1. Introduction
Nowadays, the maxillary sinus lift procedure is commonly used to increase the amount of hard tissue in the posterior region of the upper jaw before dental implants are placed. The need for this procedure arises from the phenomenon of sinus pneumatization, which decreases the amount of vertical bone available. The traditional approach to elevating the maxillary sinus involves making an incision in the lateral wall of the maxilla, then lifting the sinus membrane and inserting bone graft under direct visualization [
1].
There are several materials that can be used for the maxillary sinus lift procedure, each with its own advantages and disadvantages [
2]. Autogenous bone grafts taken from the patient’s own body are considered the gold standard for bone grafting. They have a high success rate and promote natural bone growth. However, they require a second surgical site for harvesting, which can increase the patient’s discomfort and recovery time [
3]. Donor allografts, usually from a cadaver, have the advantage of not requiring a second surgical site. They are available in different forms, such as demineralized bone matrix (DBM) or freeze-dried bone allograft (FDBA). However, the success rate of allografts may be lower than that of autogenous grafts, and there is a risk of disease transmission [
4]. Xenografts are bone materials derived from a different species, typically bovine or porcine, that are processed to remove all organic matter. They have the advantage of not requiring a second surgical site and are readily available. However, they may not be as effective as autogenous grafts and carry a small risk of an immune reaction [
5]. The most successful materials used nowadays for sinus lift procedures are synthetic bone substitutes that mimic the structure and composition of bone. They have the advantage of being readily available and not requiring a second surgical site while also eliminating the risk of disease transmission [
6]. However, their success rate may be lower than that of autogenous grafts, and their long-term stability and integration with natural bone may be a concern.
Taking into account the advantages of synthetic bone grafts, different types of such materials are used in clinical practice. Polymers, such as poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL), are biocompatible and biodegradable and can be designed to have different mechanical and chemical properties [
7]. However, their osteoconductivity may be limited, and they may not provide sufficient long-term stability [
8]. Bioactive glass, calcium aluminate, and calcium silicate, as bioceramics, are used due to their relatively high biocompatibility and osteoconductivity, as well as being able to promote bone regeneration and angiogenesis [
9]. Unfortunately, due to their low mechanical properties, their application is limited [
10]. Calcium sulfate-based material, e.g., calcium sulfate hemihydrate (CSH), in addition to biocompatibility and osteoconductivity, can be resorbed and replaced by new bone over time. However, they have a relatively short resorption time and may not provide sufficient long-term stability [
11]. The most commonly used materials in clinical practice are calcium phosphate-based materials, including hydroxyapatite (HA) and tricalcium phosphate (TCP), which are both naturally occurring minerals found in bone [
12]. Mimicking the natural bone mineral matrix, they are highly osteoconductive and osteoinductive, but HA has a higher crystallinity and slower resorption rate, while TCP has a lower crystallinity and faster resorption rate [
13].
In current research, we are investigating novel materials that combine both HA and TCP in one porous graft that can improve graft degradation and osteointegration and provide positive clinical outcomes. The formation of new bone under the influence of transplants is a key goal of sinus lifting and can be achieved through osteogenesis, osteoinduction, and osteoconduction. Osteogenesis occurs in autotransplants by providing a framework, growth factors for osteogenesis, and cells that produce bone matrix. The mechanism of osteoinduction is characteristic of autogenous bone, allogeneic bone, and xenotransplants and involves differentiation of osteogenic cells in response to osteoinductors—bone morphogenetic proteins (BMP) or other growth factors. The mechanism of osteoconduction (osteoconductivity), which involves the use of three-dimensional biological scaffolds, is based on the formation of new bone over the transplant, with the graft potentially undergoing revascularization and incorporation into new bone tissue [
14].
The clinical use of any biological materials requires compliance with key requirements for biomaterials and evidence of their safety and effectiveness. Among the mandatory requirements for the use of biological materials from bone tissue are several factors [
15]: Firstly, scaffolds must be three-dimensional and have sufficient surface area for interaction with cells and tissues in the area of use. Secondly, the material must be porous and have connections between pores. This requirement is a prerequisite for ensuring cell adhesion, migration, and proliferation of bone cells in the appropriate direction. Effective cell adhesion to the biomaterial and structural anisotropy also affect further cell orientation and cell–matrix interactions. Thirdly, the biomaterial must be non-toxic and biodegradable. However, in doing so, the biomaterial samples must have appropriate mechanical properties, and these characteristics must be comparable to the strength of cortical bone [
6].
However, questions of the immunogenicity of biomaterials deserve special attention. Regardless of the specificity of the bone transplant used for augmentation, their implementation is accompanied by an immunological response to foreign substances. It has been shown that, despite careful processing, allogeneic bone retains potential antigenicity, which induces T cell-mediated immune responses against the allograft [
16]. This is due to the presence of molecules of the major histocompatibility complex (MHC) in allogeneic bone blocks. It is important to note that the immune system’s response to bone transplants is complex and involves the participation of cells such as T lymphocytes, B lymphocytes, and macrophages.
It is important to emphasize that immune cells provide not only an inflammatory response to foreign material. There is a close relationship between the metabolism of bone tissue and the immune system. Activated T cells can influence bone resorption and osteogenesis through the action of interferon gamma (IF-gamma) or interleukin 17 (IL-17) [
17]. RANKL also plays an important role, which binds to the receptor activator of nuclear factor kappa-B (RANK) on osteoclast precursors and induces osteoclastogenesis with subsequent resorption of bone tissue [
18].
Under physiological conditions, most macrophages demonstrate the M2 phenotype, which helps maintain tissue homeostasis [
19]. Both resident M2 and inflammatory M1 macrophages can affect bone formation. Osteoclasts are traditionally considered resident macrophages in bone. In recent years, a large population of macrophages that constantly reside in bones has been identified. These macrophages are called osteomacs, which can provide pro-anabolic support to osteoblasts and promote bone formation [
20]. In the context of implant procedures, macrophages mediate both reparative processes and inflammatory responses to implanted biomaterials [
21]. Implants made of bionanomaterials induce the polarization of M1 macrophages, leading to an inflammatory response to foreign bodies and granuloma formation. The mechanisms of the influence of different bionanomaterials on integration, remodeling, and immune response after augmentation procedures are still the subject of active research [
22]. Only a small number of studies have provided actual information regarding the tissue response of the periodontal and specific structural composition of the sinus augmentation zone using allogeneic bionanomaterials. Histological evaluation of the bone healing response after the transplantation of different types of bone bionanomaterials in the human body will facilitate the use of bone graft nanomaterial by the surgeon and allow the establishment of the implant healing period according to the patient’s clinical situation. In addition, an assessment of the immune, angiogenic, and osteogenic cell responses to the novel bone grafting material will decipher the mechanisms of the morphogenetic effects of the bone bionanomaterial.
In the current research, we demonstrated a complete circle, from development to clinical application, of novel calcium deficient hydroxyapatite (HA and β-TCP mixture) with a detailed focus on tissue immune reaction and bone remodeling.
2. Materials and Methods
2.1. Materials
Calcium chloride anhydrous (CaCl2), sodium phosphate monobasic anhydrous (NaH2PO4), and sodium hydroxide (NaOH) were purchased from Pol-Aura (Warszawa, Poland), and sodium bicarbonate (NaHCO3) was purchased from Thermo Fisher Scientific (UK). The amount of pure substance was more than 99.5%. Sodium alginate was purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, P.R. China). The reactants were used as received without further purification. All media and chemicals for cell culture experiments and histological evaluation were purchased from Sigma-Aldrich (Darmstadt, Germany) and were used as received.
2.2. Bioactive Graft Synthesis
In our study, calcium deficient hydroxyapatite was produced by wet precipitation. The synthesis was carried out under the control of stirring, addition rate, pH, and temperature by the following reaction:
Two different solutions were prepared separately:
Solution 1.
CaCl2 solution (0.09 M) was prepared by dissolving an appropriate amount of CaCl2 in distilled water. The solution was heated under stirring up to 80 °C. Sodium bicarbonate (NaHCO3) was applied in an amount of 0.01 M as a reactant introducing CO32− groups.
