Production and Incorporation of Calcium-Hydrolyzed Nanoparticles in Alkali-Activated Mine Tailings
Abstract
1. Introduction
2. Materials and Methods
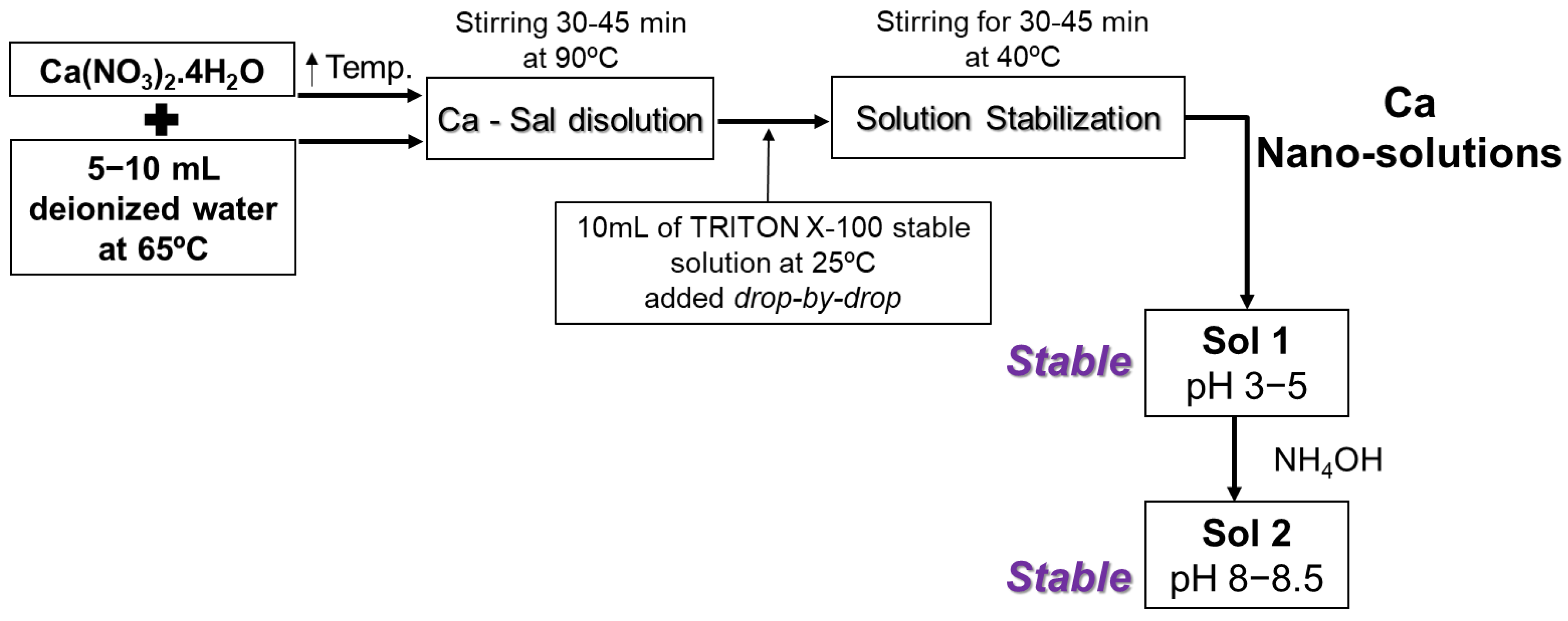
2.1. Synthesis of Calcium-Hydrolyzed Nano-Solutions
2.2. Alkaline Activation Process of MT Samples—Cubic Sample Preparation
2.3. High-Resolution Transmission Electron Microscopy (HR-TEM) Characterization
2.4. Uniaxial Compressive Tests
2.5. Fourier Transform Infrared (FTIR) Characterization
2.6. Quantitative X-ray Diffraction (QXRD) Characterization
2.7. Scanning Electron Microscopy/Energy-Dispersive Spectroscopy (SEM/EDS) Characterization
2.8. Nitrogen Adsorption–Desorption Analyses
3. Results and Discussion
3.1. Raw Material Characterization
3.2. Synthesis of Calcium-Hydrolyzed Nano-Solutions
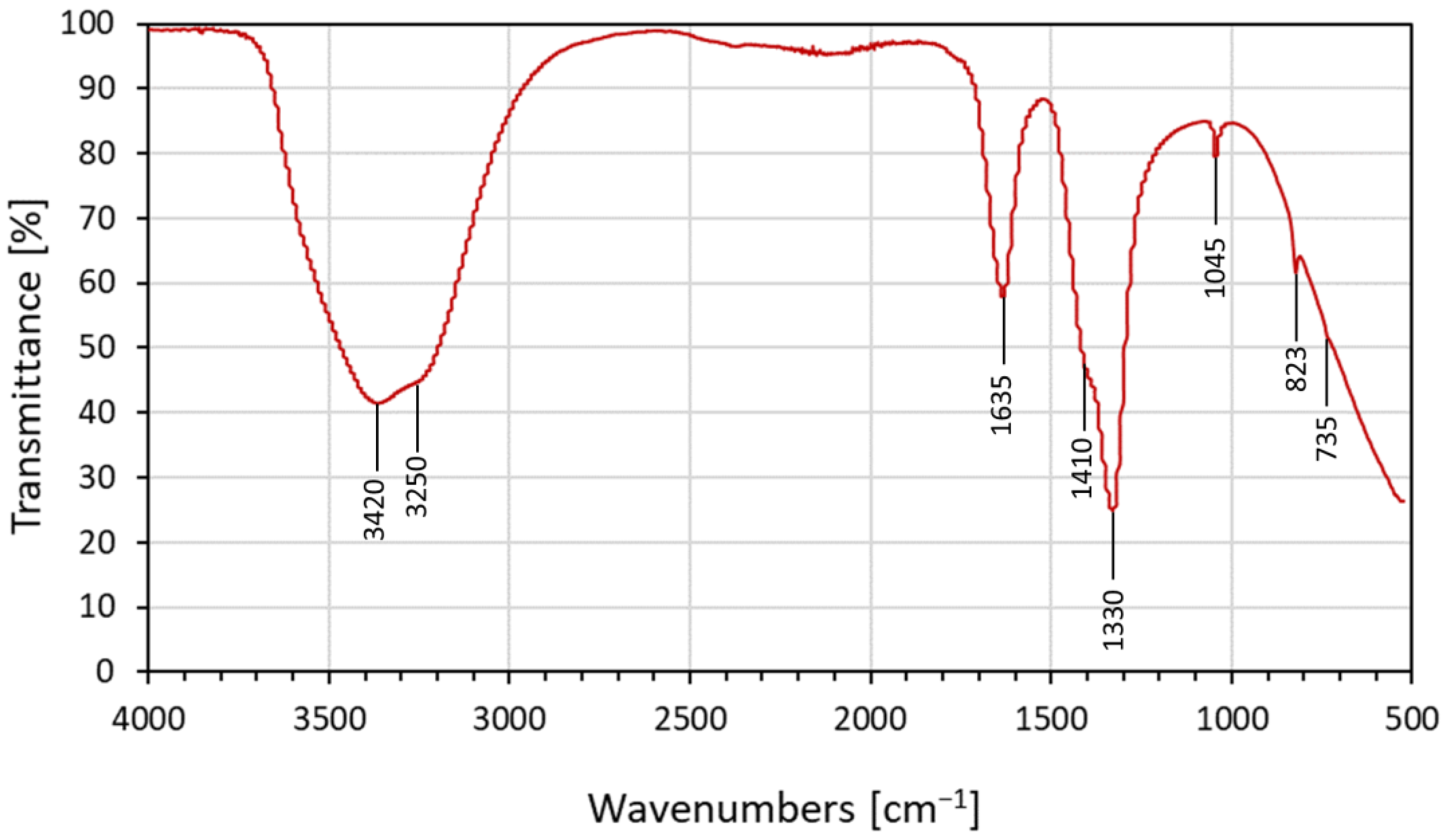
3.2.1. FTIR of Calcium-Hydrolyzed Nano-Solutions
3.2.2. HR-TEM of Calcium-Hydrolyzed Nano-Solutions
3.3. Production and Characterization of AAM Systems
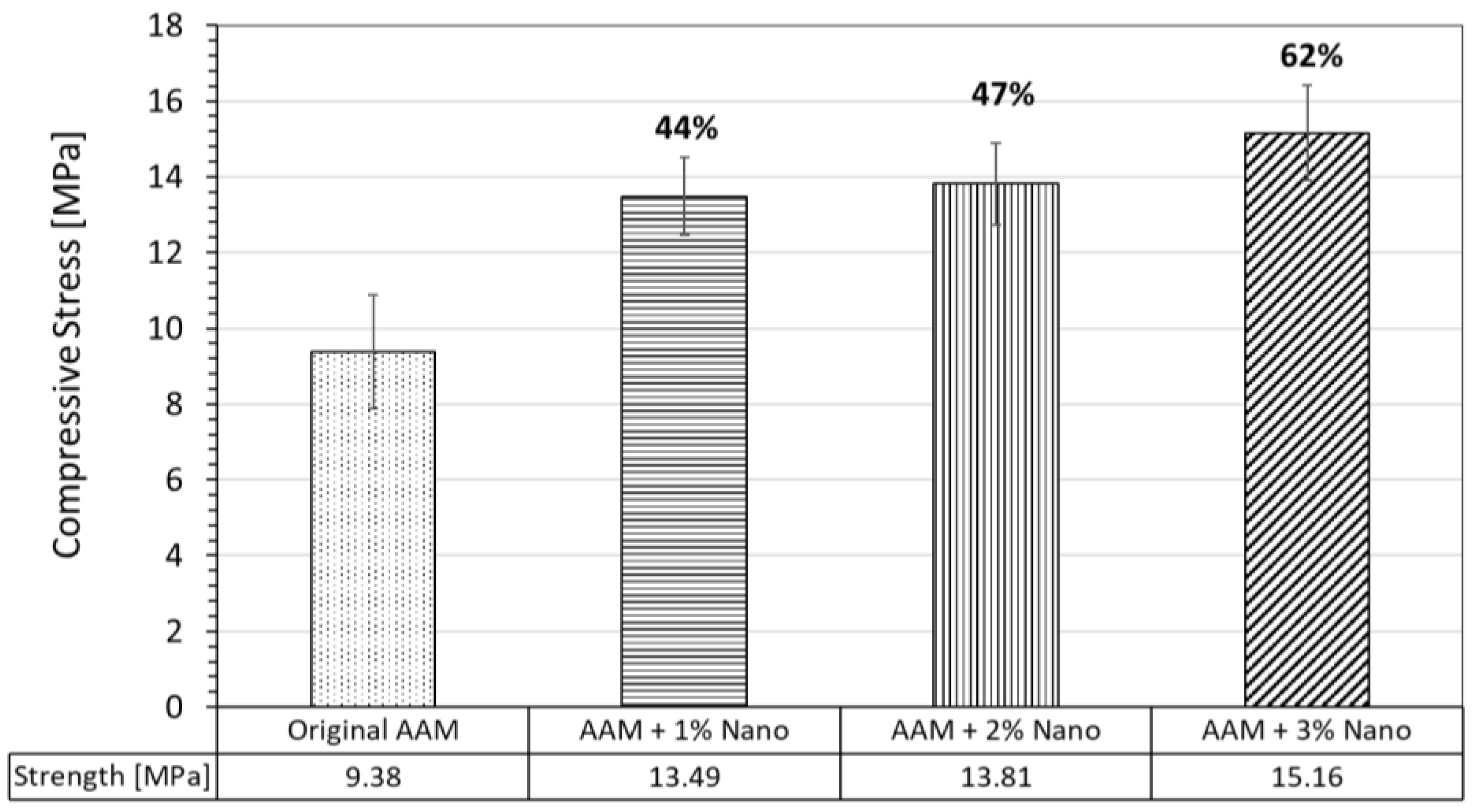
3.3.1. Compressive Strength of AAM Systems
3.3.2. Quantitative X-ray Diffraction (QXRD) Analysis of AAM Systems
3.3.3. FTIR Characterization of AAM Systems
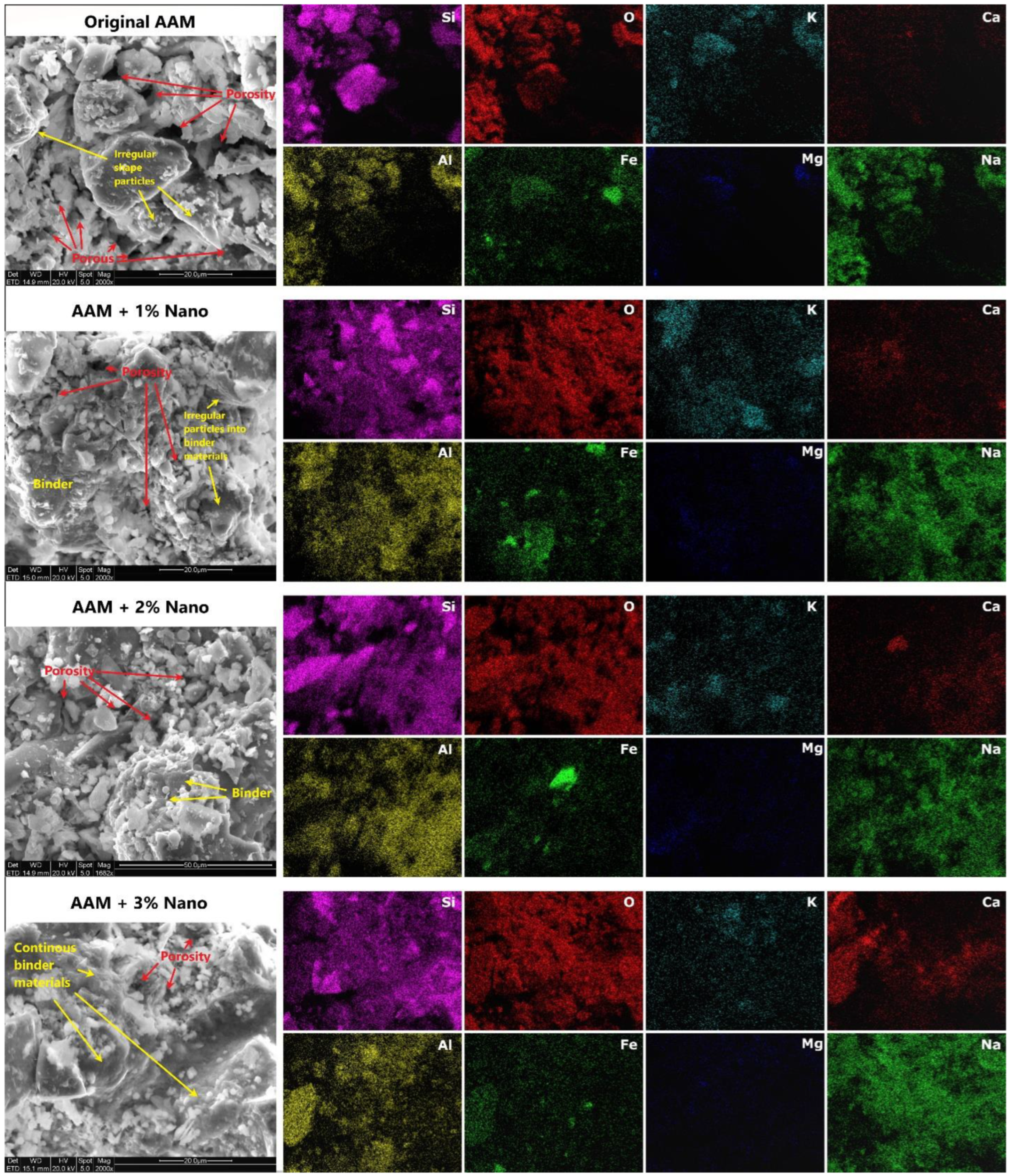
3.3.4. SEM/EDS Characterization of AAM Systems
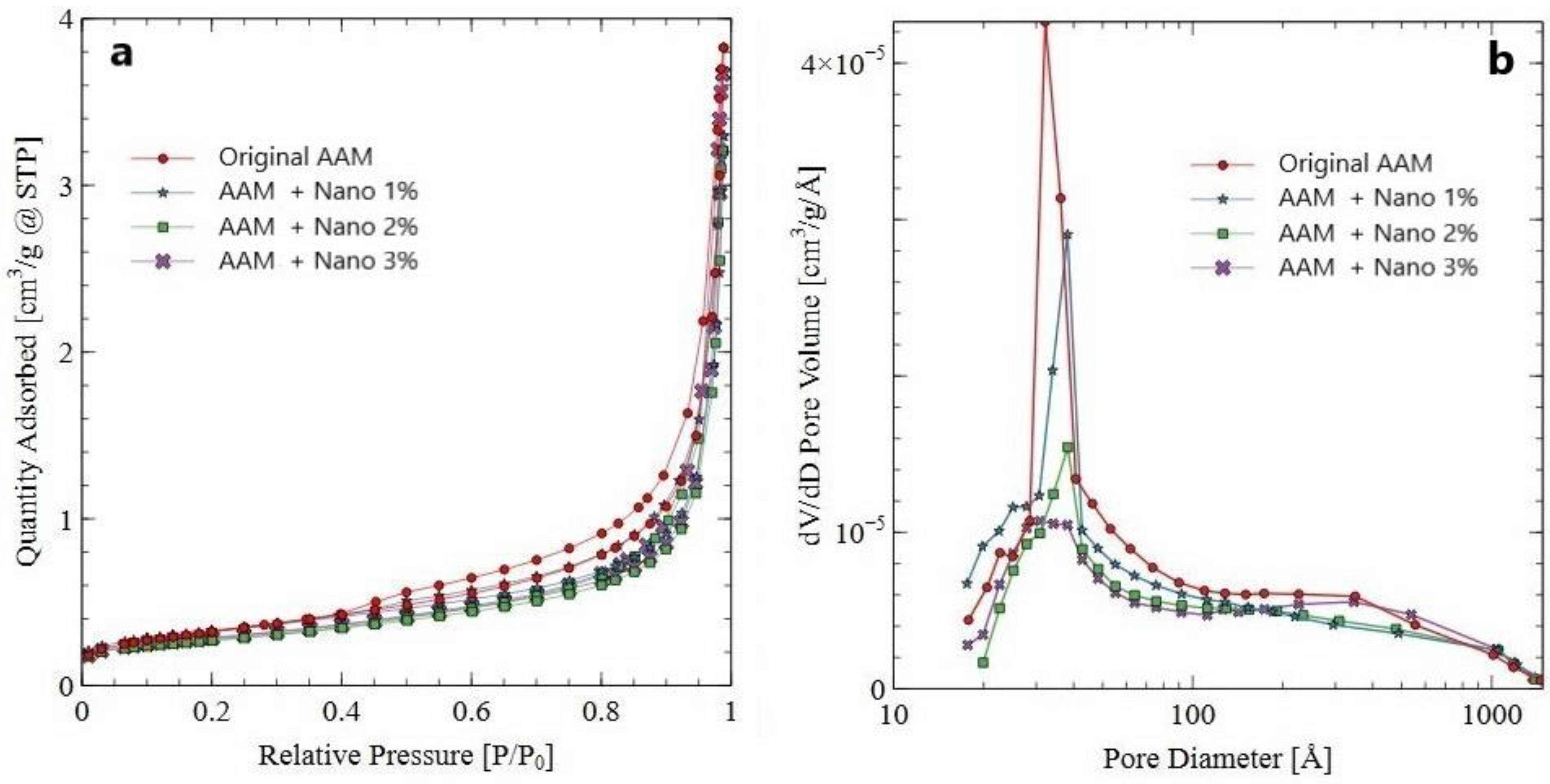
3.3.5. Nitrogen Adsorption–Desorption Analyses of AAM Systems
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perumal, P.; Kiventerä, J.; Illikainen, M. Influence of alkali source on properties of alkali activated silicate tailings. Mater. Chem. Phys. 2021, 271, 124932. [Google Scholar] [CrossRef]
