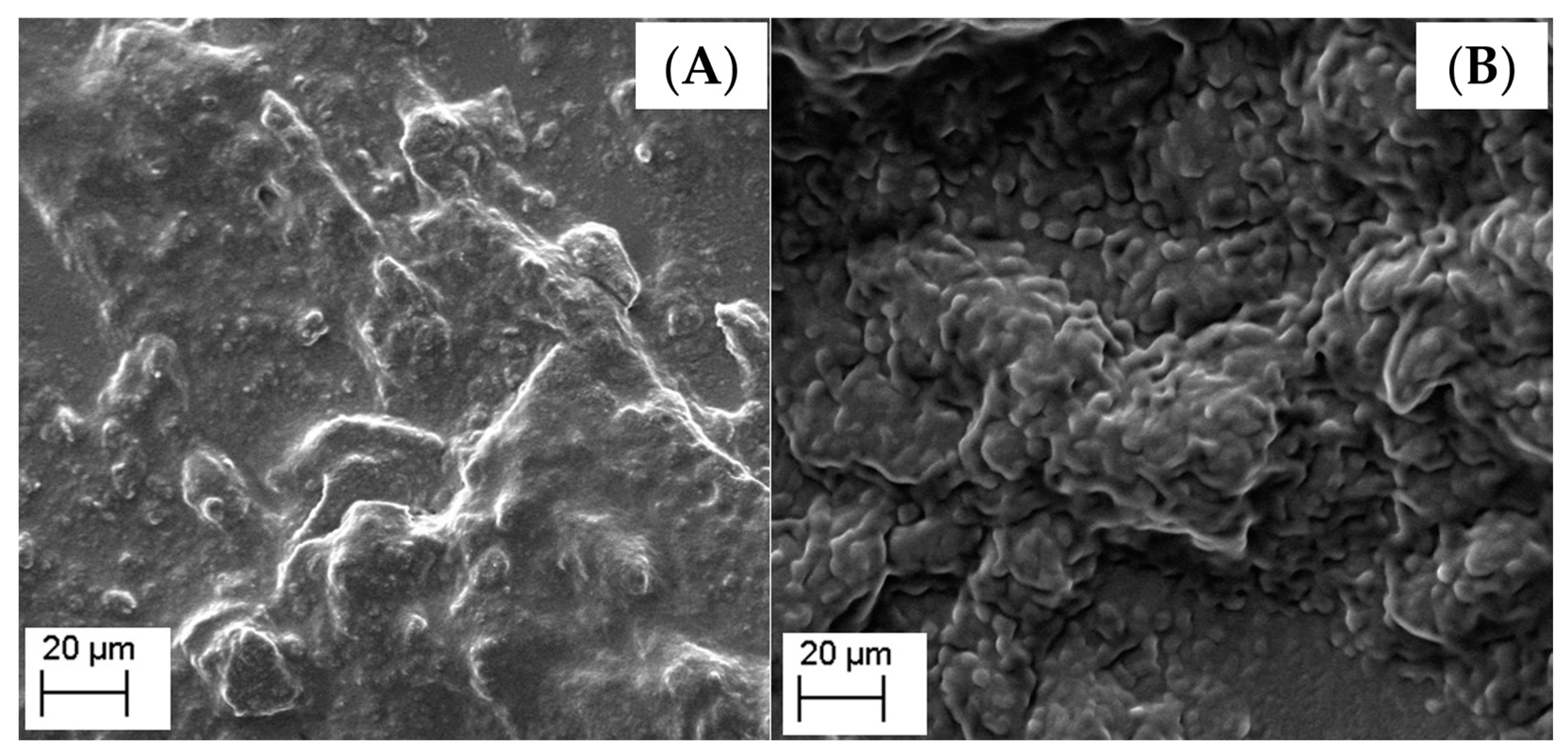
2.3.7. Scanning Electron Microscopy (SEM)
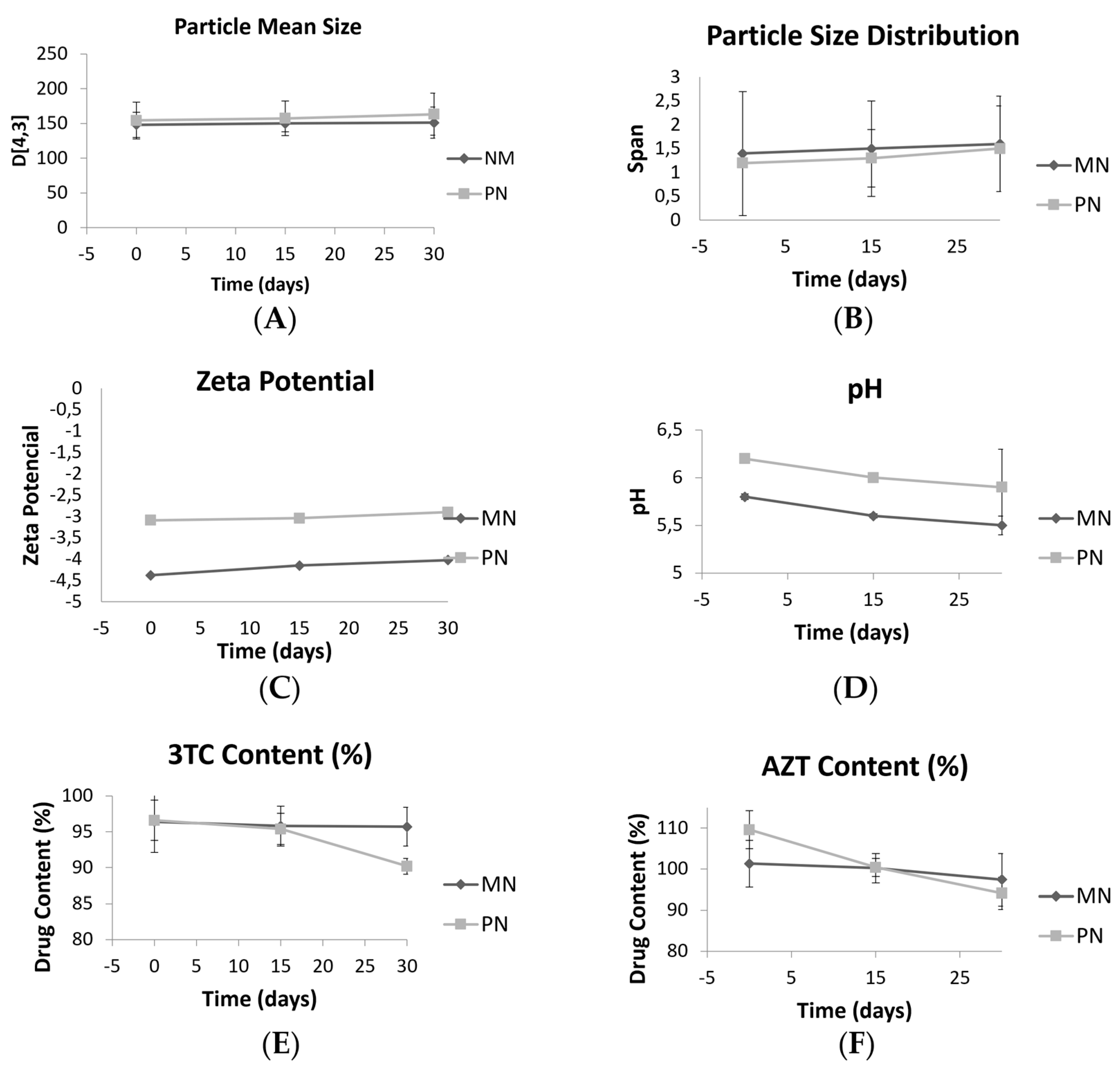
2.4. Storage Stability Studies
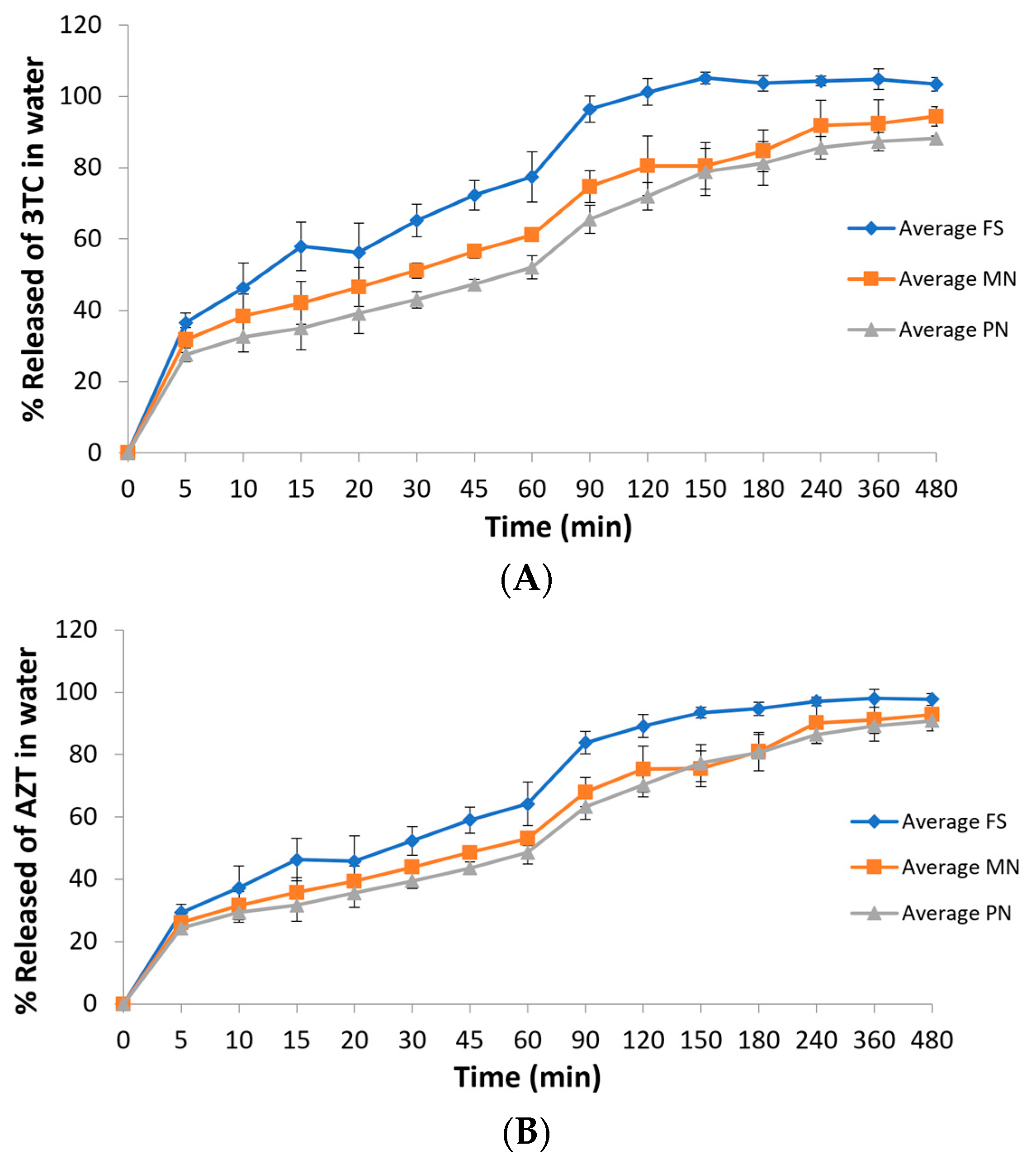
2.5. In Vitro Drug Release Profiles
2.6. Drug Degradation in Simulated Acid Medium
2.7. Mucoadhesiveness
2.8. In Vitro Taste Masking Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of the Lipid-Based Nanostructures
3.2. Storage Stability Studies
3.3. In Vitro Drug Release Profiles
3.4. Drug Degradation in Acid Medium
3.5. Mucoadhesiveness
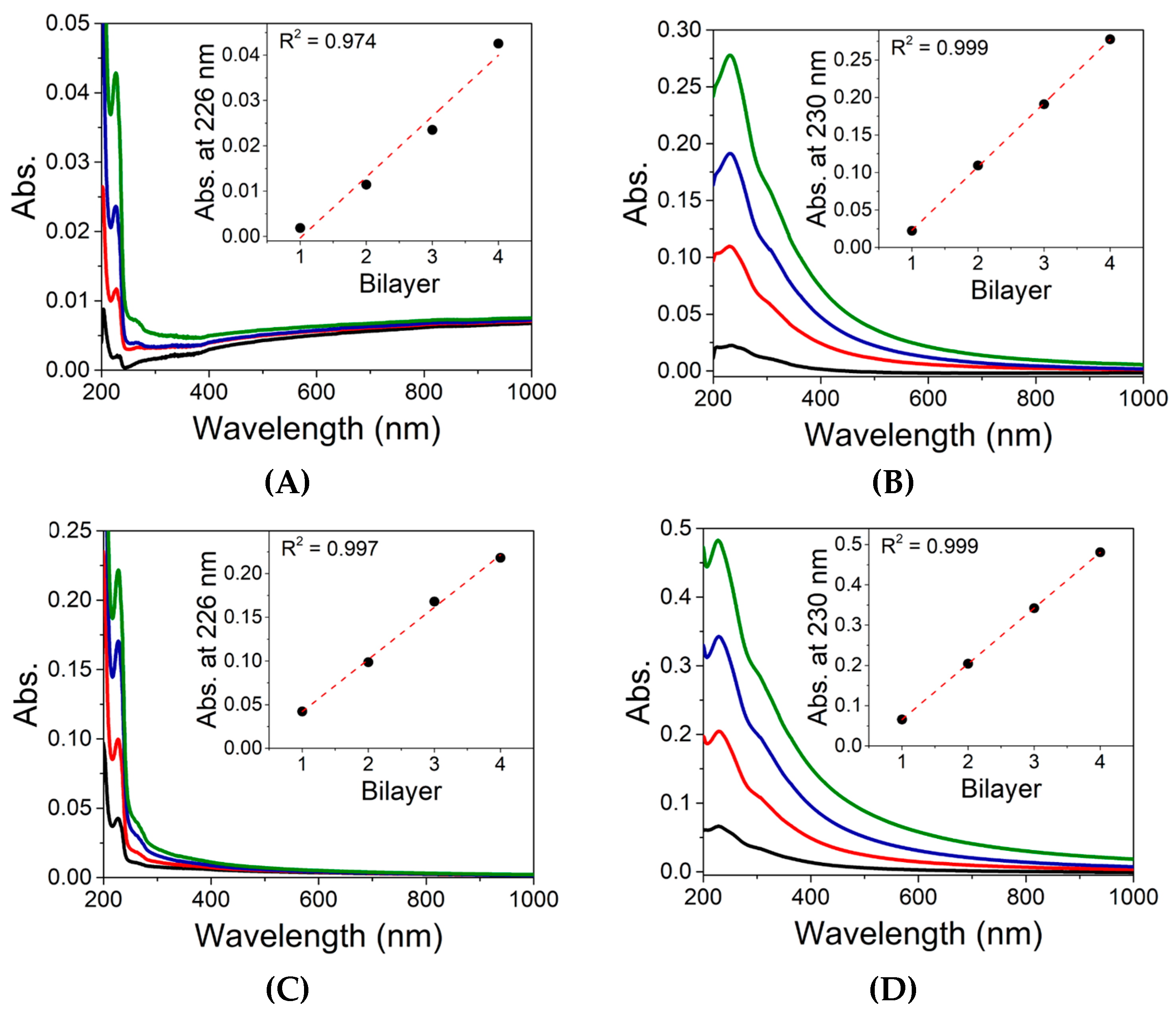
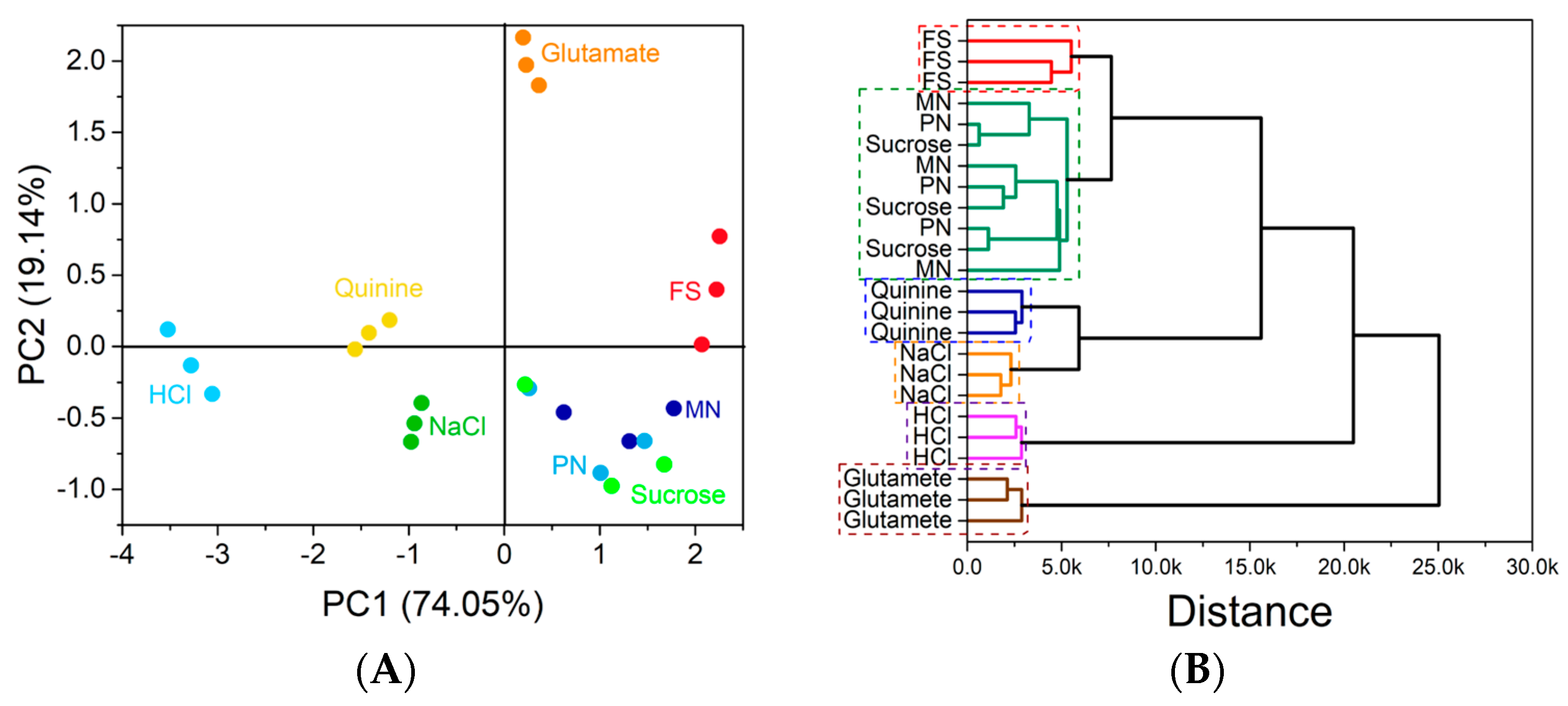
3.6. In Vitro Taste Masking Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, A.A.C.M.; Pinho, R.G.G.; de Aquino, L.M.; de Barros Perini, F.; Fonseca, F.F.; Tresse, A.S.; Pereira, G.F.M.; Avelino-Silva, V.I.; Pascom, A.R. Disparities in HIV Continuum of Care in the Paediatric Population: A Real-Life Study in Brazil. HIV Med. 2022. [Google Scholar] [CrossRef]
- Newell, M.L.; Coovadia, H.; Cortina-Borja, M.; Rollins, N.; Gaillard, P.; Dabis, F. Mortality of Infected and Uninfected Infants Born to HIV-Infected Mothers in Africa: A Pooled Analysis. Lancet 2004, 364, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- European Pregnancy and Paediatric HIV Cohort Collaboration. Safety of Zidovudine/Lamivudine Scored Tablets in Children with HIV Infection in Europe and Thailand. Eur. J. Clin. Pharmacol. 2017, 73, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.S.; Lima, L.A.; Poletto, F.; Contri, R.V.; Kulkamp Guerreiro, I.C. Nanotechnology for the Treatment of Paediatric Diseases: A Review. J. Drug. Deliv. Sci. Technol. 2022, 75, 103628. [Google Scholar] [CrossRef]
- Yih, T.C.; Al-Fandi, M. Engineered Nanoparticles as Precise Drug Delivery Systems. J. Cell. Biochem. 2006, 97, 1184–1190. [Google Scholar] [CrossRef]
- Mo, J.; Milleret, G.; Nagaraj, M. Liquid Crystal Nanoparticles for Commercial Drug Delivery. Liq. Cryst. Rev. 2017, 5, 69–85. [Google Scholar] [CrossRef]
- Ganem-Quintanar, A.; Quintanar-Guerrero, D.; Buri, P. Monoolein: A Review of the Pharmaceutical Applications. Drug Dev. Ind. Pharm. 2000, 26, 809–820. [Google Scholar] [CrossRef]
- Kulkarni, C.v.; Wachter, W.; Iglesias-Salto, G.; Engelskirchen, S.; Ahualli, S. Monoolein: A Magic Lipid? Phys. Chem. Chem. Phys. 2011, 13, 3004–3021. [Google Scholar] [CrossRef]