Solution 2.
NaH2PO4 solution (0.05 M) was prepared. Then, the second solution was added dropwise to the first solution to obtain calcium deficient hydroxyapatite under stirring and heating up to 80 °C for 2 h. During synthesis, the pH of the reaction medium was stabilized at >11 using sodium hydroxide solution. After 2 h, the pH was decreased to 9. The suspension was aged for 24 h at room temperature. The top solution was removed by decantation. The resultant precipitate was washed three times with deionized water until the solution pH became 7, and then it was used as a slurry.
The obtained calcium deficient hydroxyapatite slurry was mixed with 3% sodium alginate water solution in a relation of 3:1. The obtained mixture was added dropwise to the 0.1 M CaCl2 solution to obtain granules of HA in an alginate shell. They were frozen at −80 °C overnight followed by drying at 60 °C. The obtained samples were calcined at 900 °C to obtain a mixture of HA and β-TCP and remove the organic phase. The dried samples were ground into fine powders and used for characterization studies.
2.3. Bioactive Graft Characterization
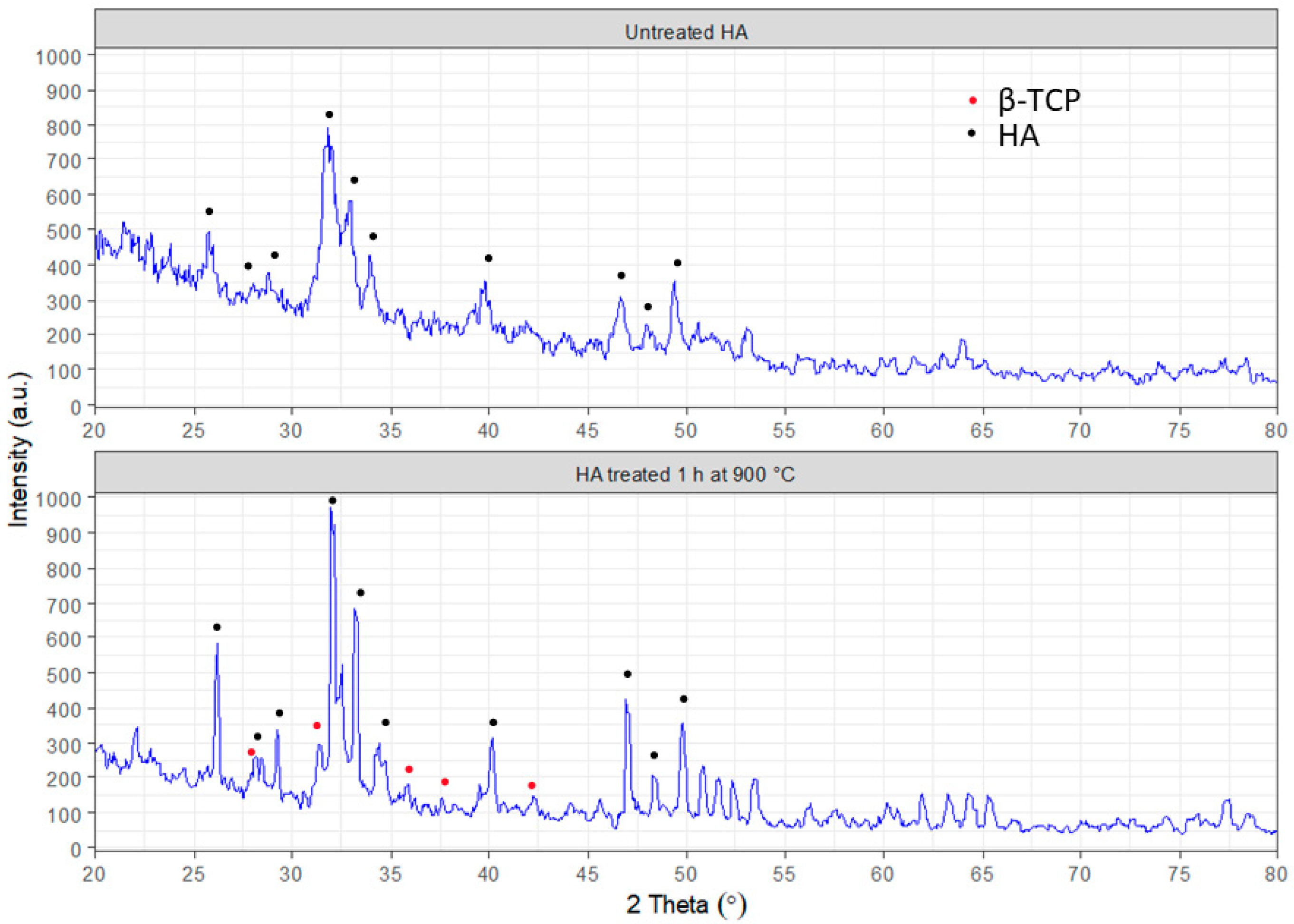
The morphology analysis of the obtained hydroxyapatite was performed with scanning electron microscopy (SEO-SEM Inspect S50-B) using an energy dispersive spectrometer AZtecOne with detector X-MaxN20 (Oxford Instruments plc, Abingdon, UK). The X-ray diffraction (XRD) analysis was carried out using an X-ray diffractometer DRON-3M (Bourevestnik, Saint-Petersburg, Russia) connected to a computer-aided system for experimental control and data processing. CuKα radiation was used (wavelength 0.154 nm) with the Bragg–Brentano focusing method. The current and voltage of the X-ray tube were 20 mA and 40 kV, respectively. The scan was performed in a continuous registration mode with a 0.02° step and 1°/min scan speed in a 2 θ range of 20–80°. All experimental data were processed using the DifWin-1 program package (Etalon-TC, Lubertsi, Russia). Phase identification was performed using the JCPDS (Joint Committee on Powder Diffraction Standards) card catalog [
23].