- Gokçe, H.S.; Tuyan, M.; Nehdi, M.L. Alkali-activated and geopolymer materials developed using innovative manufacturing techniques: A critical review. Constr. Build. Mater. 2021, 303, 124483. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Cosentino, I.; Liendo, F.; Arduino, M.; Restuccia, L.; Bensaid, S.; Deorsola, F.; Ferro, G.A. Nano CaCO3 particles in cement mortars towards developing a circular economy in the cement industry. Procedia Struct. 2020, 26, 155–165. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.L.; Brouwers, H.J.H. Assessing the chemical involvement of limestone powder in sodium carbonate activated slag. Mater. Struct. 2017, 50, 136. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilization of heavy metal in cement-based solidification/stabilization: A review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef]
- de Vargas, A.S.; Dal Molin, D.C.C.; Masuero, Â.B.; Vilela, A.C.F.; Castro-Gomes, J.; De Gutierrez, R.M. Strength development of alkali-activated fly ash produced with combined NaOH and Ca(OH)2 activators. Cem. Concr. Compos. 2014, 53, 341–349. [Google Scholar] [CrossRef]
- Mast, B.; Schroeyers, W.; Pontikes, Y.; Vandoren, B.; Schreurs, S. The Use of Alkali Activated Materials in Nuclear Industry, Comprehensive Nuclear Materials, 2nd ed.; Konings, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 6, pp. 537–556. [Google Scholar]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L. Durability of alkali-activated materials: Progress and perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Ji, T. Preliminary study on the water permeability and microstructure of concrete incorporating nano-SiO2. Cem. Concr. Res. 2005, 35, 1943–1947. [Google Scholar] [CrossRef]
- Bjornstrom, J.; Martinelli, A.; Matic, A.; Borjesson, L.; Panas, I. Accelerating effects of colloidal nano-silica for beneficial calcium-silicate-hydrate formation in cement. Chem. Phys. Lett. 2004, 39, 242–248. [Google Scholar] [CrossRef]
- Jo, B.W.; Kim, C.H.; Tae, G.H.; Park, J.B. Characteristics of cement mortar with nano-SiO2 particles. Constr. Build. Mater. 2007, 21, 1351–1355. [Google Scholar] [CrossRef]
