Recent Advances on Electrospun Nanofibers for Periodontal Regeneration
Abstract
1. Introduction
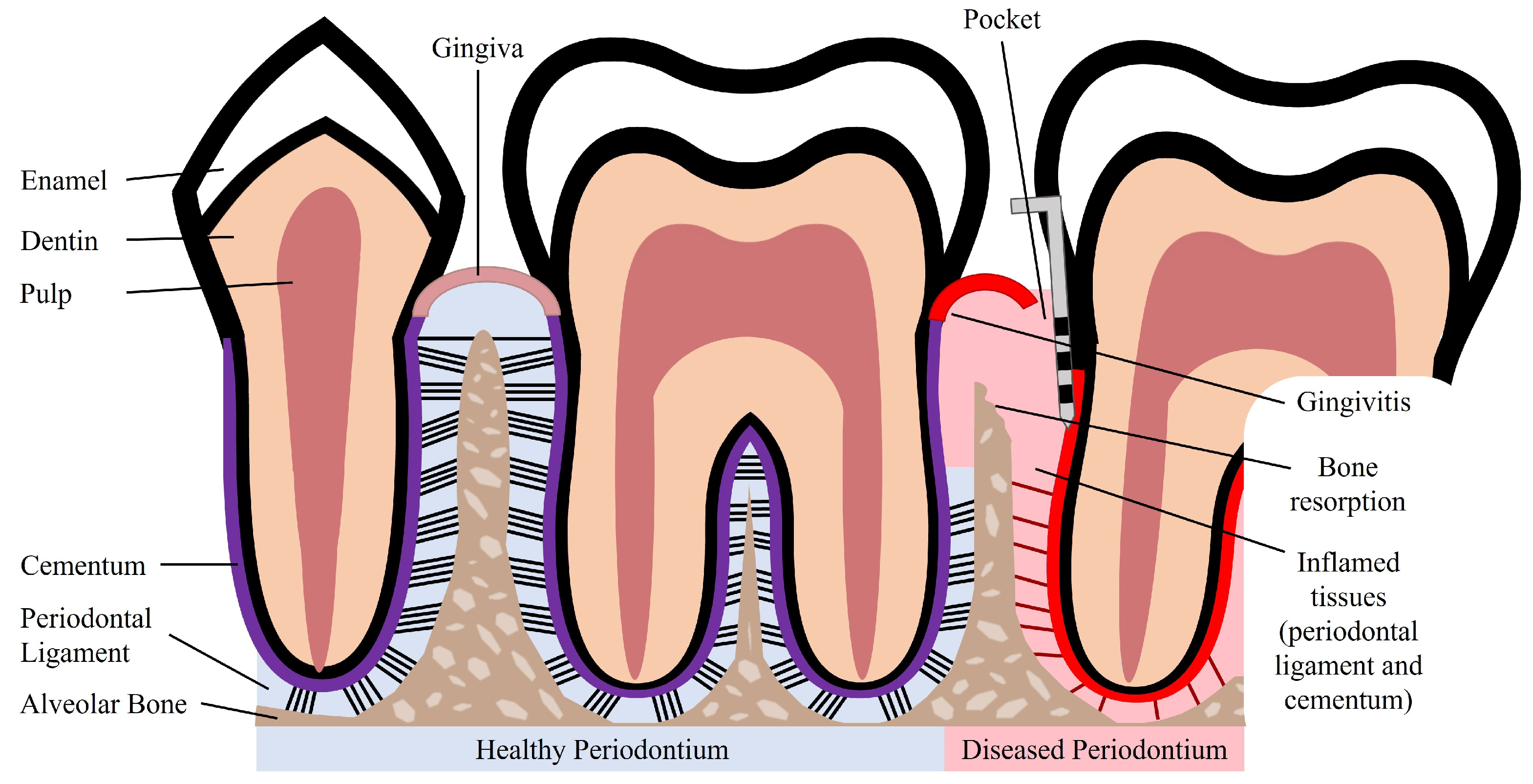
2. Periodontal Tissue Features and Periodontal Disease
3. Currently Available Treatments for Periodontal Disease
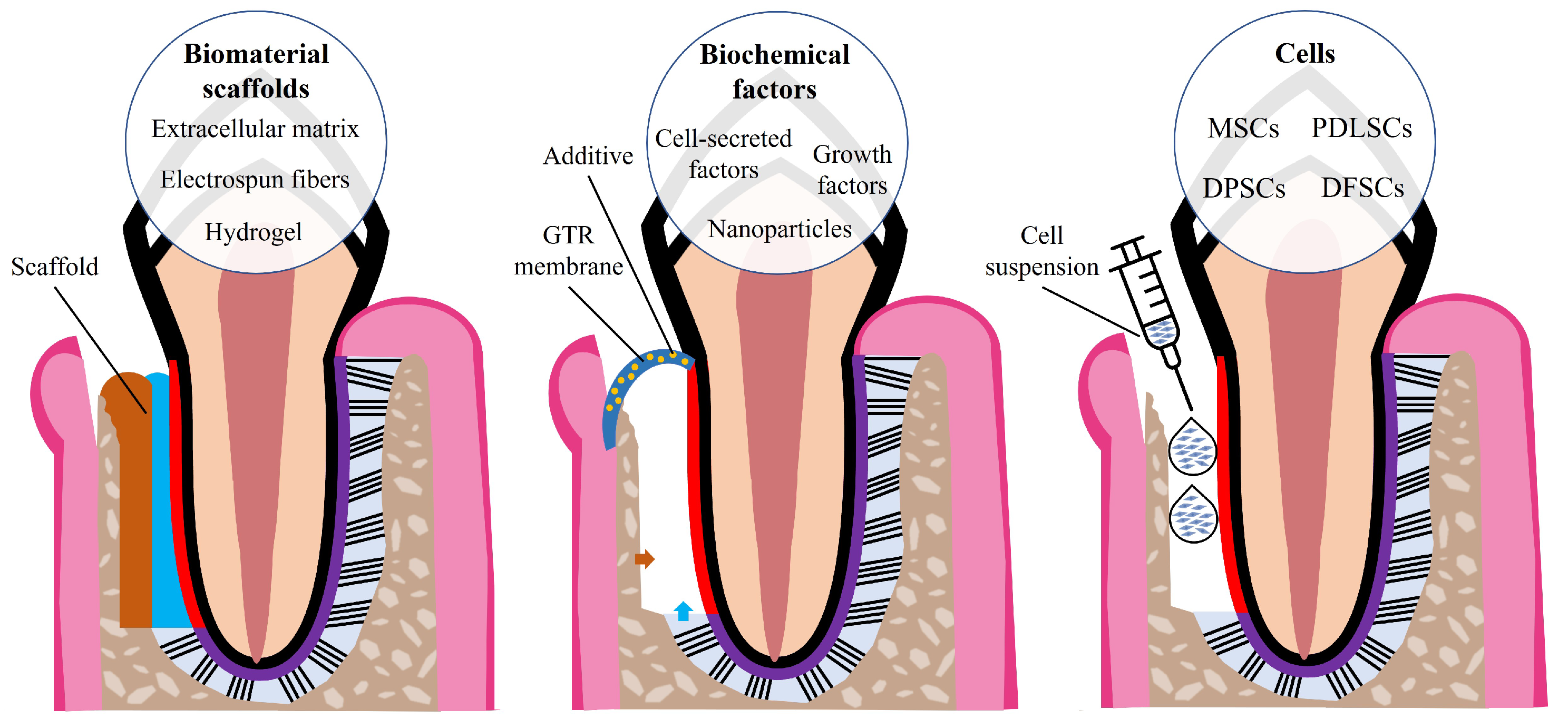
4. Periodontal Tissue Engineering
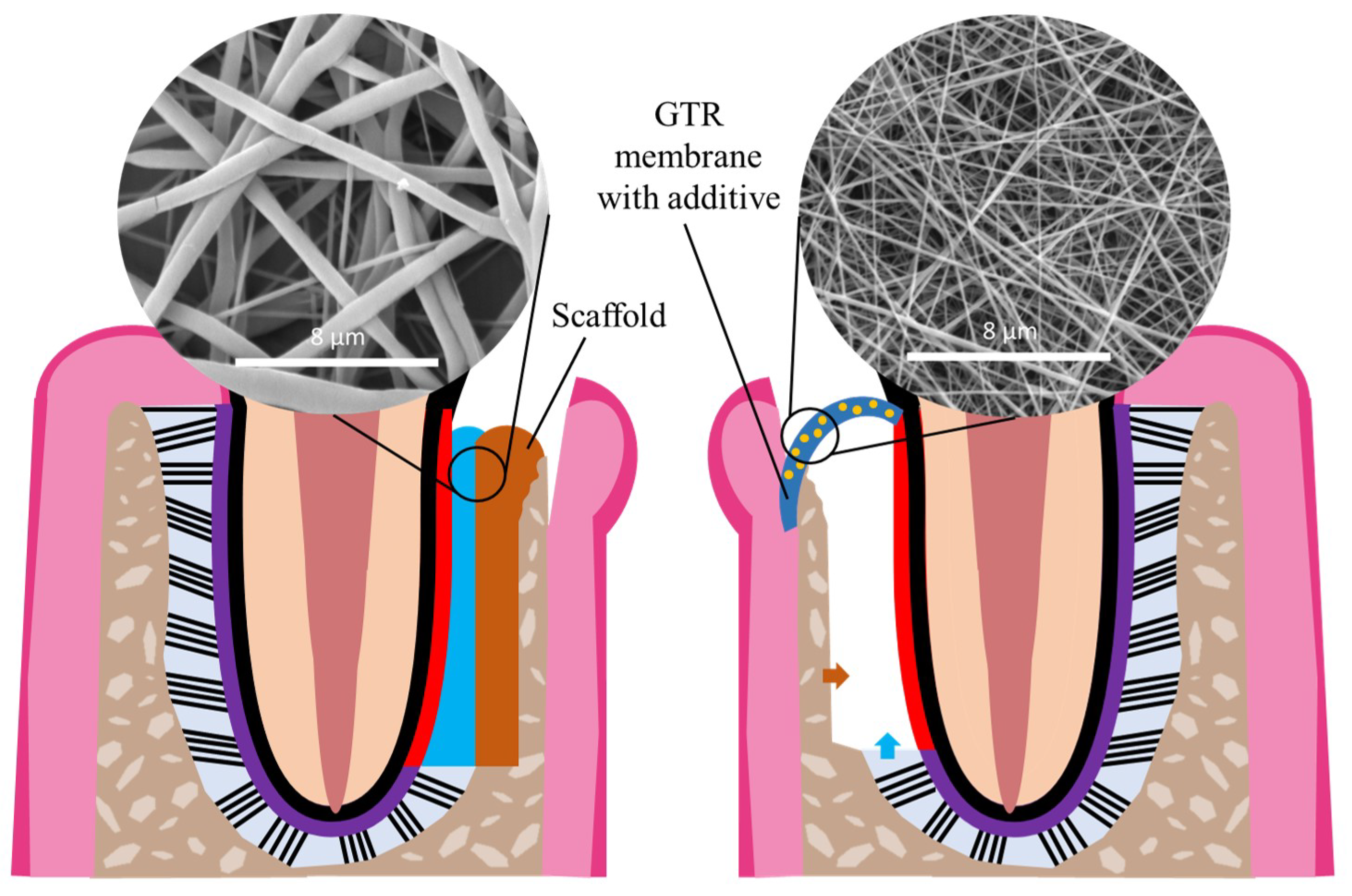
5. Electrospun Nanofibers for Periodontal Tissue Engineering
6. Current Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Preshaw, P.M.; Seymour, R.A.; Heasman, P.A. Current Concepts in Periodontal Pathogenesis. Dent. Update 2004, 31, 570–578. [Google Scholar] [CrossRef]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- De Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J. Periodontal Res. 2017, 52, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Chen, W.; Feng, Z.; Liu, Y.; Liu, P.; Xie, Y.; Yu, D.G. Electrospun Nanofibers for Periodontal Treatment: A Recent Progress. Int. J. Nanomed. 2022, 17, 4137–4162. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Lin, K.; Yu, H. Advance of Nano-Composite Electrospun Fibers in Periodontal Regeneration. Front. Chem. 2019, 7, 495. [Google Scholar] [CrossRef]
- Newman, M.G.; Takei, H.H.; Klokkevold, P.R.; Carranza, F.A. Newman and Carranza’s Clinical Periodontology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Fehrenbach, M.J.; Popowics, T. Illustrated Dental Embryology, Histology, and Anatomy; Saunders: Maryland Heights, MO, USA, 2015. [Google Scholar]
- Goudouri, O.M.; Kontonasaki, E.; Boccaccini, A.R. Layered scaffolds for periodontal regeneration. In Biomaterials for Oral and Dental Tissue Engineering; Woodhead Publishing: Sawston, UK, 2017; pp. 279–295. [Google Scholar] [CrossRef]
- Kumar, G.S. Orban’s Oral Histology and Embryology; Elsevier: Haryana, India, 2019. [Google Scholar]
- Cho, M.I.; Garant, P.R. Development and general structure of the periodontium. Periodontology 2000, 24, 9–27. [Google Scholar] [CrossRef]
- Bosshardt, D. Are Cementoblasts a Subpopulation of Osteoblasts or a Unique Phenotype? J. Dent. Res. 2005, 84, 390–406. [Google Scholar] [CrossRef]
- Jiang, N.; Guo, W.; Chen, M.; Zheng, Y.; Zhou, J.; Kim, S.G.; Embree, M.C.; Song, K.S.; Marao, H.F.; Mao, J.J. Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement. Front. Oral Biol. 2015, 18, 1–8. [Google Scholar] [CrossRef]
- Nanci, A.; Bosshardt, D.D. Structure of periodontal tissues in health and disease. Periodontology 2006, 40, 11–28. [Google Scholar] [CrossRef]
- Wu, B.; Fu, Y.; Shi, H.; Yan, B.; Lu, R.; Ma, S.; Markert, B. Tensile testing of the mechanical behavior of the human periodontal ligament. BioMed. Eng. Online 2018, 17, 172. [Google Scholar] [CrossRef]
- Jia, L.N.; Zhang, X.; Xu, H.Y.; Hua, F.; Hu, X.G.; Xie, Q.; Wang, W.; Jia, J. Development of a Doxycycline Hydrochloride-Loaded Electrospun Nanofibrous Membrane for GTR/GBR Applications. J. Nanomater. 2016, 2016, 6507459. [Google Scholar] [CrossRef]
- Sanz, M.; D’aiuto, F.; Deanfield, J.; Fernandez-Avilés, F. European workshop in periodontal health and cardiovascular disease—Scientific evidence on the association between periodontal and cardiovascular diseases: A review of the literature. Eur. Heart J. Suppl. 2010, 12, B3–B12. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Machado, V.; Leira, Y.; Proença, L.; Chambrone, L.; Mendes, J.J. Economic burden of periodontitis in the United States and Europe: An updated estimation. J. Periodontol. 2022, 93, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bui, F.Q.; da Silva, C.L.C.A.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Juliana, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines 2022, 10, 2659. [Google Scholar] [CrossRef]
- Kotronia, E.; Wannamethee, S.G.; Papacosta, A.O.; Whincup, P.H.; Lennon, L.T.; Visser, M.; Weyant, R.J.; Harris, T.B.; Ramsay, S.E. Oral Health, Disability and Physical Function: Results From Studies of Older People in the United Kingdom and United States of America. J. Am. Med. Dir. Assoc. 2019, 20, 1654.e1–1654.e9. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.J.; Lin, Z.; Villarosa, A.; Lewis, P.; Philip, P.; Sumar, B.; George, A. Countering the poor oral health of people with intellectual and developmental disability: A scoping literature review. BMC Public Health 2019, 19, 1530. [Google Scholar] [CrossRef]
- Carli, E.; Pasini, M.; Pardossi, F.; Capotosti, I.; Narzisi, A.; Lardani, L. Oral Health Preventive Program in Patients with Autism Spectrum Disorder. Children 2022, 9, 535. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Slot, D.E.; Valkenburg, C.; Van der Weijden, G.F. Mechanical plaque removal of periodontal maintenance patients: A systematic review and network meta-analysis. J. Clin. Periodontol. 2020, 47, 107–124. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Montero, E.; Citterio, F.; Romano, F.; Molina, A.; Aimetti, M. Efficacy of access flap procedures compared to subgingival debridement in the treatment of periodontitis. A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 282–302. [Google Scholar] [CrossRef]