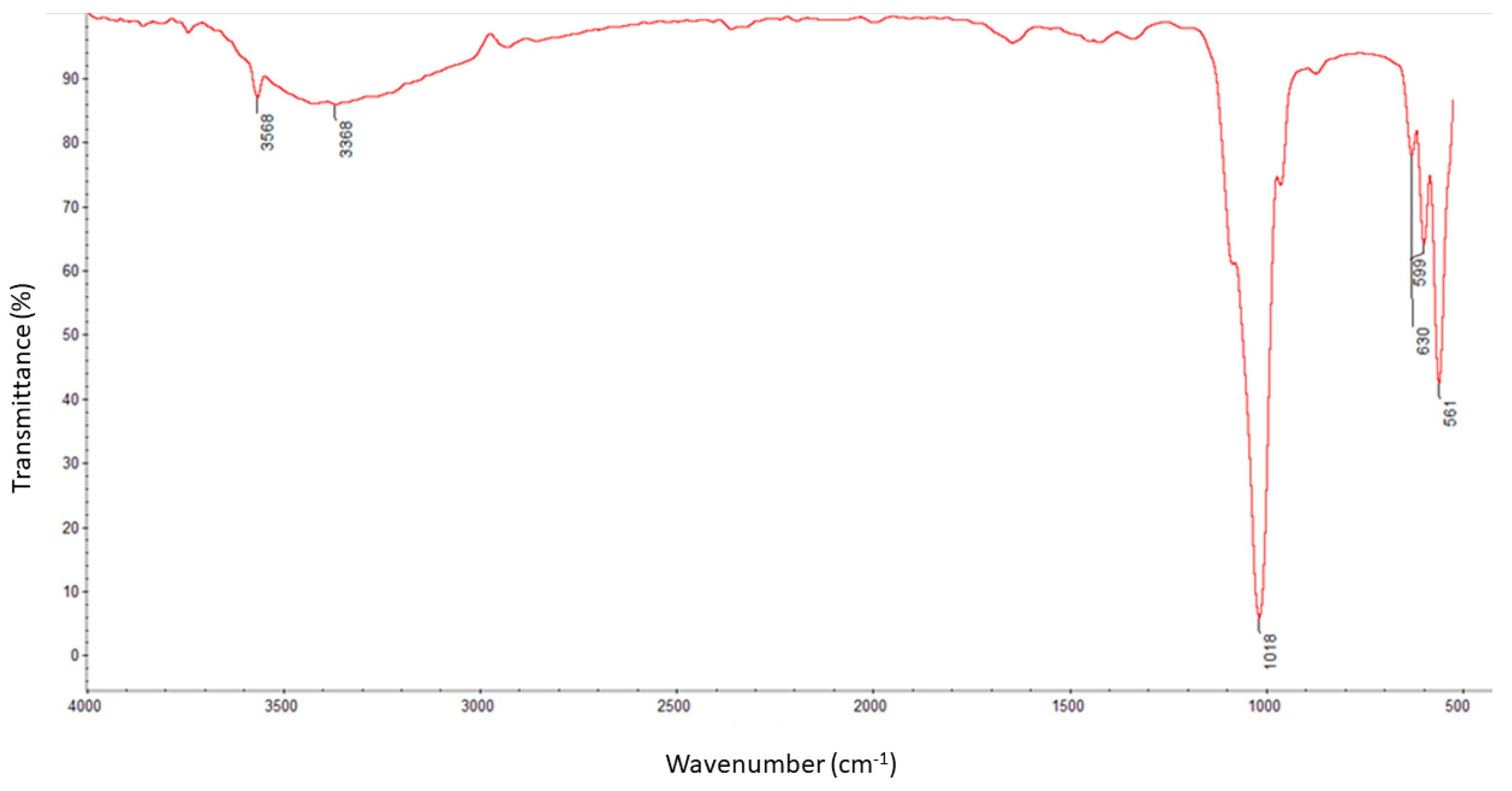
The molecule structural components were identified using the Fourier transform infrared spectroscopy method with a ThermoNicolet Nexus 470 apparatus purchased from Thermo Fisher Scientific (Waltham, MA, USA) equipped with an ATR adapter. Measurements and analysis of spectra were carried out with the use of software attached to the device. The spectra were recorded in the spectral range of 550–4000 cm
−1 with a nominal resolution of 4 cm
−1 and 32 scans for each measurement. All samples were dried before analysis [
23].
2.4. Biocompatibility Assessment
Primary osteoblasts (Passage 4) obtained from the collection of the Biomedical Research Center were used to assess the biocompatibility of as-synthesized hydroxyapatite. Before the experiment, cells were cultured in Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 (DMEM, Gibco, MA, USA) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin B (Gibco, MA, USA) under conditions of 37 °C and 5% CO
2. HA powder (100 mg) was placed on the bottom of a 96-well plate, and osteoblasts at a density of 4 × 10
4 cells/cm
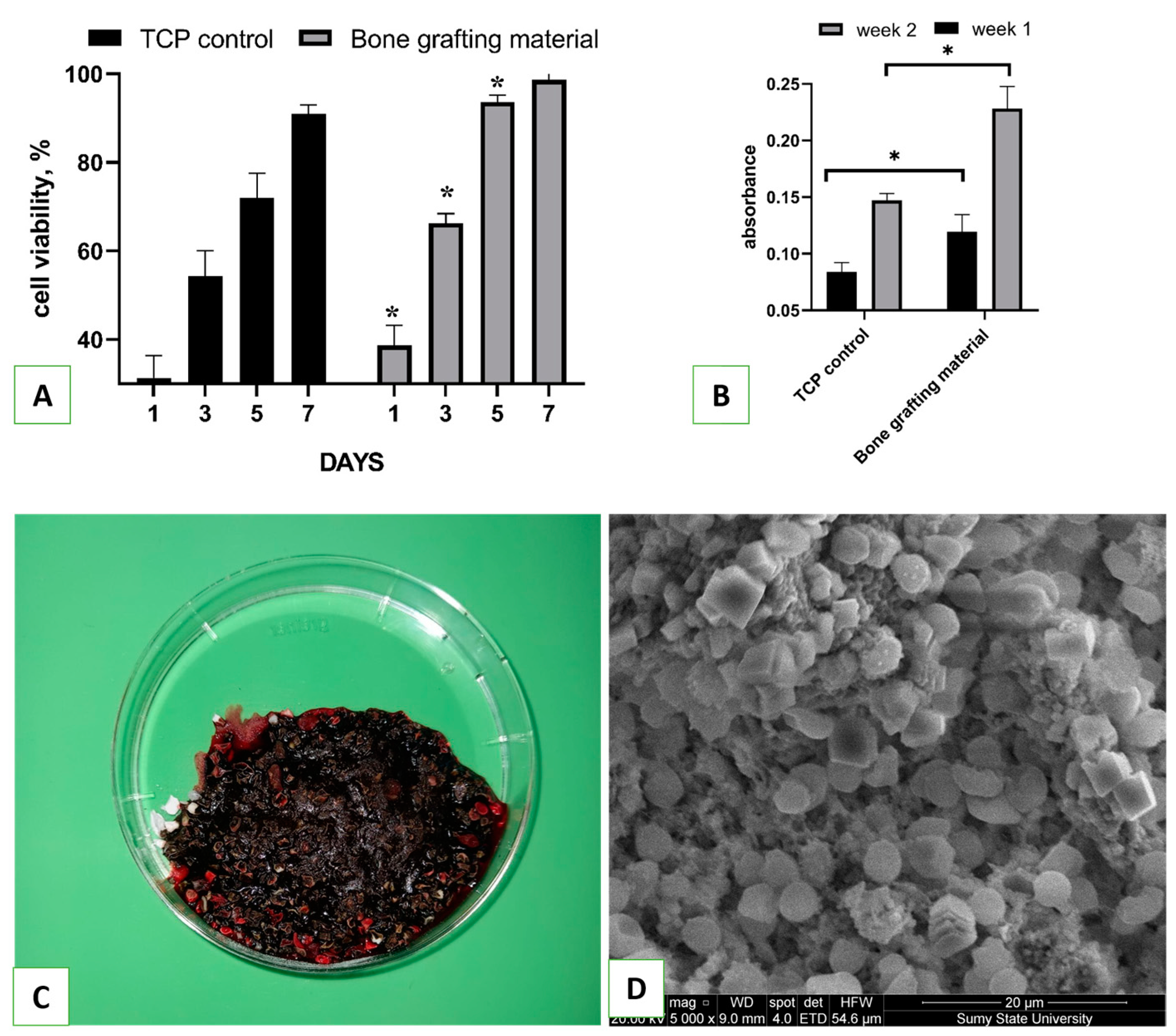
2 were seeded on over the material. The biocompatibility of the bone grafting material was evaluated using a resazurin reduction assay, as described elsewhere, on days 1, 3, 5, and 7 [
24]. Cell proliferation on the tissue culture plate (TCP) was used as a positive control. For that, resazurin was added to the cells at a 15 µg/mL final concentration and incubated for 8 h. One hundred microliters of the medium was then transferred to another 96-well plate, and the optical density (absorbance) was measured using a Multiskan FC plate reader (Thermo Fisher Scientific, Waltham, MA, USA) at 570 and 595 nm. The results were quantified using a formula from the Method for Measuring Cytotoxicity or Proliferation Using Alamar Blue by Spectrophotometry (Bio-Rad Laboratories, Hercules, CA, USA).
Collagen, which was synthesized by osteoblast cells and accumulated on samples, was detected through staining with Sirius Red dye. The staining was performed as follows [
25]: Cells were seeded on over the material at a cell density of 10⁴ cells per well, and on the 7th and 14th days of incubation, the samples were transferred to another 24-well plate and washed 3 times with ice-cold PBS (40 °C). Then, 1.5 mL of Bouin’s solution was added to each well for 1 h at room temperature. After the solution was removed, the samples were rinsed with cold tap water and dried in a fume hood overnight. On the next day, 1.5 mL of Sirius Red dye was added to the samples for 1 h, then removed, and each well was washed 4 times with 0.01 M HCl. NaOH solution (1 mL of 0.1 M) was added to each well in order to recover the bound dye. The plate was placed on a shaker for 30 min, after which 100 µL of eluted dye from each well was transferred to a 96-well plate, and the absorbance was measured using a Multiskan FC (Thermo Fisher Scientific, Waltham, MA, USA) plate reader at a wavelength of 570 nm.