- Qing, Y.; Zenan, Z.; Deyu, K.; Rongshen, C. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar] [CrossRef]
- Qing, Y.; Zenan, Z.; Li, S.; Rongshen, C. A comparative study on the pozzolanic activity between nano-SiO2 and silica fume. J. Wuhan Univ. Technol. 2006, 21, 153–157. [Google Scholar] [CrossRef]
- Kani, E.N.; Rafiean, A.H.; Alishah, A.; Astani, S.H.; Ghaffar, S.H. The effects of Nano-Fe2O3 on the mechanical, physical and microstructure of cementitious composites. Constr. Build. Mater. 2021, 266, 121137. [Google Scholar] [CrossRef]
- Sikora, P.; Horszczaruk, E.; Cendrowski, K.; Mijowska, E. The influence of Nano-Fe3O4 on the microstructure and mechanical properties of cementitious composites. Nanoscale Res. Lett. 2016, 11, 2–9. [Google Scholar] [CrossRef]
- Amer, A.A.M.; Abdullah, N.I. Behavior of Portland Cement pastes admixed with nano-iron oxide at elevated temperature. Int. Res. J. Eng. Technol. 2014, 3, 1473–1487. [Google Scholar]
- Li, Z.; Ding, S.; Yu, X.; Han, B.; Ou, J. Multifunctional cementitious composites modified with nano titanium dioxide: A review. Compos. Part A Appl. Sci. 2018, 111, 115–137. [Google Scholar] [CrossRef]
- Meng, T.; Yu, Y.; Qian, X.; Zhan, S.; Qian, K. Effect of Nano-TiO2 on the mechanical properties of cement mortar. Constr. Build. Mater. 2012, 29, 241–245. [Google Scholar] [CrossRef]
- Daniyal, M.; Akhtar, S.; Azam, A. Effect of Nano-TiO2 on the properties of cementitious composites under different exposure environments. J. Mater. Res. Technol. 2019, 8, 6158–6172. [Google Scholar] [CrossRef]
- Cuenca, E.; D’Ambrosio, L.; Lizunov, D.; Tretjakov, A.; Volobujeva, O.; Ferrara, L. Mechanical properties and self-healing capacity of ultra-high performance fibre-reinforced concrete with alumina nano-fibres: Tailoring ultra-high durability concrete for aggressive exposure scenarios. Cem. Concr. Compos. 2021, 118, 103956. [Google Scholar] [CrossRef]