- Oliveira, C.; Ferreira, C.J.O.; Sousa, M.; Paris, J.L.; Gaspar, R.; Silva, B.F.B.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M.A.; Oliveira, C.; et al. A Versatile Nanocarrier—Cubosomes, Characterization and Applications. Nanomaterials 2022, 12, 2224. [Google Scholar] [CrossRef] [PubMed]
- McLain, V.C. Final Report on the Safety Assessment of Phytantriol1. Int. J. Toxicol. 2016, 26, 107–114. [Google Scholar] [CrossRef]
- Jain, S.; Yadav, P.; Swami, R.; Swarnakar, N.K.; Kushwah, V.; Katiyar, S.S. Lyotropic Liquid Crystalline Nanoparticles of Amphotericin B: Implication of Phytantriol and Glyceryl Monooleate on Bioavailability Enhancement. AAPS PharmSciTech 2018, 19, 1699–1711. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Poechlauer, P.; Broxterman, Q.B.; Yang, B.S.; Am Ende, D.; Baird, J.; Bertsch, C.; Hannah, R.E.; Dell’Orco, P.; Noorman, H.; et al. Key Green Engineering Research Areas for Sustainable Manufacturing: A Perspective from Pharmaceutical and Fine Chemicals Manufacturers. Org. Proc. Res. Dev. 2011, 15, 900–911. [Google Scholar] [CrossRef]
- Zepon, K.M.; Marques, M.S.; da Silva Paula, M.M.; Morisso, F.D.P.; Kanis, L.A. Facile, Green and Scalable Method to Produce Carrageenan-Based Hydrogel Containing in Situ Synthesized AgNPs for Application as Wound Dressing. Int. J. Biol. Macromol. 2018, 113, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bianchin, M.D.; Prebianca, G.; Immich, M.F.; Teixeira, M.L.; Colombo, M.; Koester, L.S.; de Araújo, B.V.; Poletto, F.; Külkamp-Guerreiro, I.C. Monoolein-Based Nanoparticles Containing Indinavir: A Taste-Masked Drug Delivery System. Drug. Des. Devel. 2020, 47, 83–91. [Google Scholar] [CrossRef]
- Hao, S.; Wang, B.; Wang, Y.; Zhu, L.; Wang, B.; Guo, T. Preparation of Eudragit L 100-55 Enteric Nanoparticles by a Novel Emulsion Diffusion Method. Colloids Surf B Biointerfaces 2013, 108, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, T.H.; Gorantla, S.; Makarov, E.; Lu, Y.; Warren, G.; Vinogradov, S.v. Nanogel-Conjugated Reverse Transcriptase Inhibitors and Their Combinations as Novel Antiviral Agents with Increased Efficacy against HIV-1 Infection. Mol. Pharm. 2015, 12, 4226–4236. [Google Scholar] [CrossRef] [PubMed]
- Wibel, R.; Braun, D.E.; Hämmerle, L.; Jörgensen, A.M.; Knoll, P.; Salvenmoser, W.; Steinbring, C.; Bernkop-Schnürch, A. In Vitro Investigation of Thiolated Chitosan Derivatives as Mucoadhesive Coating Materials for Solid Lipid Nanoparticles. Biomacromolecules 2021, 22, 3980–3991. [Google Scholar] [CrossRef]