2.5. Blood Interaction Test
A total of 4.0 g of bone grafting nanomaterial was weighed and placed in six Petri dishes with a diameter of 6 cm. In order to control the speed of blood clotting, six additional empty Petri dishes were prepared. Whole venous blood (60 mL) was collected from a male volunteer who provided consent for the study. Prior to conducting the study, the Ethics Committee on Medical Research of the Medical Institute of Sumy State University approved the protocol. Next, 5 mL of blood was immediately added to each dish containing the bone grafting nanomaterial. The samples were gently stirred with a glass rod to ensure even distribution of the blood. A timer was started as soon as the blood was added, and it was stopped once a clot had formed. For scanning electron microscopy (SEM) analysis, bone grafting nanomaterial with coagulated blood weighing approximately 0.5 g was fixed in a 2.5% glutaraldehyde solution and then dehydrated in alcohols of increasing concentration for 24 h. Once dry, the samples were covered with a 30–50 nm layer of silver using a vacuum set-up VUP-5M (SELMI, Sumy, Ukraine). The SEM images of the blood clot on the hydroxyapatite were captured using an FEI Inspect S50B (FEI, Brno, Czech Republic) with an Everhart–Thornley secondary electron detector.
2.6. Animal Experiment
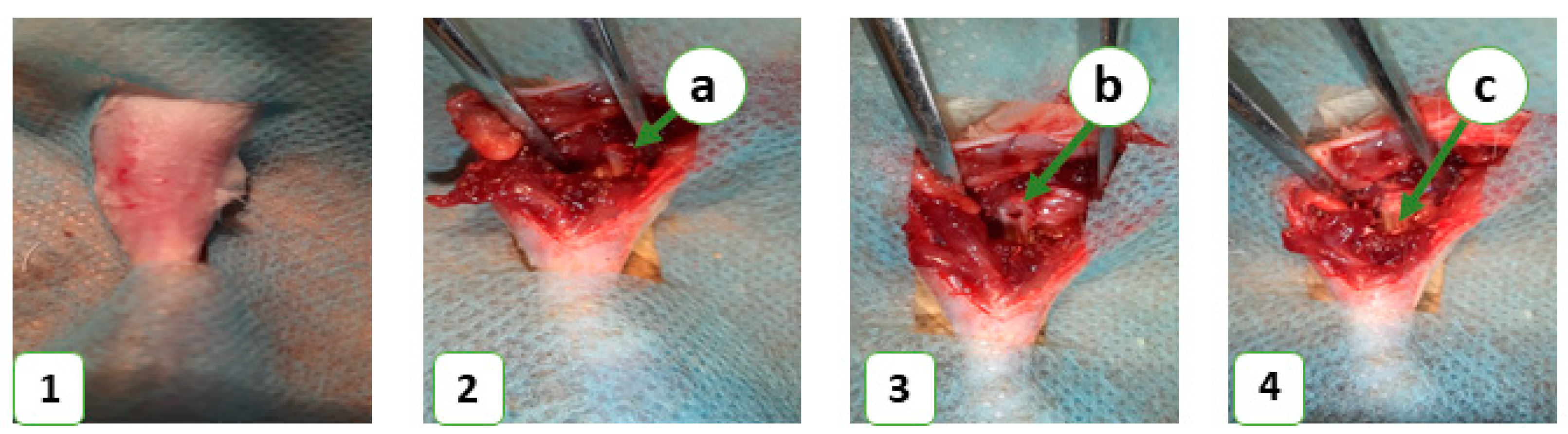
In this study, 36 laboratory rats were obtained from the Vivarium of Sumy State University. The animals were housed at 22 ± 2 °C on a 12-h light/dark cycle and had free access to food and water. Each animal was kept in a separate cage in accordance with the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. The study was approved by the Commission on Bioethics Compliance in Experimental and Clinical Research. The animals were randomized into control and experimental groups, with 18 animals in each. The control group consisted of animals that did not receive any bone substitute after the operation, while the experimental group included animals that received a novel bioactive bone graft nanomaterial to replace a bone defect in the middle third of the tibia. Prior to the operation, the animals’ legs were shaved under anesthesia (ketamine, 10 mg per 1 kg). The surgical field was treated with 70% ethanol to prevent bacterial contamination and then surrounded with a sterile cloth. A bone defect was created in the middle third of the tibia using a stomatological drill (d-2.2 mm) and filled with the novel bone grafting nanomaterial in the experimental group (
Figure 1). The wound was closed with simple interrupted sutures, and an aseptic dressing was applied. The animals were euthanized on the 7th, 14th, and 28th days of the experiment with an overdose of ketamine (70 mg/kg).
The material was fixed in a 10% neutral (buffered) formaldehyde solution for 24 h. All tissue processing procedures (fixation, decalcification, paraffin saturation, and embedding) were performed according to generally accepted methods. Serial sections with a thickness of 4–5 μm were stained with Mayer’s hematoxylin and eosin.