- Rashad, A.M. A synopsis about the effect of nano-Al2O3, nano-Fe2O3, nano-Fe3O4 and nano-clay on some properties of cementitious materials—A short guide for civil engineers. Mater. Des. 2013, 52, 143–157. [Google Scholar] [CrossRef]
- Wu, Z.; Khayat, K.H.; Shi, C.; Tutikian, B.F.; Chen, Q. Mechanisms underlying the strength enhancement of UHPC modified with nano-SiO2 and nano-CaCO3. Cem. Concr. Compos. 2021, 119, 103992. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Liu, A.; Wang, X. Effect of nano-CaCO3 on properties of cement paste. Energy Procedia 2011, 16, 991–996. [Google Scholar] [CrossRef]
- Kim, G.M.; Nam, I.W.; Yang, B.; Yoon, H.N.; Lee, H.K.; Park, S. Carbon nanotube (CNT) incorporated cementitious composites for functional construction materials: The state of the art. Compos. Struct. 2019, 227, 111244. [Google Scholar] [CrossRef]
- da Silva Andrade-Neto, J.; Santos, T.A.; De Andrade-Pinto, S.; Ribeiro-Dias, C.M.; Véras-Ribeiro, D. Effect of the combined use of carbon nanotubes (CNT) and metakaolin on the properties of cementitious matrices. Constr. Build. Mater. 2021, 271, 121903. [Google Scholar] [CrossRef]
- Niu, X.-J.; Li, Q.-B.; Hu, Y.; Tan, Y.-S.; Liu, C.-F. Properties of cement-based materials incorporating nano-clay and calcined nano-clay: A. review. Constr. Build. Mater. 2021, 284, 122820. [Google Scholar] [CrossRef]
- Seifan, M.; Mendoza, S.; Berenjian, A. Mechanical properties and durability performance of fly ash based mortar containing nano- and micro-silica additives. Constr. Build. Mater. 2020, 252, 119121. [Google Scholar] [CrossRef]
- Collins, A. Nanotechnology Cookbook: Practical, Reliable and Jargon-Free Experimental Procedures, 1st ed.; Elsevier Science: London, UK, 2012; pp. 1–324. [Google Scholar]