- Giaretta, M.; Bianchin, M.D.; Kanis, L.A.; Contri, R.V.; Külkamp-Guerreiro, I.C. Development of Innovative Polymer-Based Matricial Nanostructures for Ritonavir Oral Administration. J. Nanomater. 2019, 2019, 8619819. [Google Scholar] [CrossRef]
- European Medicines Agency. Reflection Paper: Formulations of Choice for the Paediatric Population. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-formulations-choice-paediatric-population_en.pdf (accessed on 29 December 2022).
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.v.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-Ionic Surfactants for Stabilization of Polymeric Nanoparticles for Biomedical Uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef]
- de Morais, J.M.; dos Santos, O.D.H.; Delicato, T.; da Rocha-Filho, P.A. Characterization and Evaluation of Electrolyte Influence on Canola Oil/Water Nano-Emulsion. J. Dispers. Sci. Technol. 2007, 27, 1009–1014. [Google Scholar] [CrossRef]
- Gomes, G.S.; Frank, L.A.; Contri, R.V.; Longhi, M.S.; Pohlmann, A.R.; Guterres, S.S. Nanotechnology-Based Alternatives for the Topical Delivery of Immunosuppressive Agents in Psoriasis. Int. J. Pharm. 2023, 631, 122535. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Lakshmi, Y.S.; Kondapi, A.K. Triple Drug Combination of Zidovudine, Efavirenz and Lamivudine Loaded Lactoferrin Nanoparticles: An Effective Nano First-Line Regimen for HIV Therapy. Pharm. Res. 2017, 34, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, P.; Giorgini, E.; Adamo, F.C.; Vita, F.; Logrippo, S.; Francescangeli, O.; Pisani, M. Effects of a Cationic Surfactant Incorporation in Phytantriol Bulk Cubic Phases and Dispersions Loaded with the Anticancer Drug 5-Fluorouracil. J. Mol. Liq. 2019, 286, 110954. [Google Scholar] [CrossRef]
- Gavini, V. Combination Therapy of Lamivudine and Zidovudine Using Sterically Stabilized Liposomes: Development and Characterization. Asian J. Pharm. 2016, 9, S31–S38. [Google Scholar] [CrossRef]
- Sankar, V.; Madhura Keerthi, L. Nilaykumar Parmar Formation and In-Vitro Evaluation of Zidovudine-Lamivudine Nanoparticles. Indian J. Pharm. Educ. Res. 2012, 46, 192–196. [Google Scholar]