2.7. Clinical Application
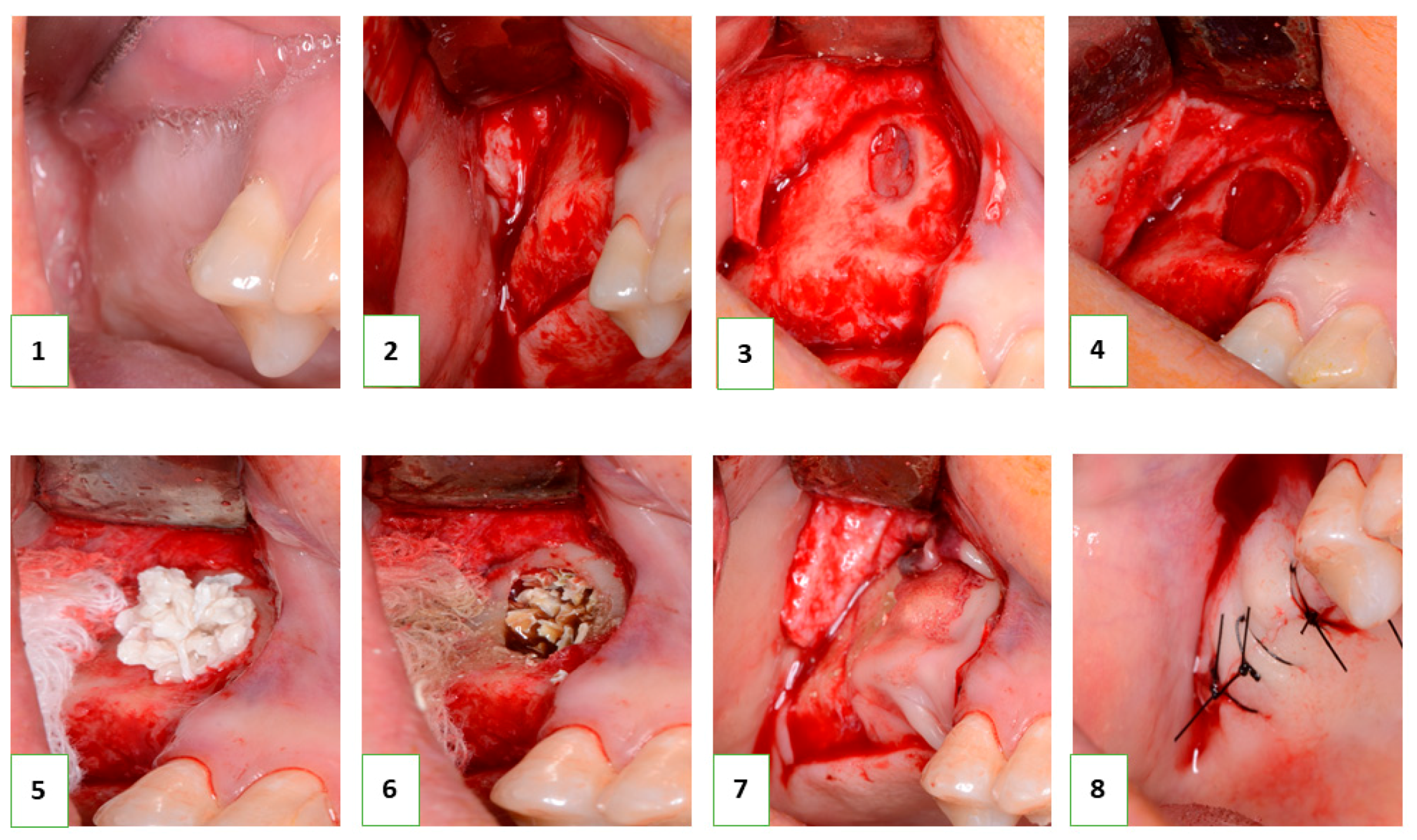
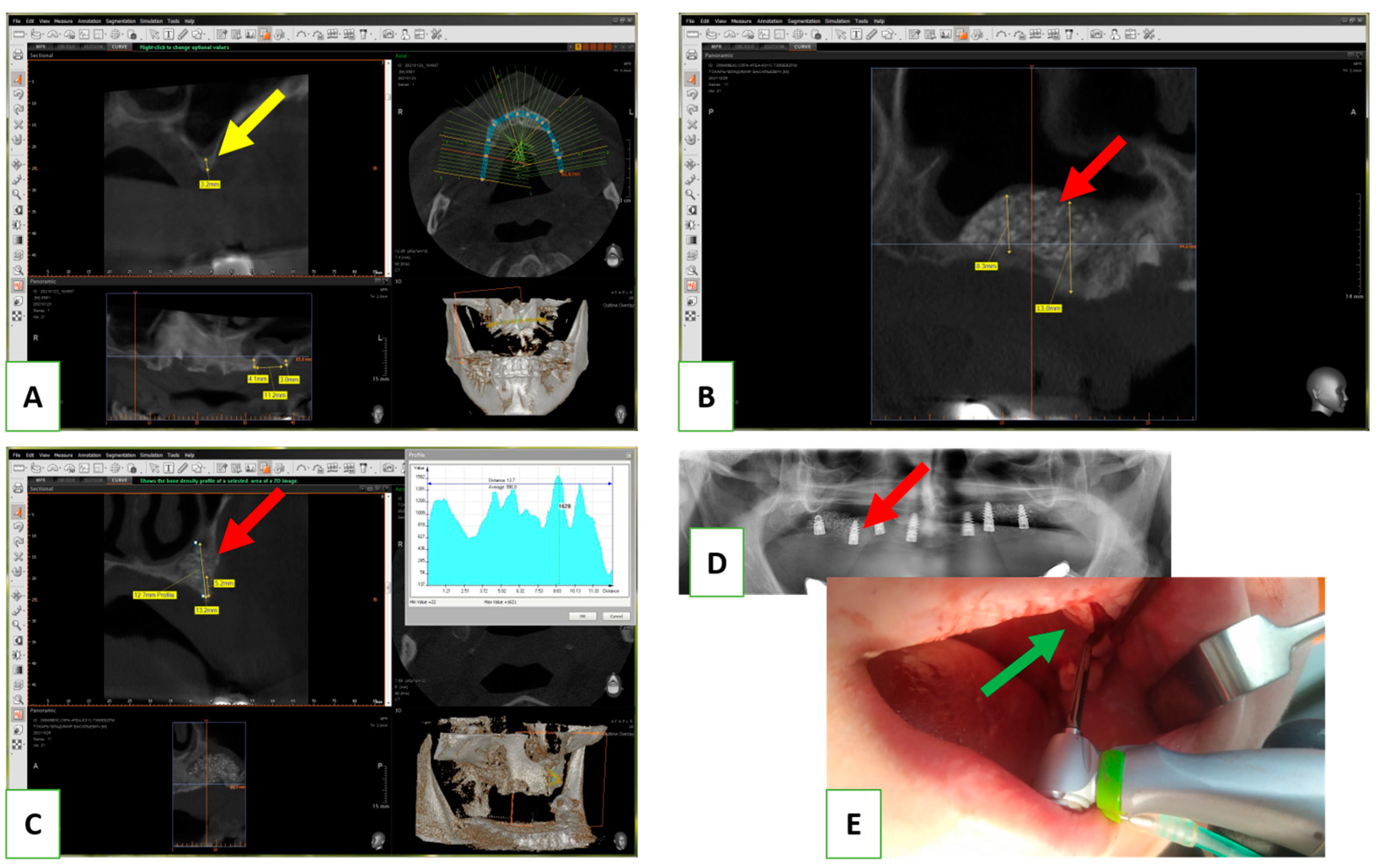
Following the successful in vitro biocompatibility assessment and in vivo evaluation of effectiveness, the bone grafting nanomaterial was approved for clinical application under protocol #12-75/19 (Zaporizhzhia State Medical University). The open sinus lifting operation was carried out according to the following protocol (
Figure 2): After sedation with an analgesic (Ketanov) and application of a hemostatic agent (Dicinon), a full-thickness mucoperiosteal flap was formed in the area of the anterior wall of the maxillary sinus, followed by skeletonization of the anterior wall of the maxillary sinus. Using a round bur with irrigation, the cortical layer of the bone was removed to expose the sinus membrane (Schneider’s membrane). The membrane was then peeled from the floor of the maxillary sinus and the side wall of the nose using sinus elevators and raised to the height of the desired augmentation. The space formed between the bottom of the sinus and the dome of the membrane was filled with a graft that had been pre-moistened with an antibiotic and antiseptic solution (dioxidine, chlorhexidine bigluconate 0.05%). The graft was evenly distributed throughout the volume and condensed with a force of up to 150 g/cm
2. The integrity of the Schneiderian membrane was monitored, as well as an assessment of the degree of vascularization of the recipient zone by the rate of wetting of the augmentate with blood. The window in the anterior wall of the maxillary sinus was closed with a membrane (PLA), the mucoperiosteal flap was placed in place and sutured, and standard anti-inflammatory therapy was prescribed. The sutures were removed on the tenth day, and no complications were observed in the postoperative period.
In all cases, implantation was recommended to patients after 8 months. In the next stage, the installation of screw implants was carried out according to the standard protocol, with simultaneous sampling of a bone fragment from the augmentation area using a tubular burr. The terms of implantation varied from 8 to 12 months and were determined based on the clinical situation. This clinical study included 6 clinical cases with a detailed analysis of tissue biopsy before implantation.
2.8. Histological Evaluation of Bone Augmentation with HA/β-TCP
Intervention and further biopsy were performed in line with the Declaration of Helsinki. The study protocol was approved by the ethics committee (protocol #12-75/19, Zaporizhzhia State Medical University). All patients gave their informed consent before enrollment in the study, and all patients completed the study successfully and received the opportunity for free follow-up visits after the intervention.