- Geisinger, M.L.; Kaur, M.; Basma, H. Nonsurgical Periodontal Therapy: A Review of Current Standards of Care and Innovations to Improve Gingival and Periodontal Health. Curr. Oral Health Rep. 2019, 6, 177–187. [Google Scholar] [CrossRef]
- Polak, D.; Wilensky, A.; Antonoglou, G.N.; Shapira, L.; Goldstein, M.; Martin, C. The efficacy of pocket elimination/reduction compared to access flap surgery: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Liaw, A.; Miller, C.; Nimmo, A. Comparing the periodontal tissue response to non-surgical scaling and root planing alone, adjunctive azithromycin, or adjunctive amoxicillin plus metronidazole in generalized chronic moderate-to-severe periodontitis: A preliminary randomized controlled trial. Aust. Dent. J. 2019, 64, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, L.; Li, X.; Liu, S.; Du, J.; Xu, J.; Hu, J.; Liu, Y. Challenges and Tissue Engineering Strategies of Periodontal-Guided Tissue Regeneration. Tissue Eng. Part C Methods 2022, 28, 405–419. [Google Scholar] [CrossRef]
- Valenti, C.; Pagano, S.; Bozza, S.; Ciurnella, E.; Lomurno, G.; Capobianco, B.; Coniglio, M.; Cianetti, S.; Marinucci, L. Use of the Er:YAG Laser in Conservative Dentistry: Evaluation of the Microbial Population in Carious Lesions. Materials 2021, 14, 2387. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef]
- Mancini, L.; Fratini, A.; Marchetti, E. Periodontal Regeneration. Encyclopedia 2021, 1, 87–98. [Google Scholar] [CrossRef]
- Xu, X.; Ren, S.; Li, L.; Zhou, Y.; Peng, W.; Xu, Y. Biodegradable engineered fiber scaffolds fabricated by electrospinning for periodontal tissue regeneration. J. Biomater. Appl. 2021, 36, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.A.; Rozental, T.D. Bone Graft Substitutes. Hand Clin. 2012, 28, 457–468. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone grafts and substitutes in dentistry: A review of current trends and developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Cranio-Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef]
- Li, Q.; Yang, G.; Li, J.; Ding, M.; Zhou, N.; Dong, H.; Mou, Y. Stem cell therapies for periodontal tissue regeneration: A network meta-Analysis of preclinical studies. Stem Cell Res. Ther. 2020, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Moura, C.S.; Borrecho, G.; de Matos, A.P.A.; Cabral, J.M.S.; Linhardt, R.J.; Ferreira, F.C. Effects of glycosaminoglycan supplementation in the chondrogenic differentiation of bone marrow- and synovial- derived mesenchymal stem/stromal cells on 3D-extruded poly (ε-caprolactone) scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 207–222. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Poundarik, A.A.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Biomimetic matrices for rapidly forming mineralized bone tissue based on stem cell-mediated osteogenesis. Sci. Rep. 2018, 8, 14388. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Couto de Carvalho, L.A.; Tosta dos Santos, S.L.; Sacramento, L.V.; de Almeida, V.R.; de Aquino Xavier, F.C.; dos Santos, J.N.; Águida Cristina Gomes Henriques Leitão. Mesenchymal stem cell markers in periodontal tissues and periapical lesions. Acta Histochem. 2020, 122, 151636. [Google Scholar] [CrossRef]
- Kadkhoda, Z.; Rafiei, S.C.; Azizi, B.; Khoshzaban, A. Assessment of Surface Markers Derived from Human Periodontal Ligament Stem Cells: An In Vitro Study. J. Dent. 2016, 13, 325–332. [Google Scholar]
- Zhou, T.; Pan, J.; Wu, P.; Huang, R.; Du, W.; Zhou, Y.; Wan, M.; Fan, Y.; Xu, X.; Zhou, X.; et al. Dental Follicle Cells: Roles in Development and beyond. Stem Cells Int. 2019, 2019, 9159605. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Li, X.; Wang, J.; He, X.T.; Sun, H.H.; Chen, F.M. Concise Review: Periodontal Tissue Regeneration Using Stem Cells: Strategies and Translational Considerations. Stem Cells Transl. Med. 2018, 8, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.Y.; Ning, Y.; Huang, X. Advanced technologies in periodontal tissue regeneration based on stem cells: Current status and future perspectives. J. Dent. Sci. 2021, 16, 501–507. [Google Scholar] [CrossRef]
- Takewaki, M.; Kajiya, M.; Takeda, K.; Sasaki, S.; Motoike, S.; Komatsu, N.; Matsuda, S.; Ouhara, K.; Mizuno, N.; Fujita, T.; et al. MSC/ECM Cellular Complexes Induce Periodontal Tissue Regeneration. J. Dent. Res. 2017, 96, 984–991. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, X.; Zhou, H.; Zhang, C.; Wang, Y.; Huang, J.; Liu, M.; Yang, P.; Song, A. Enhancement of periodontal tissue regeneration by conditioned media from gingiva-derived or periodontal ligament-derived mesenchymal stem cells: A comparative study in rats. Stem Cell Res. Ther. 2020, 11, 42. [Google Scholar] [CrossRef]