- Jiang, J.; Zheng, Q.; Yan, Y.; Guo, D.; Wang, F.; Wu, S.; Sun, W. Design of a Novel Nanocomposite with C-S-H@LA for Thermal Energy Storage: A Theoretical and Experimental Study. Appl. Energy 2018, 220, 395–407. [Google Scholar] [CrossRef]
- AbouSamra, M.M.; Afifi, S.M.; Galal, A.F.; Kamel, R. Rutin-Loaded Phyto-Sterosomes as a Potential Approach for the Treatment of Hepatocellular Carcinoma: In-Vitro and in-Vivo Studies. J. Drug Deliv. Sci. Technol. 2023, 79, 104015. [Google Scholar] [CrossRef]
- Sun, R.-G.; Zhang, J. Effect of Sphingomyeline and Monosialioganglioside-Gm1 on the Phase Behavior of the Super-Molecular Structures of 1,2-Dielaidoyl-Sn-Glycero-3-Phosphatidyl-Ethanolamine: Cubic Phase Im3m to Pn3m Transition in Liquid Cryst. Acta Chim. Sin. 2007, 65, 246–252. [Google Scholar]
- Muller, F.; Salonen, A.; Glatter, O. Phase Behavior of Phytantriol/Water Bicontinuous Cubic Pn3m Cubosomes Stabilized by Laponite Disc-like Particles. J. Colloid. Interface Sci. 2010, 342, 392–398. [Google Scholar] [CrossRef]
- Mori, A.; Yamamoto, E.; Kubo, K.; Ujiie, S.; Baumeister, U.; Tschierske, C. Bicontinuous Cubic Phase with the Pn3m Space Group Formed by N,N,N-Tris(5-Alkoxytroponyl)-1,5,9-Triazacyclododecanes. Liq. Cryst. 2010, 37, 1059–1065. [Google Scholar] [CrossRef]
- Briggs, J.; Caffrey, M. The Temperature-Composition Phase Diagram and Mesophase Structure Characterization of Monopentadecenoin in Water. Biophys. J. 1994, 67, 1594. [Google Scholar] [CrossRef] [PubMed]
- Barauskas, J.; Landh, T. Phase Behavior of the Phytantriol/Water System. Langmuir 2003, 19, 9562–9565. [Google Scholar] [CrossRef]
- Mariani, P.; Rustichelli, F.; Saturni, L.; Cordone, L. Stabilization of the Monoolein Pn3m Cubic Structure on Trehalose Glasses. Eur. Biophys. J. 1999, 28, 294–301. [Google Scholar] [CrossRef]
- Rappolt, M.; Gregorio, G.M.D.I.; Almgren, M.; Amenitsch, H.; Pabst, G.; Laggner, P.; Mariani, P. Non-Equilibrium Formation of the Cubic Pn3m Phase in a Monoolein/Water System. Eur. Lett. 2006, 75, 267. [Google Scholar] [CrossRef]
- Magana, J.R.; Homs, M.; Esquena, J.; Freilich, I.; Kesselman, E.; Danino, D.; Rodríguez-Abreu, C.; Solans, C. Formulating Stable Hexosome Dispersions with a Technical Grade Diglycerol-Based Surfactant. J. Colloid. Interface Sci. 2019, 550, 73–80. [Google Scholar] [CrossRef]