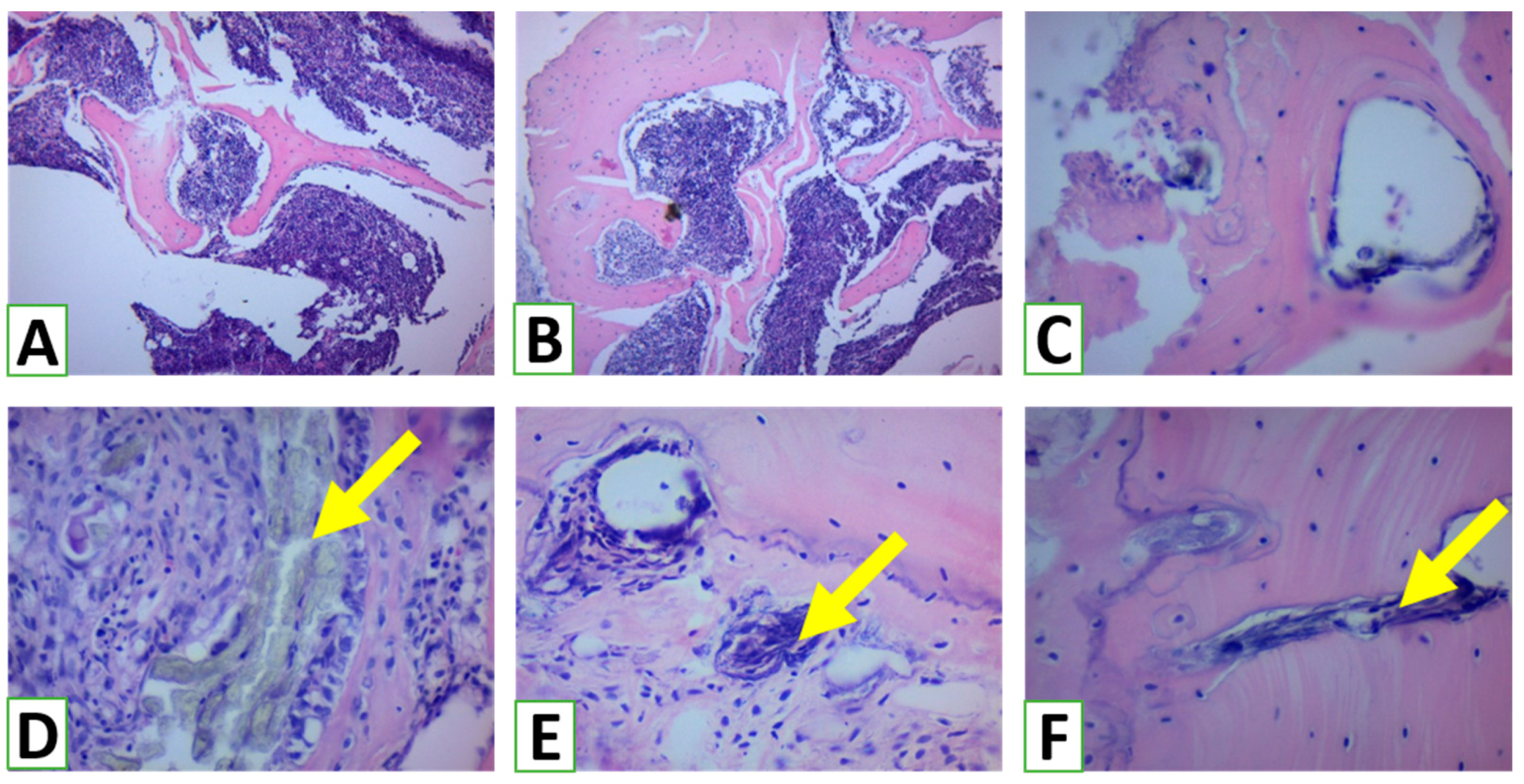
The samples obtained after biopsy were fixed immediately by immersion in 10% buffered formalin for 24–48 h with further decalcification in EDTA (4.1% disodium ethylenediaminetetraacetic acid solution). After completion of decalcification, the biopsies were processed according to the standard protocol with further embedding in paraffin (Paraplast). Paraffin blocks were cut at 4–5 µm, and histological slides were stained using hematoxylin and eosin, as well as toluidine blue, for routine histological examination. Examination of histological specimens was performed using parameters adapted from those used for the assessment of bone healing and remodeling. The shares of space filled with bone trabeculae, graft nanomaterial, and connective tissue were measured histomorphometrically [
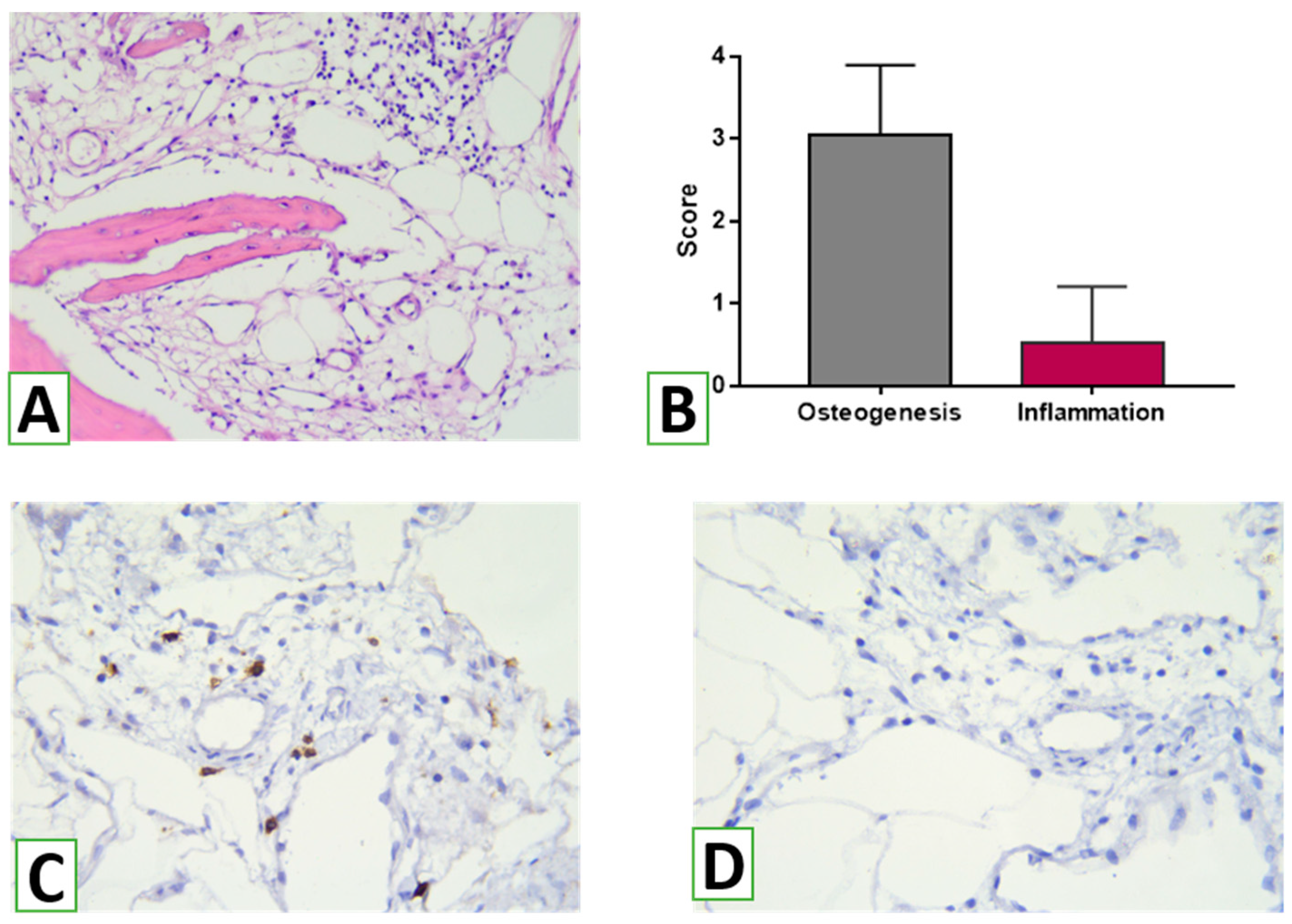
26]. In addition, the thickness of the bone trabeculae was measured in three areas of three serial sections at three representative areas at high magnification (Leica Microsystems GmbH, Wetzlar, Germany). The intensity of osteogenesis was assessed semi-qualitatively using the following scoring system: 0 = no features of osteogenesis; 1 = bone formation around graft (e.g., granules) with the appearance of osteoblasts and deposition of osteoid; 2 = bone formation around graft with primary bone features; 3 = bone trabeculae formation around grafts with features of active remodeling (detection of lamellar bone with primary osteons and osteocytes, vascular detection, fibrous bone remnants in the lamellar bone, and remains of bone replacement nanomaterial embedded in/on the bone); 4 = mature lamellar bone with osteons [
14]. In addition, the immune reaction to NG was assessed using a semi-quantitative score according to the following scheme: 0 = none; 1 = loose infiltrates, disseminated or focal; 2 = dense, moderately extensive lymphocytic infiltrates; 3 = extensive, dense lymphocytic infiltrates with edema and focal giant cells; 4 = pronounced inflammatory reaction including giant cells and necrosis [
27]. The assessment was performed by two independent observers blindly
2.9. Immunohistochemical Study
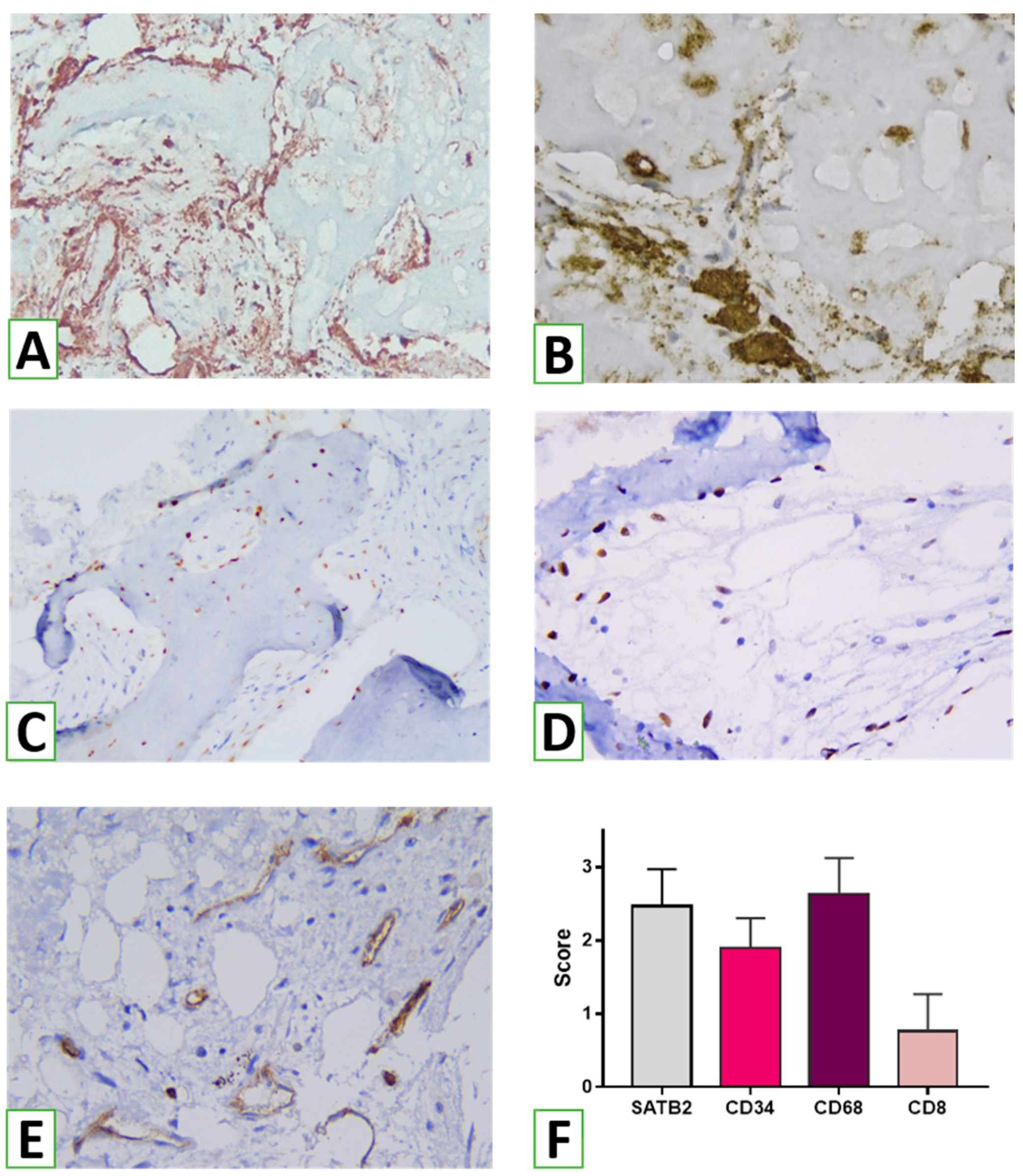