- Flores, M.G.; Yashiro, R.; Washio, K.; Yamato, M.; Okano, T.; Ishikawa, I. Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J. Clin. Periodontol. 2008, 35, 1066–1072. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Ding, G.; Fang, D.; Zhang, C.; Bartold, P.M.; Gronthos, S.; Shi, S.; Wang, S. Periodontal Ligament Stem Cell-Mediated Treatment for Periodontitis in Miniature Swine. Stem Cells 2008, 26, 1065–1073. [Google Scholar] [CrossRef]
- Hu, J.; Cao, Y.; Xie, Y.; Wang, H.; Fan, Z.; Wang, J.; Zhang, C.; Wang, J.; Wu, C.T.; Wang, S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res. Ther. 2016, 7, 130. [Google Scholar] [CrossRef]
- Gao, X.; Shen, Z.; Guan, M.; Huang, Q.; Chen, L.; Qin, W.; Ge, X.; Chen, H.; Xiao, Y.; Lin, Z. Immunomodulatory Role of Stem Cells from Human Exfoliated Deciduous Teeth on Periodontal Regeneration. Tissue Eng. Part A 2018, 24, 1341–1353. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Hu, Y.; Sun, J.; Guo, W.; Li, H.; Chen, J.; Huo, F.; Tian, W.; Li, S. Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent. Mater. 2019, 35, 1238–1253. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Chung, H.Y.; Wang, J.H.; Shih, J.C.; Kuo, M.Y.P.; Chang, P.C.; Huang, Y.D.; Wang, P.C.; Chang, C.C. Amniotic membrane and adipose-derived stem cell co-culture system enhances bone regeneration in a rat periodontal defect model. J. Formos. Med. Assoc. 2016, 115, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun Nanofibers of Natural and Synthetic Polymers as Artificial Extracellular Matrix for Tissue Engineering. Nanomaterials 2021, 11, 21. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Chinnappan, B.A.; Krishnaswamy, M.; Xu, H.; Hoque, M.E. Electrospinning of Biomedical Nanofibers/Nanomembranes: Effects of Process Parameters. Polymers 2022, 14, 3719. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A. Electrospun Nanofibers as Carriers of Microorganisms, Stem Cells, Proteins, and Nucleic Acids in Therapeutic and Other Applications. Front. Bioeng. Biotechnol. 2020, 8, 130. [Google Scholar] [CrossRef]
- Wu, X.; Miao, L.; Yao, Y.; Wu, W.; Liu, Y.; Chen, X.; Sun, W. Electrospun fibrous scaffolds combined with nanoscale hydroxyapatite induce osteogenic differentiation of human periodontal ligament cells. Int. J. Nanomed. 2014, 9, 4135–4143. [Google Scholar] [CrossRef]
- Samadian, H.; Mobasheri, H.; Azami, M.; Faridi-Majidi, R. Osteoconductive and electroactive carbon nanofibers/hydroxyapatite nanocomposite tailored for bone tissue engineering: In vitro and in vivo studies. Sci. Rep. 2020, 10, 14853. [Google Scholar] [CrossRef]
- Mirzaeei, S.; Mansurian, M.; Asare-Addo, K.; Nokhodchi, A. Metronidazole-and amoxicillin-loaded plga and pcl nanofibers as potential drug delivery systems for the treatment of periodontitis: In vitro and in vivo evaluations. Biomedicines 2021, 9, 975. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; Chagas, P.A.; Leite, I.S.; Inada, N.M.; de Annunzio, S.R.; Fontana, C.R.; Campana-Filho, S.P.; Correa, D.S. Core-sheath nanostructured chitosan-based nonwovens as a potential drug delivery system for periodontitis treatment. Int. J. Biol. Macromol. 2020, 142, 521–534. [Google Scholar] [CrossRef]
- Cristo, F.D.; Valentino, A.; Luca, I.D.; Peluso, G.; Bonadies, I.; Calarco, A.; Salle, A.D. PLA Nanofibers for Microenvironmental-Responsive Quercetin Release in Local Periodontal Treatment. Molecules 2022, 27, 2205. [Google Scholar] [CrossRef]
- Sun, M.; Liu, Y.; Jiao, K.; Jia, W.; Jiang, K.; Cheng, Z.; Liu, G.; Luo, Y. A periodontal tissue regeneration strategy via biphasic release of zeolitic imidazolate framework-8 and FK506 using a uniaxial electrospun Janus nanofiber. J. Mater. Chem. B 2022, 10, 765–778. [Google Scholar] [CrossRef]
- He, Z.; Liu, S.; Li, Z.; Xu, J.; Liu, Y.; Luo, E. Coaxial TP/APR electrospun nanofibers for programmed controlling inflammation and promoting bone regeneration in periodontitis-related alveolar bone defect models. Mater. Today Bio 2022, 16, 100438. [Google Scholar] [CrossRef]
- Shang, L.; Liu, Z.; Ma, B.; Shao, J.; Wang, B.; Ma, C.; Ge, S. Dimethyloxallyl glycine/nanosilicates-loaded osteogenic/angiogenic difunctional fibrous structure for functional periodontal tissue regeneration. Bioact. Mater. 2021, 6, 1175–1188. [Google Scholar] [CrossRef]
- Xie, Q.; Jia, L.N.; Xu, H.Y.; Hu, X.G.; Wang, W.; Jia, J. Fabrication of Core-Shell PEI/pBMP2-PLGA Electrospun Scaffold for Gene Delivery to Periodontal Ligament Stem Cells. Stem Cells Int. 2016, 2016, 5385137. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ding, Z.; Xia, S.; Liu, Y.; Lei, S.; Zhong, M.; Chen, X. Poly lactic-co-glycolic acid scaffold loaded with plasmid DNA encoding fibroblast growth factor-2 promotes periodontal ligament regeneration of replanted teeth. J. Periodontal Res. 2020, 55, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Rehman, I.U. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; He, L.; Yang, R.; Chen, B.; Xie, X.; Jiang, B.; Weidong, T.; Ding, Y. Enhanced effects of electrospun collagen-chitosan nanofiber membranes on guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2020, 31, 155–168. [Google Scholar] [CrossRef]