- Bouchemal, K.; Briançon, S.; Perrier, E.; Fessi, H. Nano-Emulsion Formulation Using Spontaneous Emulsification: Solvent, Oil and Surfactant Optimisation. Int. J. Pharm. 2004, 280, 241–251. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Nanoemulsions for Health, Food, and Cosmetics: A Review. Env. Chem. Lett. 2021, 19, 3381–3395. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, F.; Zhang, J.; Yu, L.; Zhang, G.; Liu, C.; Wang, N.; Xu, B. Development of Polyglycerol Fatty Acid Ester-Based Low-Energy Nanoemulsion for the Improvement of Curcumin Stability. J. Dispers. Sci. Technol. 2022, 43, 605–611. [Google Scholar] [CrossRef]
- Donida, B.; Tauffner, B.; Raabe, M.; Immich, M.F.; de Farias, M.A.; de Sá Coutinho, D.; Machado, A.Z.; Kessler, R.G.; Portugal, R.v.; Bernardi, A.; et al. Monoolein-Based Nanoparticles for Drug Delivery to the Central Nervous System: A Platform for Lysosomal Storage Disorder Treatment. Eur. J. Pharm. Biopharm. 2018, 133, 96–103. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Esposito, E.; Cortesi, R. Challenges in the Physical Characterization of Lipid Nanoparticles. Pharmaceutics 2021, 13, 549. [Google Scholar] [CrossRef]
- Neto, C.; Aloisi, G.; Baglioni, P.; Larsson, K. Imaging Soft Matter with the Atomic Force Microscope: Cubosomes and Hexosomes. J. Phys. Chem. B 1999, 103, 3896–3899. [Google Scholar] [CrossRef]
- Demurtas, D.; Guichard, P.; Martiel, I.; Mezzenga, R.; Hébert, C.; Sagalowicz, L. Direct Visualization of Dispersed Lipid Bicontinuous Cubic Phases by Cryo-Electron Tomography. Nat. Commun. 2015, 6, 8915. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Matsko, N.B. Chapter Three—Electron Microscopy of Pharmaceutical Systems. Adv. Imaging Electron Phys. 2014, 181, 125–208. [Google Scholar] [CrossRef]
- Rahimi, S.; Khoee, S.; Ghandi, M. Development of Photo and PH Dual Crosslinked Coumarin-Containing Chitosan Nanoparticles for Controlled Drug Release. Carbohydr. Polym. 2018, 201, 236–245. [Google Scholar] [CrossRef]
- Umeyor, C.; Attama, A.; Uronnachi, E.; Kenechukwu, F.; Nwakile, C.; Nzekwe, I.; Okoye, E.; Esimone, C. Formulation Design and in Vitro Physicochemical Characterization of Surface Modified Self-Nanoemulsifying Formulations (SNEFs) of Gentamicin. Int. J. Pharm. 2016, 497, 161–198. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.J.; Holkar, C.R.; Karekar, S.E.; Pinjari, D.v.; Pandit, A.B. Ultrasound Assisted Manufacturing of Paraffin Wax Nanoemulsions: Process Optimization. Ultrason. Sonochem. 2015, 23, 201–207. [Google Scholar] [CrossRef]