- Li, H.; Xiao, H.-G.; Yuan, J.; Ou, J. Microstructure of cement mortar with nano-particles. Compos. Part B Eng. 2004, 352, 185–189. [Google Scholar] [CrossRef]
- Sobolev, K.; Flores, I.; Hermosillo, R.; Torres-Martínez, L.M. Nanomaterials and nanotechnology for high-performance cement composites. ACI Mater. 2006, 254, 93–120. [Google Scholar]
- Nakama, Y. Surfactants. In Cosmetic Science and Technology: Theoretical Principles and Applications, 1st ed.; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 231–244. [Google Scholar]
- Ambrosi, M.; Dei, L.; Giorgi, R.; Neto, C.; Baglioni, P. Colloidal particles of Ca(OH)2: Properties and applications to restoration of frescoes. Langmuir 2001, 17, 4251–4255. [Google Scholar] [CrossRef]
- Perera, Y. Preparation of nano-ceramics via aqueous sol-gel method modified with surfactants: An overview. Mater. Sci. Forum 2010, 644, 79–84. [Google Scholar]
- Taglieri, G.; Daniele, V.; Del Re, G.; Volpe, R. A new and original method to produce Ca(OH)2 nanoparticles by using an anion exchange resin. Adv. Nanoparticles 2015, 4, 17–24. [Google Scholar] [CrossRef]
- Paradles, H.H. Shape and size of a nonionic surfactant micelle. Triton X-100 in aqueous solution. J. Phys. Chem. 1980, 84, 599–607. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Park, I.; Villacorte-Tabelin, M. Synthesis and characterization of coal fly ash and palm oil fuel ash modified artisanal and small-scale gold mine (ASGM) tailings based geopolymer using sugar mill lime sludge as Ca-based activator. Heliyon 2021, 7, e06654. [Google Scholar] [CrossRef]
- Michalopoulou, A.; Maravelaki, P.N.; Kilikoglou, V.; Karatasios, I. Morphological characterization of water-based nanolime dispersions. J. Cult. Herit. 2020, 46, 11–20. [Google Scholar] [CrossRef]
- Jeon, D.; Jun, Y.; Jeong, Y.; Oh, J.E. Microstructural and strength improvements through the use of Na2CO3 in a cement less Ca(OH)2—Activated Class F fly ash system. Cem. Concr. Res. 2015, 67, 215–225. [Google Scholar] [CrossRef]