- Sousa, F.B.D.; Barud, H.S.; Delgado, A.; Dieterle, M.P.; Steinberg, T.; Tomakidi, P.; Nohava, J.; Vach, K.; Schulz, S.D.; Hellwig, E.; et al. Novel In Situ-Cross-Linked Electrospun Gelatin/Hydroxyapatite Nonwoven Scaffolds Prove Suitable for Periodontal Tissue Engineering. Pharmaceutics 2022, 14, 1286. [Google Scholar] [CrossRef]
- Yang, M.; Gao, X.; Shen, Z.; Shi, X.; Lin, Z. Gelatin-assisted conglutination of aligned polycaprolactone nanofilms into a multilayered fibre-guiding scaffold for periodontal ligament regeneration. RSC Adv. 2019, 9, 507–518. [Google Scholar] [CrossRef]
- Peng, W.; Ren, S.; Zhang, Y.; Fan, R.; Zhou, Y.; Li, L.; Xu, X.; Xu, Y. MgO Nanoparticles-Incorporated PCL/Gelatin-Derived Coaxial Electrospinning Nanocellulose Membranes for Periodontal Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Ren, S.; Zhou, Y.; Zheng, K.; Xu, X.; Yang, J.; Wang, X.; Miao, L.; Wei, H.; Xu, Y. Cerium oxide nanoparticles loaded nanofibrous membranes promote bone regeneration for periodontal tissue engineering. Bioact. Mater. 2022, 7, 242–253. [Google Scholar] [CrossRef]
- Huang, T.Y.; Shahrousvand, M.; Hsu, Y.T.; Su, W.T. Polycaprolactone/Polyethylene glycol blended with dipsacus asper wall extract nanofibers promote osteogenic differentiation of periodontal ligament stem cells. Polymers 2021, 13, 2245. [Google Scholar] [CrossRef]
- Tondnevis, F.; Ketabi, M.A.; Fekrazad, R.; Sadeghi, A.; Keshvari, H.; Abolhasani, M.M. In Vitro Characterization of Polyurethane-Carbon Nanotube Drug Eluting Composite Scaffold for Dental Tissue Engineering Application. J. Biomimetics Biomater. Biomed. Eng. 2020, 47, 13–24. [Google Scholar] [CrossRef]
- Serôdio, R.; Schickert, S.L.; Costa-Pinto, A.R.; Dias, J.R.; Granja, P.L.; Yang, F.; Oliveira, A.L. Ultrasound sonication prior to electrospinning tailors silk fibroin/PEO membranes for periodontal regeneration. Mater. Sci. Eng. C 2019, 98, 969–981. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, H.; Zhang, R.; Wang, K.; Liu, J. Construction and characterization of antibacterial PLGA/wool keratin/ornidazole composite membranes for periodontal guided tissue regeneration. J. Biomater. Appl. 2020, 34, 1267–1281. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, K.; Gao, T.; Zhang, R.; Cai, Z.; Liu, J.; Ma, H.; Zhang, W. Controlled release of bFGF loaded into electrospun core-shell fibrous membranes for use in guided tissue regeneration. Biomed. Mater. 2020, 15, 35021. [Google Scholar] [CrossRef]
- Münchow, E.A.; Albuquerque, M.T.P.; Zero, B.; Kamocki, K.; Piva, E.; Gregory, R.L.; Bottino, M.C. Development and characterization of novel ZnO-loaded electrospun membranes for periodontal regeneration. Dent. Mater. 2015, 31, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Xu, W.; Xue, Y.; Chen, L.; Ye, H.; Zhong, E.; Ye, Z.; Gao, J.; Yan, Y. The use of chitosan/PLA nano-fibers by emulsion eletrospinning for periodontal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Xu, W.; Shen, R.; Yan, Y. Emulsion electrospun PLA/calcium alginate nanofibers for periodontal tissue engineering. J. Biomater. Appl. 2020, 34, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Liu, X.; Sui, B.; Liu, C.; Mo, X.; Sun, J. Development of fish collagen/bioactive glass/chitosan composite nanofibers as a GTR/GBR membrane for inducing periodontal tissue regeneration. Biomed. Mater. 2017, 12, 55004. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Klymov, A.; Shao, J.; Zhang, Y.; Ji, W.; Kolwijck, E.; Jansen, J.A.; Leeuwenburgh, S.C.; Yang, F. Electrospun Nanofibrous Silk Fibroin Membranes Containing Gelatin Nanospheres for Controlled Delivery of Biomolecules. Adv. Healthc. Mater. 2017, 6, 1700014. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Miao, L.; Wang, Y.; Ren, S.; Yang, X.; Hu, Y.; Sun, W. Controlled release of recombinant human cementum protein 1 from electrospun multiphasic scaffold for cementum regeneration. Int. J. Nanomed. 2016, 11, 3145–3158. [Google Scholar] [CrossRef]
- Yang, F.; Miao, Y.; Wang, Y.; Zhang, L.M.; Lin, X. Electrospun zein/gelatin scaffold-enhanced cell attachment and growth of human periodontal ligament stem cells. Materials 2017, 10, 1168. [Google Scholar] [CrossRef]
- Sundaram, M.N.; Sowmya, S.; Deepthi, S.; Bumgardener, J.D.; Jayakumar, R. Bilayered construct for simultaneous regeneration of alveolar bone and periodontal ligament. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 761–770. [Google Scholar] [CrossRef]