- Divsalar, A.; Saboury, A.A.; Nabiuni, M.; Zare, Z.; Kefayati, M.E.; Seyedarabi, A. Characterization and Side Effect Analysis of a Newly Designed Nanoemulsion Targeting Human Serum Albumin for Drug Delivery. Colloids Surf. B Biointerfaces 2012, 98, 80–84. [Google Scholar] [CrossRef]
- Katzer, T.; Chaves, P.; Bernardi, A.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Castor Oil and Mineral Oil Nanoemulsion: Development and Compatibility with a Soft Contact Lens. Pharm. Dev. Technol. 2014, 19, 232–237. [Google Scholar] [CrossRef]
- Farmacopéia Brasileira, 6th ed.; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2019.
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef]
- Lopes, C.M.; Lobo, J.M.S.; Costa, P. Formas Farmacêuticas de Liberação Modificada: Polímeros Hidrifílicos. Rev. Bras. De Ciências Farm. 2005, 41, 143–154. [Google Scholar] [CrossRef]
- De Lyra, M.A.M.; Soares-Sobrinho, J.L.; Brasileiro, M.T.; De, M.F.; Roca, L.; Barraza, J.A.; Viana, O.D.S.; Rolim-Neto, P.J. Sistemas Matriciais Hidrofílicos e Mucoadesivos Para Liberação Controlada de Fármacos. Lat. Am. J. Pharm. 2007, 26, 784–793. [Google Scholar]
- Valadares, C.P.; Pinto Silva, R.A.; Tavares, W.C.; Duarte, M.A. Apresentação Da Técnica de Estudo Do Tempo de Esvaziamento Gástrico Por Meio Da Ultra-Sonografia. Radiol. Bras. 2006, 39, 15–18. [Google Scholar] [CrossRef]
- Avachat, A.M.; Parpani, S.S. Formulation and Development of Bicontinuous Nanostructured Liquid Crystalline Particles of Efavirenz. Colloids Surf B Biointerfaces 2015, 126, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Teubl, B.J.; Meindl, C.; Eitzlmayr, A.; Zimmer, A.; Fröhlich, E.; Roblegg, E. In-Vitro Permeability of Neutral Polystyrene Particles via Buccal Mucosa. Small 2013, 9, 457–466. [Google Scholar] [CrossRef]
- Sosnik, A.; Imperiale, J.C.; Vázquez-González, B.; Raskin, M.M.; Muñoz-Muñoz, F.; Burillo, G.; Cedillo, G.; Bucio, E. Mucoadhesive Thermo-Responsive Chitosan-g-Poly(N-Isopropylacrylamide) Polymeric Micelles via a One-Pot Gamma-Radiation-Assisted Pathway. Colloids Surf. B Biointerfaces 2015, 136, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Eliyahu, S.; Aharon, A.; Bianco-Peled, H. Acrylated Chitosan Nanoparticles with Enhanced Mucoadhesion. Polymers 2018, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Grießinger, J.; Dünnhaupt, S.; Cattoz, B.; Griffiths, P.; Oh, S.; Gómez, S.B.I.; Wilcox, M.; Pearson, J.; Gumbleton, M.; Abdulkarim, M.; et al. Methods to Determine the Interactions of Micro- and Nanoparticles with Mucus. Eur. J. Pharm. Biopharm. 2015, 96, 464–476. [Google Scholar] [CrossRef]