- Akturk, B.; Kizilkanat, A.B.; Kabay, N. Effect of calcium hydroxide on fresh state behavior of sodium carbonate activated blast furnace slag pastes. Constr. Build Mater. 2019, 21, 388–399. [Google Scholar] [CrossRef]
- Palomo, A.; Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A review on alkaline activation: New analytical perspectives. Mater. De Construcción 2014, 64, e022. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Do geopolymers actually contain nanocrystalline zeolites?—A re-examination of existing results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Puertas, F.; Sobrados, I.; Sanz, J. Structure of calcium silicate hydrate formed in alkaline activated slag. Influence of the type of alkaline activator. J. Am. Ceram. Soc. 2003, 86, 1389–1394. [Google Scholar] [CrossRef]
- Kiventerä, J.; Sreenivasan, H.; Cheeseman, C.; Kinnunen, P.; Illikainen, M. Immobilization of sulfates and heavy metals in gold mine tailings by sodium silicate and hydrated lime. J. Environ. Chem. Eng. 2018, 6, 6530–6536. [Google Scholar] [CrossRef]
- Blyszko, J.; Kiernozycki, W.; Guskos, N.; Zolnierkiewicz, G.; Typek, J.; Narkiewicz, U.; Podsiadl, M. Study of mechanical properties of concrete with low concentration of magnetic nanoparticles. J. Non-Cryst. Solids 2008, 354, 4515–4518. [Google Scholar] [CrossRef]
- Zhang, N.; Hedayat, A.; Sosa, H.G.B.; Cardenas, J.J.G.; Álvarez, G.E.S.; Rivera, V.A. Fracture and failure processes of geopolymerized mine tailings under uniaxial compression. In Proceedings of the 54th US Rock Mechanics/Geomechanics Symposium, Golden, CO, USA, 28 June–1 July 2020; Volume 1923, pp. 1–9. [Google Scholar]
- ASTM C109/C109M-20; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM: West Conshohocken, PA, USA, 2016.