- Lam, L.R.W.; Schilling, K.; Romas, S.; Misra, R.; Zhou, Z.; Caton, J.G.; Zhang, X. Electrospun core-shell nanofibers with encapsulated enamel matrix derivative for guided periodontal tissue regeneration. Dent. Mater. J. 2021, 40, 1208–1216. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Zhang, N.; Shi, J.; Zhang, X.; Qi, C.; Midgley, A.C.; Wang, S. Potentials of sandwich-like chitosan/polycaprolactone/gelatin scaffolds for guided tissue regeneration membrane. Mater. Sci. Eng. C 2020, 109, 110618. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Yang, B.; Li, L.; Luo, X.; Zhang, X.; Feng, L.; Jiang, Z.; Yu, M.; Guo, W.; et al. Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials 2015, 52, 56–70. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; Dias, L.M.; Surur, A.K.; de Moraes, D.A.; Pavarina, A.C.; Fontana, C.R.; Correa, D.S. Electrospun Composite Bead-on-String Nanofibers Containing CaO2 Nanoparticles and MnO2 Nanosheets as Oxygen-Release Systems for Biomedical Applications. ACS Appl. Nano Mater. 2022, 5, 14425–14436. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; de Annunzio, S.R.; Carmello, J.C.; Pavarina, A.C.; Fontana, C.R.; Correa, D.S. Combining Coaxial Electrospinning and 3D Printing: Design of Biodegradable Bilayered Membranes with Dual Drug Delivery Capability for Periodontitis Treatment. ACS Appl. Bio Mater. 2022, 5, 146–159. [Google Scholar] [CrossRef]
- Jenvoraphot, T.; Thapsukhon, B.; Daranarong, D.; Molloy, R.; Supanchart, C.; Krisanaprakornkit, S.; Topham, P.D.; Tighe, B.; Mahomed, A.; Punyodom, W. Tetracycline-Loaded Electrospun Poly(l-lactide-co-ε-caprolactone) Membranes for One-Step Periodontal Treatment. ACS Appl. Polym. Mater. 2022, 4, 2459–2469. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Zamani, M.; Prabhakaran, M.; Bahrami, S.H.; Ramakrishna, S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater. Sci. Eng. C 2016, 58, 521–531. [Google Scholar] [CrossRef]
- Dikici, B.A.; Dikici, S.; Reilly, G.C.; MacNeil, S.; Claeyssens, F. A novel bilayer polycaprolactone membrane for guided bone regeneration: Combining electrospinning and emulsion templating. Materials 2019, 12, 2643. [Google Scholar] [CrossRef]
- Budai-szűcs, M.; Ruggeri, M.; Faccendini, A.; Léber, A.; Rossi, S.; Varga, G.; Bonferoni, M.C.; Vályi, P.; Burián, K.; Csányi, E.; et al. Electrospun scaffolds in periodontal wound healing. Polymers 2021, 13, 307. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Feng, Y.; Jiang, P.; Su, J.; Huang, C. Dual micelles-loaded gelatin nanofibers and their application in lipopolysaccharide-induced periodontal disease. Int. J. Nanomed. 2019, 14, 963–976. [Google Scholar] [CrossRef]
- Shoba, E.; Lakra, R.; Kiran, M.S.; Korrapati, P.S. 3 D nano bilayered spatially and functionally graded scaffold impregnated bromelain conjugated magnesium doped hydroxyapatite nanoparticle for periodontal regeneration. J. Mech. Behav. Biomed. Mater. 2020, 109, 103822. [Google Scholar] [CrossRef]
- Ho, M.H.; Claudia, J.C.; Tai, W.C.; Huang, K.Y.; Lai, C.H.; Chang, C.H.; Chang, Y.C.; Wu, Y.C.; Kuo, M.Y.P.; Chang, P.C. The treatment response of barrier membrane with amoxicillin-loaded nanofibers in experimental periodontitis. J. Periodontol. 2021, 92, 886–895. [Google Scholar] [CrossRef]
- Hakimi, F.; Hashemikia, S.; Sadighian, S.; Ramazani, A. Nanofibrous chitosan/polyethylene oxide silver/hydroxyapatite/silica composite as a potential biomaterial for local treatment of periodontal disease. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Jiang, W.; Li, L.; Zhang, D.; Huang, S.; Jing, Z.; Wu, Y.; Zhao, Z.; Zhao, L.; Zhou, S. Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015, 25, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Wang, K.; Xu, L. A bilayered PLGA/multiwall carbon nanotubes/bacterial cellulose composite membrane for tissue regeneration of maxillary canine periodontal bone defects. Mater. Lett. 2018, 212, 118–121. [Google Scholar] [CrossRef]
- Deepak, A.; Goyal, A.K.; Rath, G. Development and Characterization of Novel Medicated Nanofiber for the Treatment of Periodontitis. AAPS PharmSciTech 2018, 19, 3687–3697. [Google Scholar] [CrossRef]
- Liu, X.; He, X.; Jin, D.; Wu, S.; Wang, H.; Yin, M.; Aldalbahi, A.; El-Newehy, M.; Mo, X.; Wu, J. A biodegradable multifunctional nanofibrous membrane for periodontal tissue regeneration. Acta Biomater. 2020, 108, 207–222. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, J.H.; Kim, H.W. Osteoinductive Fibrous Scaffolds of Biopolymer/Mesoporous Bioactive Glass Nanocarriers with Excellent Bioactivity and Long-Term Delivery of Osteogenic Drug. ACS Appl. Mater. Interfaces 2015, 7, 1140–1152. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, X.; Meng, S.; Dai, X.; Han, B.; Deng, X. Enhanced Critical Size Defect Repair in Rabbit Mandible by Electrospun Gelatin/β-TCP Composite Nanofibrous Membranes. J. Nanomater. 2015, 2015, 396916. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Ma, H.; Zhou, Y.; Ma, X.; Liu, J.; Huang, J.; Yu, N. Bilayered PLGA/Wool Keratin Composite Membranes Support Periodontal Regeneration in Beagle Dogs. ACS Biomater. Sci. Eng. 2016, 2, 2162–2175. [Google Scholar] [CrossRef]