- Sosnik, A.; Augustine, R. Challenges in Oral Drug Delivery of Antiretrovirals and the Innovative Strategies to Overcome Them. Adv. Drug Deliv. Rev. 2016, 103, 105–120. [Google Scholar] [CrossRef]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A Promising Approach in Drug Delivery System. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Escuder-Gilabert, L.; Peris, M. Review: Highlights in Recent Applications of Electronic Tongues in Food Analysis. Anal. Chim. Acta 2010, 665, 15–25. [Google Scholar] [CrossRef]
- Riul, A.; Dantas, C.A.R.; Miyazaki, C.M.; Oliveira, O.N. Recent Advances in Electronic Tongues. Analyst 2010, 135, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Daikuzono, C.M.; Dantas, C.A.R.; Volpati, D.; Constantino, C.J.L.; Piazzetta, M.H.O.; Gobbi, A.L.; Taylor, D.M.; Oliveira, O.N.; Riul, A. Microfluidic Electronic Tongue. Sens. Actuators B Chem. 2015, 207, 1129–1135. [Google Scholar] [CrossRef]
- Manzoli, A.; Shimizu, F.M.; Mercante, L.A.; Paris, E.C.; Oliveira, O.N.; Correa, D.S.; Mattoso, L.H.C. Layer-by-Layer Fabrication of AgCl–PANI Hybrid Nanocomposite Films for Electronic Tongues. Phys. Chem. Chem. Phys. 2014, 16, 24275–24281. [Google Scholar] [CrossRef] [PubMed]
- Borato, C.E.; Riul, A.; Ferreira, M.; Oliveira, O.N.; Mattoso, L.H.C. Exploiting the Versatility of Taste Sensors Based on Impedance Spectroscopy. Instrum. Sci. Technol. 2007, 32, 21–30. [Google Scholar] [CrossRef]
- Riul, A.; Malmegrim, R.R.; Fonseca, F.J.; Mattoso, L.H.C. Nano-Assembled Films for Taste Sensor Application. Artif. Organs 2003, 27, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Riul, A.; dos Santos, D.S.; Wohnrath, K.; di Tommazo, R.; Carvalho, A.C.P.L.F.; Fonseca, F.J.; Oliveira, O.N.; Taylor, D.M.; Mattoso, L.H.C. Artificial Taste Sensor: Efficient Combination of Sensors Made from Langmuir−Blodgett Films of Conducting Polymers and a Ruthenium Complex and Self-Assembled Films of an Azobenzene-Containing Polymer. Langmuir 2001, 18, 239–245. [Google Scholar] [CrossRef]
| Formulation | Monoolein | Phytantriol | Poloxamer 407 | Zidovudine | Lamivudine |
|---|---|---|---|---|---|
| MN | 2 g | - | 1 g | 20 mg | 10 mg |
| PN | - | 2 g | 1 g | 20 mg | 10 mg |