- Snellings, R.; Salze, A.; Scrivener, K.L. Use of X-ray diffraction to quantify amorphous supplementary cementitious materials in anhydrous and hydrated blended cements. Cem. Concr. Res. 2014, 64, 89–98. [Google Scholar] [CrossRef]
- Haha, M.B.; De Weerdt, K.; Lothenbach, B. Quantification of the degree of reaction of fly ash. Cem. Concr. Res. 2010, 40, 1620–1629. [Google Scholar] [CrossRef]
- Williams, R.P.; Riessen, A.V. Determination of the reactive component of fly ashes for geopolymer production using XRF and XRD. Fuel 2010, 89, 3683–3692. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Kato, H. Selective growth of silver particles on the facets of synthetic diamond. CrystEngComm 2016, 18, 7430–7434. [Google Scholar] [CrossRef]
- Zhang, N.; Hedayat, A.; Sosa, H.G.B.; Tunnah, J.; Cárdenas, J.J.G.; Álvarez, G.E.S. Estimation of the mode I fracture toughness and evaluations on the strain behaviors of the compacted mine tailings from full-field displacement fields via digital image correlation. Theor. Appl. Fract. Mech. 2021, 114, 103014. [Google Scholar] [CrossRef]
- Perera-Mercado, Y.; Hedayat, A.; Tunstall, L.; Clements, C.; Hylton, J.; Figueroa, L.; Zhang, N.; Sosa, H.G.B.; Tupa, N.; Morales, I.Y.; et al. Effect of the Class C Fly Ash on Low-Reactive Gold Mine Tailing Geopolymers. Polymers 2022, 14, 2809. [Google Scholar] [CrossRef]
- Matalkah, F.; Soroushian, P. Synthesis and characterization of alkali aluminosilicate hydraulic cement that meets standard requirements for general use. Constr. Build. Mater. 2018, 158, 42–49. [Google Scholar] [CrossRef]
- Perera-Mercado, Y.A.; Betancourt-Galindo, R.; Saucedo-Salazar, E.M.; Puente-Urbina, B.A.; Medellín-Banda, D.I.; Neira-Velázquez, M.G.; Gutierrez-Villarreal, M.H.; García-Rodríguez, S.P. Production of micrometer-sized composite polymer-magnetic spheres using as precursor metallurgical wastes. Polym. Polym. Compos. 2014, 22, 387–392. [Google Scholar] [CrossRef]
- Hayes, S.M.; Root, R.A.; Perdrial, N.; Maier, R.M. Surficial weathering of iron sulfide mine tailings under semi-arid climate. Geochim. Cosmochim. Acta 2014, 141, 240–257. [Google Scholar] [CrossRef]
- Irish, D.E.; Walrafen, G.E. Raman and Infrared Spectral Studies of Aqueous Calcium Nitrate Solutions. J. Chem. Phys. 1967, 46, 378. [Google Scholar] [CrossRef]
- Amer, A.A.; El-Didamony, H.; El-Sokkary, T.M.; Wahdan, M.I. Synthesis and characterization of some calcium aluminate phases from nano-size starting materials. Boletín Soc. Española Cerámica Vidr. 2022, 61, 6106–6198. [Google Scholar] [CrossRef]
- Moreira, A.P.D.; Teixeira, A.M.R.F. An investigation on the formation of calcium naphthenate from commercial naphthenic acid solutions by thermogravimetric analysis. Braz. J. Pet. Gas 2009, 3, 051–056. [Google Scholar]
- Wu, H.B.; Chan, M.N.; Chan, C.K. FTIR characterization of polymorphic transformation of ammonium nitrate. Aerosol Sci. Technol. 2007, 41, 81–588. [Google Scholar] [CrossRef]
- Dua, Y.; Meng, Q.; Hou, R.; Yan, J.; Dai, H.; Zhang, T. Fabrication of nano-sized Ca(OH)2 with excellent adsorption ability for N2O4. Particuology 2012, 10, 737–743. [Google Scholar] [CrossRef]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in construction—Recent developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- Sumesh, M.; Alengaram, U.J.; Jumaat, M.Z.; Mo, K.H.; Alnahha, M.F. Incorporation of nano-materials in cement composite and geopolymer based paste and mortar—A review. Constr. Build. Mater. 2017, 148, 62–84. [Google Scholar] [CrossRef]
- Tsai, C.-J.; Huang, R.; Lin, W.-T.; Wang, H.-N. Mechanical and cementitious characteristics of ground granulated blast furnace slag and basic oxygen furnace slag blended mortar. Mater. Des. 2014, 60, 267–273. [Google Scholar] [CrossRef]