- Lotfi, G.; Shokrgozar, M.A.; Mofid, R.; Abbas, F.M.; Ghanavati, F.; Baghban, A.A.; Yavari, S.K.; Pajoumshariati, S. Biological Evaluation (In Vitro and In Vivo) of Bilayered Collagenous Coated (Nano Electrospun and Solid Wall) Chitosan Membrane for Periodontal Guided Bone Regeneration. Ann. Biomed. Eng. 2016, 44, 2132–2144. [Google Scholar] [CrossRef]
- Assunção, M.; Dehghan-Baniani, D.; Yiu, C.H.K.; Später, T.; Beyer, S.; Blocki, A. Cell-Derived Extracellular Matrix for Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 602009. [Google Scholar] [CrossRef]
- Guan, Y.; Yang, B.; Xu, W.; Li, D.; Wang, S.; Ren, Z.; Zhang, J.; Zhang, T.; Liu, X.; Li, J.; et al. Cell-Derived Extracellular Matrix Materials for Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 1007–1021. [Google Scholar] [CrossRef]
- Xing, H.; Lee, H.; Luo, L.; Kyriakides, T.R. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol. Adv. 2020, 42, 107421. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, S.; Bishop, J.; Khademhosseini, A.; Yang, Y. The osteogenic differentiation of human bone marrow MSCs on HUVEC-derived ECM and β-TCP scaffold. Biomaterials 2012, 33, 6998–7007. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Silva, J.C.; Udangawa, R.N.; Cabral, J.M.; Ferreira, F.C.; da Silva, C.L.; Linhardt, R.J.; Vashishth, D. Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 99, 479–490. [Google Scholar] [CrossRef]
- Silva, J.C.; Carvalho, M.S.; Udangawa, R.N.; Moura, C.S.; Cabral, J.M.S.; da Silva, C.L.; Ferreira, F.C.; Vashishth, D.; Linhardt, R.J. Extracellular matrix decorated polycaprolactone scaffolds for improved mesenchymal stem/stromal cell osteogenesis towards a patient-tailored bone tissue engineering approach. J. Biomed. Mater. Res. Part Appl. Biomater. 2020, 108, 2153–2166. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.M.; Huang, J.P.; Lu, K.X.; Sun, W.L.; Tan, J.Y.; Li, B.X.; Chen, L.L.; Wu, Y.M. Regeneration potential of decellularized periodontal ligament cell sheets combined with 15-deoxy-Δ 12,14-prostaglandin J2 nanoparticles in a rat periodontal defect. Biomed. Mater. 2021, 16, 45008. [Google Scholar] [CrossRef]
- Farag, A.; Vaquette, C.; Theodoropoulos, C.; Hamlet, S.M.; Hutmacher, D.W.; Ivanovski, S. Decellularized periodontal ligament cell sheets with recellularization potential. J. Dent. Res. 2014, 93, 1313–1319. [Google Scholar] [CrossRef]
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Bartold, P.M.; Hutmacher, D.W.; Ivanovski, S. The effect of decellularized tissue engineered constructs on periodontal regeneration. J. Clin. Periodontol. 2018, 45, 586–596. [Google Scholar] [CrossRef]
- Chen, C.C.; Lee, S.Y.; Teng, N.C.; Hu, H.T.; Huang, P.C.; Yang, J.C. In Vitro and In Vivo Studies of Hydrophilic Electrospun PLA95/β-TCP Membranes for Guided Tissue Regeneration (GTR) Applications. Nanomaterials 2019, 9, 599. [Google Scholar] [CrossRef]
- George, P.; Jayakumar, N.; Kaarthikeyan, G. Effectiveness of electrospun Ocimum sanctum nanofibers as an adjunct to scaling and root planning in the management of chronic periodontitis: A randomized controlled clinical trial. J. Int. Oral Health 2021, 13, 115–121. [Google Scholar] [CrossRef]
- Fraser, D.; Caton, J.; Benoit, D.S.W. Periodontal Wound Healing and Regeneration: Insights for Engineering New Therapeutic Approaches. Front. Dent. Med. 2022, 3, 815810. [Google Scholar] [CrossRef]
- Aveic, S.; Craveiro, R.B.; Wolf, M.; Fischer, H. Current Trends in In Vitro Modeling to Mimic Cellular Crosstalk in Periodontal Tissue. Adv. Healthc. Mater. 2021, 10, 2001269. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Masante, B.; Putame, G.; Massai, D.; Mussano, F. Challenges of Periodontal Tissue Engineering: Increasing Biomimicry through 3D Printing and Controlled Dynamic Environment. Nanomaterials 2022, 12, 3878. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Cuggia, G.; Scribante, A. Domiciliary Use of Chlorhexidine vs. Postbiotic Gels in Patients with Peri-Implant Mucositis: A Split-Mouth Randomized Clinical Trial. Appl. Sci. 2022, 12, 2800. [Google Scholar] [CrossRef]

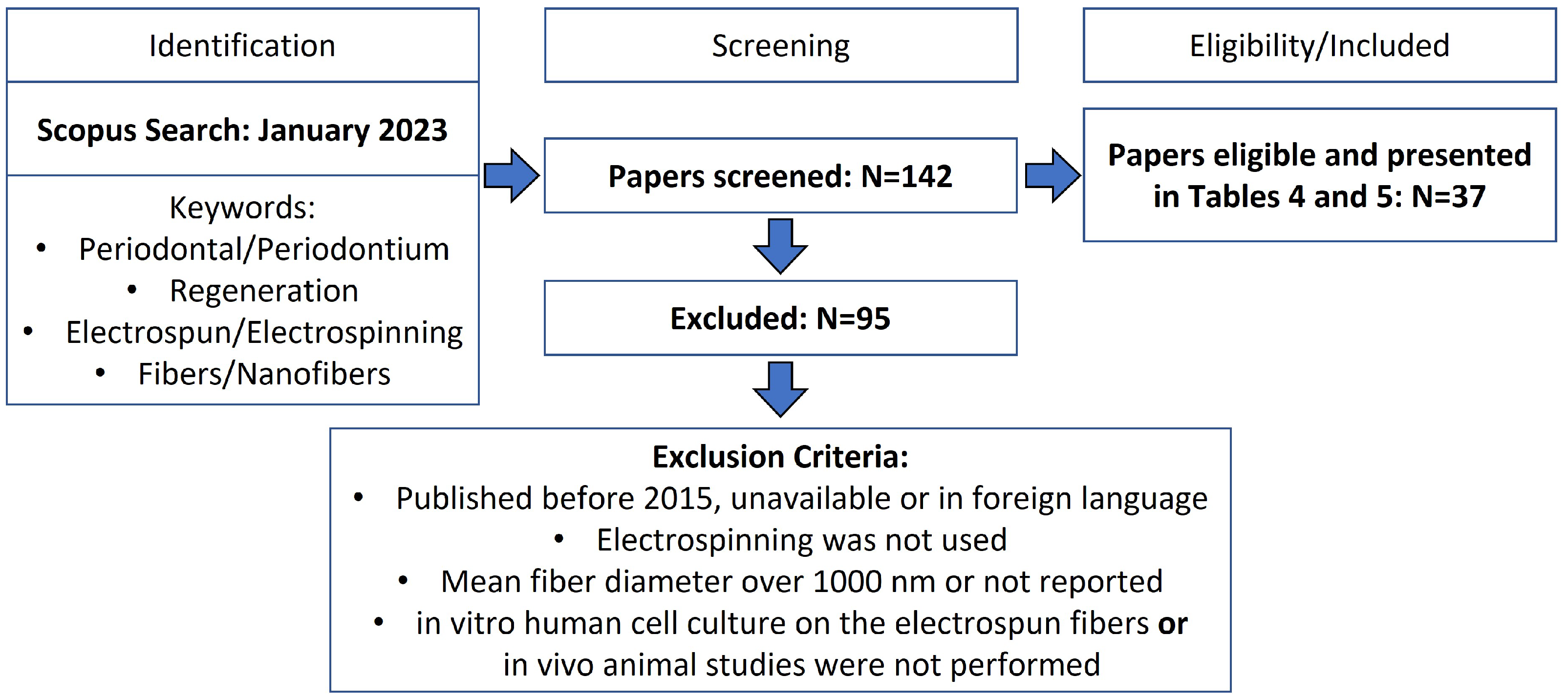
| Non-Surgical | Surgical |
|---|---|
| Plaque control [26] | Open flap surgery [27] |
| Scaling and root planing [28] | Pocket reduction [29] |
| Adjunctive therapies [30] | Guided tissue regeneration [31] |
| Laser treatment [32] | Bone grafting [33] |
| Dental Tissues | Other Tissues | ||
|---|---|---|---|
| Periodontal Ligament [51] | Dental Pulp [53] | Gingiva [50] | Bone Marrow [49] |
| Exfoliated Deciduous Teeth [54] | Dental Follicle [55] | Adipose Tissue [56] | |
| Physical | Mechanical | Chemical |