To analyze the host tissues’ and cells’ reactions to NanoGraft, an immunohistochemical (IHC) study was performed. Serial sections 4 μm in thickness were cut, deparaffinized, and hydrated. Endogenous peroxidase activity was blocked using 3% methanol in hydrogen peroxide. After antigen retrieval, incubation with primary antibodies was performed. After washing, labeled polymer secondary antibodies (Envision Detection System, Dako) were added to the slides. Peroxidase activity was detected using diaminobenzidine (DAB), yielding a brown staining product. Slides were counterstained with Mayer’s hematoxylin. The following biomarkers were used for IHC: CD8 (DAKO; Clone C8/144B) was used for visualizing cytotoxic T cells as effector cells of cell-mediated immunity, and FOXP3 (Cell Marque, Clone EP340) was used for the assessment of T regulatory lymphocytes producing anti-inflammatory and profibrogenic agents. CD68 (DAKO, Clone KP1) and CD163 (Cell Marque, Clone MRQ-26) were used to assess macrophages of the M1 and M2 types. For evaluating angiogenesis, we used antibodies against CD34 (DAKO, Clone QBEnd 10). Finally, to analyze the osteogenic potential of the graft, SATB2 (Cell Marque, Clone EP281) as a marker of osteogenic lineage differentiation was applied.
To evaluate the effect of augmentation on the overall outcome, histomorphometry was performed with an evaluation of the proportion of the specimen filled with bone tissues, graft nanomaterial, and connective tissue. For assessing the potential immunogenic effect of the bionanomaterial used for augmentation, the intensity of inflammation was examined using semi-quantitative analysis. In addition, the presence and density of innate and adaptive immunity cells were considered adaptive. Reactions of pro- (CD68+) and anti-inflammatory (CD163+) subtypes of macrophages were assessed. In addition, the density of immunoreactive (CD8+) and immunosuppressive (FOXP3+) T cells was scored. To consider angiogenesis, the number of vessels was evaluated using cells positive for vascular biomarker CD34. The characteristics of the biomarkers are presented in
Table 1.
To make a judgment about the osteogenic properties of the graft, we assessed bone tissue formation by measuring the proportion of bone trabeculae in the specimen and the density and spatial distribution of osteogenic cells highlighted by SATB2. Digital photographs of the histological and immunohistochemical specimens were taken using a digital camera (Leica Microsystems) placed over a light microscope (Leica Microsystems).
2.10. Statistical Analysis
Quantitative data were expressed as mean ± standard deviation. Comparisons between groups were performed using the t-test. In addition, the Kruskal–Wallis test was used when working with categorical data. A p < 0.05 was determined to be statistically significant. Statistical analyses were performed using GraphPad Prism 8.0 (V8.0.1).