| Monoolein-Based Nanostructures | Phytantriol-Based Nanostructures | |||
|---|---|---|---|---|
| Ultrasonication Time (min) | D[4,3] (v) (nm) | Span (v) | D[4,3] (v) | Span (v) |
| 5 | 288 ± 62 | 3.0 ± 0.6 | 1253 ± 389 | 8.8 ± 0.5 |
| 10 | 191 ± 32 | 1.9 ± 0.4 | 563 ± 15 | 7.6 ± 1.1 |
| 15 | 148 ±18 | 1.4 ± 1.3 | 437 ± 42 | 6.4 ± 0.8 |
| 20 | ND | ND | 259 ± 53 | 3.1 ± 1.7 |
| 25 | ND | ND | 171 ± 35 | 1.4 ± 0.4 |
| 30 | ND | ND | 154 ± 27 | 1.2 ± 0.1 |
| Formulation | Drug Analyzed | AUC (In Vitro Release Profile) |
|---|---|---|
| FS | Lamivudine | 46,539 ± 1411 a |
| MN | Lamivudine | 39,590 ± 2548 b |
| PN | Lamivudine | 36,743 ± 1380 b |
| FS | Zidovudine | 42,437 ± 692 a |
| MN | Zidovudine | 38,016 ± 2545 b |
| PN | Zidovudine | 36,763 ± 1297 b |
| Formulation | Drug analyzed | Drug Degradation in Acid Medium (%) |
|---|---|---|
| FS | Lamivudine | 18.2 ± 2.7a |
| MN | Lamivudine | 15.0 ± 6.8a |
| PN | Lamivudine | 6.3 ± 5.4b |
| FS | Zidovudine | 18.0 ± 3.1a |
| MN | Zidovudine | 13.6 ± 8.7a |
| PN | Zidovudine | 4.9 ± 6.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedes, M.D.V.; Marques, M.S.; Berlitz, S.J.; Facure, M.H.M.; Correa, D.S.; Steffens, C.; Contri, R.V.; Külkamp-Guerreiro, I.C. Lamivudine and Zidovudine-Loaded Nanostructures: Green Chemistry Preparation for Pediatric Oral Administration. Nanomaterials 2023, 13, 770. https://doi.org/10.3390/nano13040770
Guedes MDV, Marques MS, Berlitz SJ, Facure MHM, Correa DS, Steffens C, Contri RV, Külkamp-Guerreiro IC. Lamivudine and Zidovudine-Loaded Nanostructures: Green Chemistry Preparation for Pediatric Oral Administration. Nanomaterials. 2023; 13(4):770. https://doi.org/10.3390/nano13040770
Chicago/Turabian StyleGuedes, Marina D. V., Morgana S. Marques, Simone J. Berlitz, Murilo H. M. Facure, Daniel S. Correa, Clarice Steffens, Renata V. Contri, and Irene C. Külkamp-Guerreiro. 2023. "Lamivudine and Zidovudine-Loaded Nanostructures: Green Chemistry Preparation for Pediatric Oral Administration" Nanomaterials 13, no. 4: 770. https://doi.org/10.3390/nano13040770
APA StyleGuedes, M. D. V., Marques, M. S., Berlitz, S. J., Facure, M. H. M., Correa, D. S., Steffens, C., Contri, R. V., & Külkamp-Guerreiro, I. C. (2023). Lamivudine and Zidovudine-Loaded Nanostructures: Green Chemistry Preparation for Pediatric Oral Administration. Nanomaterials, 13(4), 770. https://doi.org/10.3390/nano13040770