|---|---|---|
| Fiber diameter | Ultimate tensile stress | Composition Hydrophilicity |
| Fiber alignment | Elastic modulus Elongation | Functionalization (e.g., additives) |
| Polymers + Additives | MFD (nm) | Alignment | Cells | Main Outcomes from In Vitro Culture Studies | Year; Ref |
|---|---|---|---|---|---|
| CTS, PEG | 410 ± 163 288 ± 107 | aligned non-aligned | ES-MPs | Non-aligned fibers: ↑ calcium deposition by ES-MPs; Aligned fibers: ↑ cell viability | 2017; [74] |
| COL, CTS, PCL | 239 ± 26 | non-aligned | PDLCs | Membranes with CTS → ↑ cell viability | 2020; [75] |
| GEL, PEG + HAP | 528 ± 17 | non-aligned | BMMSCs PDLCs | ↑ porosity → ↑ cell viability; ↑ POSTN and OPN expression in cocultures | 2022; [76] |
| PCL, GEL | 599 ± 95 590 ± 167 | aligned non-aligned | PDLSCs | Both types non-cytotoxic; cells elongated along fiber alignment on aligned fibers | 2019; [77] |
| PCL//GEL + MgO | ≈400 | non-aligned | PDLSCs | MgO NPs in the core → antibacterial + ↑ mineralization + ↑ ALP and RUNX2 | 2021; [78] |
| PCL, GEL + CeO2 | 378 ± 204 355 ± 181 | non-aligned | PDLSCs | ↑ cell proliferation and ALP activity in membranes with CeO2 NPs | 2022; [79] |
| PCL, PEG | 522 ± 159 | non-aligned | PDLSCs | ↑ ALP, RUNX2, OC gene expression | 2021; [80] |
| PU + MET | 200–300 | non-aligned | DPSCs | ↑ metronidazole content→ ↓ cell viability | 2020; [81] |
| PLGA + pFGF-2 | 165 ± 60 | non-aligned | PDLCs | pFGF-2 → ↑ cell viability; ↑ COL I and scleraxis gene expression | 2020; [73] |
| SF, PEG | 300–400 | non-aligned | PDLCs | Sonication+↓ PEG→ ↑ cell proliferation | 2019; [82] |
| PLGA, WK + ORN or bFGF | 600–700 | non-aligned | PDLCs | ORN or bFGF→ ↑ proliferation; ↑ ALP; cytocompatible but ↓ with ↑ ORN/bFGF | 2020; [83] [84] |
| PCL, GEL + ZnO | 250–650 | non-aligned | DPSCs | Antibacterial due to ZnO NPs; cytocompatible (but ↓ with ↑ ZnO) | 2015; [85] |
| PLA, CTS | ≈200 | non-aligned | PDLCs; BMMSCs | ↑ pro-inflammatory mediators expression by PDLCs; ↑ CTS NPs → ↑ hydrophilic, ↑ OPG and mineralization by BMMSCs | 2018; [86] |
| PLA, Calcium alginate | 250 ± 90 | non-aligned | PDLCs; BMMSCs | ↑ COL I, RUNX2 and OPG by BMMSCs and ↑ pro-inflammatory levels by PDLCs; Alginate → ↑ hydrophilic, ↑ cell viability | 2020; [87] |
| COL, CTS + BG | 159 ± 59 | non-aligned | PDLCs | ↑ cell viability; ↑ RUNX2, OPN, OC, ALP gene expression; antibacterial activity | 2017; [88] |
| SF, PEG + vancomycin | ≈500 | non-aligned | PDLCs | Antibacterial due to vancomycin loaded GEL NSs; ↑ GEL NSs → ↑ cell proliferation | 2017; [89] |
| PCL + DOX | 150–300 | non-aligned | PDLCs | ↑ DOX → antibacterial, but ↓ cell viability | 2016; [16] |
| PCL, COL, PEG + rCMP1 | ≈200 | non-aligned | PDLCs | rCMP1 → ↑ CMP1, CAP; ↓ OC, OPN | 2016; [90] |
| Zein, GEL | 407 ± 140 | non-aligned | PDLSCs | GEL → ↑ cell proliferation; ↑ ALP activity | 2017; [91] |
| PCL | 377 ± 3 | non-aligned | DFSCs | Fibroblastic differentiation | 2016; [92] |
| PLGA + DMOG, nSi | 1069 922 | non-aligned | PDLSCs | DMOG→ ↑ VEGF; nSi→ ↑ ALP, OC, OPN RUNX2; induced angio- and osteogenesis | 2021; [71] |
| PCL//PEG + EMD | 500–1000 | non-aligned | PDLSCs | Enamel Matrix Derivative in the core → ↑ OC, RUNX2, ALP and OPN expression | 2021; [93] |
| PCL, GEL | 500–600 | non-aligned | GFs | ↑ cell viability; ↑ COL secretion/deposition | 2020; [94] |
| PLGA, GEL | 322 ± 58 | aligned | DFSCs | Cell proliferation along fiber direction; ↓ ALP, RUNX2; ↑ VEGF gene expression | 2015; [95] |
| PLGA or PCL + MET, AMX | 240 ± 48 282 ± 68 | non-aligned | GFs | Both: > 80 % cell viability with different drug concentrations; PCL → ↑ cell viability | 2021; [66] |
| PLA + CaO2, MnO2 | ≈300 | non-aligned | NOK | Sustained oxygen release; 7.5% CaO2 + MnO2 → antibacterial and ↑ cell viability | 2022; [96] |
| PCL, Zein + β-GP//PEG + Curc, TH | 150–300 | non-aligned | NOK | Coaxial fibers on the surface of 3D-printed layer; TH → antibacterial; Biocompatible but ↑ Curc/TH release → ↓ cell viability | 2022; [97] |
| PLCL + TH | ≈600 | non-aligned | OK; OF | OF > OK viability; ↑ TH → ↓ cell viability | 2022; [98] |
| CTS//PVA + TH | 150–300 | non-aligned | DF | Cytocompatible; TH → antibacterial | 2020; [67] |
| PLGA//GT + TH | 200–400 | non-aligned | DF | Coaxial → ↑ prolonged TH release than blend; cytocompatible; TH → ↓ cell viability | 2016; [99] |
| PCL | 300–800 | non-aligned | DF | Biocompatible; cell barrier properties | 2019; [100] |
| GEL, CTS + CA | 350–500 | non-aligned | DF SAOS-2 | Biocompatible; only scaffolds with low molecular weight CTS → antibacterial | 2021; [101] |
| GEL + SP600125, SB203580 | ≈350 | non-aligned | PDLCs | Cytocompatible: similar cell proliferation on loaded fibers, pure GEL fibers and TCP; ↓ MMP-2 and MMP-13 expression | 2019; [102] |
| PVA + BR, Mg-HAP | 200 ± 15 300 ± 14 | non-aligned | GF | Surface of a porous scaffold; BR/Mg-HAP →↑ cell adhesion, proliferation, migration | 2020; [103] |