4. Discussion
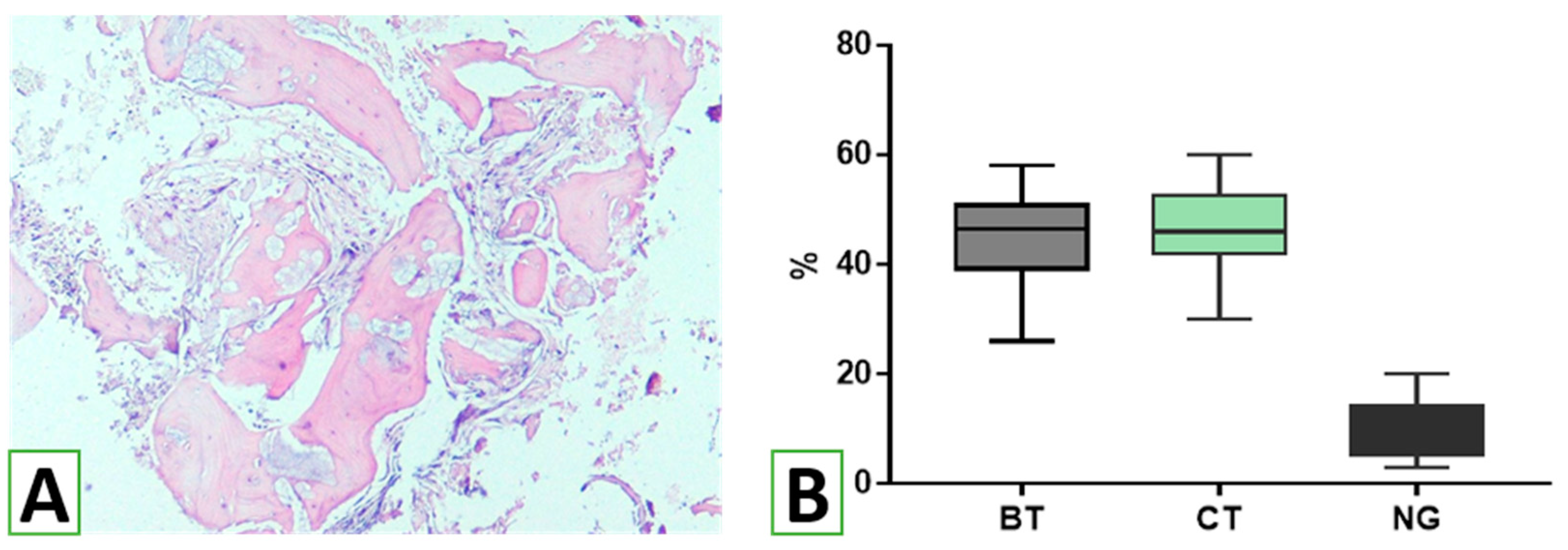
The data obtained in the study indicate the effective use of HA/β-TCP nanomaterial with cells and tissues in alveolar processes. In most cases, the remnants of the biomaterial were determined within the bone trabeculae, and only a small portion was found extra-trabecularly, demonstrating high integration of the nanomaterial with new bone formation in the sinus lifting zone. It should be emphasized that the biomaterial used for sinus augmentation was immunologically inactive. According to histomorphometric analysis, a large area of the transplant residues in the augmentation zones was in contact with the newly formed bone tissue, reflecting the mechanisms of the osteoconductive effect of the novel HA/β-TCP bioactive nanomaterial. As previous studies have shown, the amount of newly formed bone, transplant residues, and connective tissue components varies widely when using different materials for sinus lifting [
36]. For example, the use of autogenous bone stimulated osteogenesis to a greater extent compared to biphasic calcium phosphate (BCP) [
37]. According to this study, 6–8 months after sinus lifting, the percentage of newly formed bone in the augmentation zone was 28.2% and 36.8% for BCP and autogenous bone, respectively. The majority of the areas of interest were occupied by connective tissue, forming 38.9% and 58.4%, respectively. In this case, fragments of BCP residues accounted for up to 32.9%, reflecting the limited biodegradation of the material. Instead, when using autogenous bone, fragments of its residues formed an average of 4.8% of the total volume of biopsies from the augmentation zones, which is the basis for positioning this material as the gold standard [
38]. The results obtained in the study significantly exceed the indicators for BCP and approach the parameters when using autologous bone tissue, which actually reflects the high biocompatibility and, at the same time, appropriate biodegradation of novel HA/β-TCP, which acted as a conductor for the formation of its own bone tissue. The results demonstrated the advantages of using novel HA/β-TCP compared to allogeneic bone, which resulted in only 18.65 ± 12.20% new bone formation, 25.93 ± 12.36% residual allogeneic material, and 53.45 ± 10.34% connective tissue [
39]. However, it should be noted that recent clinical studies on the use of combined scaffolds based on hydroxyapatite and polylactic acid or polyethyleneimine, which combine the characteristics of biodegradable polymers and bioceramics, have yielded results comparable to those presented in this study [
40].
Histomorphometric analysis made it possible to evaluate not only the proportion and interaction between newly formed bone and the novel HA/β-TCP material’s residues but also to determine the degree of maturation of newly formed bone tissue in the area of transplant use. According to the data of the study, the majority of bone trabeculae in the biopsies of the augmentation zones corresponded to the third stage of osteogenesis—bone remodeling with the replacement of coarse fibrous bone tissue with lamellar bone. At the same time, signs of high maturity with the presence of osteons were found in more than a quarter of the trabeculae. The obtained data were compared with those in a study of five other bone materials [
14]. The formation of bone and effective direct osteogenesis are actually a result of the activity of bone tissue cells—primarily osteoblasts and osteoclasts involved in the processes of osteogenesis and bone remodeling.
In this study, a significant number of SATB2-expressing osteogenic cells were identified in the augmentation zone. In addition to SATB2+ cells on the surface of bone trabeculae, a significant number of committed osteogenic cells were found freely in the connective tissue between trabeculae. These SATB2-positive cells may actually correspond to induced precursor cells that have been involved in the process of osteogenic differentiation. Such a pattern may reflect the osteoinductive potential of the HA/β-TCP nanomaterial, which stimulated the differentiation and migration of osteogenic cells to areas of osteogenesis.
According to the results of the histological and immunohistochemical studies, use of the novel HA/β-TCP nanomaterial was accompanied by signs of a slight inflammatory reaction. Within the biopsy specimens, only small diffuse lymphohistiocytic infiltrates were identified, which, according to various authors, may be a consequence of a transient weak immune response in response to damage in the augmentation zone and a normal bone remodeling process (Schmidt-Bleek K et al., 2012). Moreover, only a small number of CD8+ T cells were detected, which also play a role in osteogenesis and bone remodeling [
41]. Similar data were obtained by Solakoglu Ö et al., who demonstrated the presence of a small number of infiltrates and the presence of CD3, CD4+, and CD8+ lymphocytes when using different variants of bone allografts.
It is important to emphasize that T regulatory cells responsible for the mechanisms of immune tolerance were not detected in the tissue biopsy samples from the augmentation zones during the study. The obtained results may indicate the primary low immunogenicity of the HA/β-TCP bone nanomaterial used. The role of other mechanisms of anti-inflammatory action associated with the activity of different subtypes of macrophages cannot be excluded either.
In addition to T lymphocytes, macrophages play an important role and have crucial significance for bone metabolism and bone tissue remodeling [
22]. Macrophages represent a numerous population of immune cells present in different tissues and organs. Traditionally, macrophages quickly accumulate in damaged areas or areas of infection, where they play a critical role in innate immunity [
42]. In addition, macrophages regulate tissue homeostasis and the implementation of various pathophysiological processes, including innate and adaptive immunity, regeneration, angiogenesis, and carcinogenesis. Moreover, macrophages not only initiate tissue inflammation but also promote tissue repair and remodeling [
43]. In bone tissue, macrophages are an integral component of the bone remodeling process, as they coordinate the communication between osteoclasts and osteoblasts and stimulate anabolic processes critical for bone formation [
44].
It is generally accepted that macrophages represent a spectrum of activated phenotypes rather than discrete stable subpopulations. Indeed, numerous studies have documented their programming flexibility, whereby macrophages switch from one functional phenotype to another in response to variable signals from the local microenvironment [
45]. Schematically, macrophages are classified into two subsets: classically activated macrophages (M1) and alternatively activated macrophages (M2), although this is an oversimplification, and the actual spectrum of macrophage phenotypes is more complex [
43].
As shown by the results of this study, numerous macrophages were detected in the biopsy samples from the augmentation zone, both CD68+ and CD163+. The dense network of CD163-positive M2 macrophages was of particular interest. Xia Z et al. (2006) previously showed that macrophages are the dominant cell type in the infiltration formed in response to the implantation of bionanomaterials in both soft and hard tissues. These cells and their variants, including multinucleated giant foreign body cells, are part of the inflammatory response and reaction to foreign material that occurs in any interventions involving biological materials. In addition, macrophages play an important role in the biodegradation of biomaterials used for implantation through the initiation of phagocytosis and extracellular degradation mechanisms.