| PLA + AMX | 737 ± 128 775 ± 174 | non-aligned | PDLCs | Cell viability similar to TCP; ↑ cyclin D and ROCK II expression; AMX → antibacterial | 2021; [104] |
| PLGA//PEI + pBMP-2 | 300–500 | non-aligned | PDLSCs | Blend and coaxial show similar cell viability; Coaxial → ↑ BMP-2, RUNX2 expression, ↑ calcium levels, ↑ transfection efficiency | 2016; [72] |
| PLA + QUE | ≈500 | non-aligned | GF | QUE release in acidic conditions; QUE → ↓ pro-inflammatory mediator expression | 2022; [68] |
| CTS, PEG + Ag, HAP, Si, DOXH | 150–200 | non-aligned | GF | Biocompatible; DOXH → ↓ cell viability; antibacterial due to DOXH and Ag/HAP/Si | 2022; [105] |
| Polymers + Additives | MFD (nm) | Alignment | Model | Main Outcomes from In Vivo Studies | Year; Ref. |
|---|---|---|---|---|---|
| PCL, COL, PEG + rCMP1 | ≈200 | non-aligned | rat | rCMP1 → ↑ cementum-like tissue and ↓ new bone formation in defect | 2016; [90] |
| COL, CTS, PCL | 239 ± 26 | non-aligned | rat | Membranes with CTS → ↑ new bone formed; ↑ bone ALP and OC expression | 2020; [75] |
| PCL, GEL | 599 ± 95 590 ± 167 | aligned non-aligned | rat | Aligned Fibers → ↑ new oriented PDL fibers similar to natural PDL, ↑ POSTN | 2019; [77] |
| COL, CTS + BG | 159 ± 59 | non-aligned | dog | ↑ new bone formation; ↓ inflammation compared to the control (no membrane) | 2017; [88] |
| PCL, GEL + CeO2 | 378 ± 204 355 ± 181 | non-aligned | rat | Membranes with CeO2 NPs accelerated new bone formation | 2022; [79] |
| PLGA + pFGF-2 | 165 ± 60 | non-aligned | dog | pFGF-2 → ↓ root surface resorption; more regular PDL-like tissues formed | 2020; [73] |
| PLGA + DMOG, nSi | 1069 922 | non-aligned | rat | DMOG/nSi→ functional cementum- PDL-bone complex; ↑ new bone formed | 2021; [71] |
| PCL, PEG | 616 ± 213 574 ± 218 | aligned non-aligned | rat | Aligned fibers: ↑ new PDL-like oriented fibers; ↑ COL I, ↓ COL III; ↑ POSTN | 2015; [106] |
| PCL, GEL | 500–600 | non-aligned | rat | Biodegradable; strong cell barrier effects | 2020; [94] |
| PLGA, GEL | 322 ± 58 | aligned | swine | Layer of a p-DFSCs seeded construct → cementum and PDL-like tissues formed | 2015; [95] |
| PLGA + MWNTs | <1000 | non-aligned | dog | ↑ new cementum, PDL and bone formed compared to the control (no membrane) | 2018; [107] |
| PVA + HAP, MET | 200–300 | non-aligned | rat | MET → antibacterial, disease control; HAP → significant ↓ in pocket depth | 2018; [108] |
| PLA + MgO | ≈500 | non-aligned | rat | MgO NPs → ↑ new bone formation | 2020; [109] |
| PCL, GEL + BG, DEX | <1000 | non-aligned | rat | Biocompatible with and without DEX; DEX→ ↑ bone volume and surface density | 2015; [110] |
| GEL + β-TCP | ≈400 | non-aligned | rabbit | β-TCP → ↑ bone volume; ↑ OC | 2015; [111] |
| PLGA, WK | 200–500 | non-aligned | dog | Results similar to COL membrane: ↑ new cementum, PDL and bone; ↑ bone density than with periodontal flap surgery | 2016; [112] |
| PCL, PVP + ZIF-8, FK506 | 797 ± 138 654 ± 332 | non-aligned | rat | ZIF-8/FK506 → ↑ bone volume; ↑ OC; antibacterial + anti-inflammatory effects | 2022; [69] |
| PLGA, GEL + TP, APR | 200–400 | non-aligned | rat | TP/APR → ↑ bone volume; ↑ dense PDL with ↑ COL fibers; anti-inflammatory | 2022; [70] |
| COL | 50–300 | non-aligned | rabbit | Layer of COL nanofibers on CTS film → ↑ new bone formed (similar to Bio-Gide) | 2016; [113] |
| PLGA or PCL + MET, AMX | 240 ± 48 282 ± 68 | non-aligned | rat rabbit | Prolonged drug release; Biocompatible; ↓ inflammation in rabbits than in rats | 2021; [66] |
| GEL + rBMP-2, SP600125, SB203580 | ≈350 | non-aligned | dog | Both inhibitors → ↑ bone volume; larger angulation of the regenerated PDL fibers, closer to natural PDL | 2019; [102] |
| PVA + BR, Mg-HAP | 200 ± 15 300 ± 14 | non-aligned | rat | BR/Mg-HAP → ↑ oral wound healing rate; more regular arrangement of COL fibers; BR → antibacterial agent | 2020; [103] |
| PLA + AMX | 737 ± 128 775 ± 174 | non-aligned | rat | AMX → ↑ dense-packed and well aligned regenerated COL fibers; ↓ inflammation | 2021; [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.S.; Carvalho, M.S.; Silva, J.C. Recent Advances on Electrospun Nanofibers for Periodontal Regeneration. Nanomaterials 2023, 13, 1307. https://doi.org/10.3390/nano13081307
Santos MS, Carvalho MS, Silva JC. Recent Advances on Electrospun Nanofibers for Periodontal Regeneration. Nanomaterials. 2023; 13(8):1307. https://doi.org/10.3390/nano13081307
Chicago/Turabian StyleSantos, Mafalda S., Marta S. Carvalho, and João C. Silva. 2023. "Recent Advances on Electrospun Nanofibers for Periodontal Regeneration" Nanomaterials 13, no. 8: 1307. https://doi.org/10.3390/nano13081307
APA StyleSantos, M. S., Carvalho, M. S., & Silva, J. C. (2023). Recent Advances on Electrospun Nanofibers for Periodontal Regeneration. Nanomaterials, 13(8), 1307. https://doi.org/10.3390/nano13081307