- Kamath, M.; Prashant, S.; Kumar, M. Micro-characterization of alkali activated paste with fly ash-GGBS-metakaolin binder system with ambient setting characteristics. Constr. Build. Mater. 2021, 277, 122323. [Google Scholar] [CrossRef]
- Zhou, Z.; Sofi, M.; Liu, J.; Li, S.; Zhong, A.; Mendis, P. Nano-CSH modified high volume fly ash concrete: Early-age properties and environmental impact analysis. J. Clean. Prod. 2021, 286, 124924. [Google Scholar] [CrossRef]
- Madrid, J.A.; Lanzón, M. Synthesis and morphological examination of high-purity Ca(OH)2 nanoparticles suitable to consolidate porous surfaces. Appl. Surf. Sci. 2017, 424, 2–8. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Taufiq-Yap, Y.H.; Lee, H.V. Synthesis of clamshell derived Ca(OH)2 nano-particles via simple surfactant-hydration treatment. J. Chem. Eng. 2015, 262, 1043–1051. [Google Scholar] [CrossRef]
- Kouvelos, E.; Kesore, K.; Steriotis, T.; Grigoropoulou, H.; Bouloubasi, D.; Theophilou, N.; Tzintzos, S.; Kanelopoulos, N. High pressure N2/CH4 adsorption measurements in clinoptilolites. Microporous Mesoporous Mater. 2007, 99, 106–111. [Google Scholar] [CrossRef]
- Montes-Luna, A.J.; Fuentes-López, N.C.; Castruita-de-León, G.; Pérez-Camacho, O.; Yeverino-Miranda, C.Y.; Perera-Mercado, Y.A. PBI/Clinoptilolite mixed matrix composite membranes for binary (CH4/N2) and ternary (CH4/N2/CO2) gas separations. J. Appl. Polym. Sci. 2020, 138, 50155. [Google Scholar]
- Falah, M.; Obenaus-Emler, R.; Kinnunen, P.; Illikainen, M. Effects of activator properties and curing conditions on alkali-activation of low-alumina mine tailings. Waste Biomass Valorization 2020, 11, 5027–5039. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Rózycka, A.; Gołek, Ł. Incorporation of Al in C-A-S-H gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR, and TEM analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The effect of alkali metals on the formation of geopolymeric gels from alkali-feldspars. Colloids Surf. A Physicochem. Eng. 2003, 216, 27–44. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, G.; Li, H.; Wu, Q.; Zhang, C.; Yin, Z.; Hua, S. Insights to the sulfate resistance and microstructures of alkali-activated metakaolin/slag pastes. Appl. Clay Sci. 2021, 202, 105968. [Google Scholar] [CrossRef]
- Canfield, G.M.; Eichler, J.; Griffith, K.; Hearn, J.D. The role of calcium in blended fly ash geopolymers. J. Mater. Sci. 2014, 49, 5922–5933. [Google Scholar] [CrossRef]
- Nikolov, A. Physical properties and powder XRD characterization of coal fly ash-based geopolymer heated up to 1150 °C. Rev. Bulg. Geol. Soc. 2019, 80, 36–38. [Google Scholar]
- Allel, A.; Naceur, M.W.; Benguergoura, H.; Ledoux, A.; Saeed, W.S.; Al-Odayni, A.B.; Aouak, T. Pervaporative separation of water–ethanol mixtures using an Algerian Na+ montmorillonite nanoclay-incorporated poly(vinyl alcohol) nanocomposite membrane. RSC Adv. 2020, 10, 39531. [Google Scholar] [CrossRef]
- Jin, Y.; Stephan, D.; Lu, Z. The effects of calcium formate on the early hydration of alkali silicate activated slag. Mater. Struct. 2019, 52, 37. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, A.; Cerdán, M.; Jordá, J.D.; Amat, B.; Cortina, J. Characterization of soil mineralogy by FTIR: Application to the analysis of mineralogical changes in soils affected by vegetation patches. Plant Soil 2019, 439, 447–458. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of calcium phosphates using Fourier transform infrared spectroscopy. In Infrared Spectroscopy, 1st ed.; Theophanides, T., Ed.; IntechOpen: London, UK, 2012; pp. 123–149. [Google Scholar]
- Castro, P.M.; Jagodzinski, P.W. FTIR and Raman spectra and structure of Cu(NO3)+ in aqueous solution and acetone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1991, 47, 1707–1720. [Google Scholar] [CrossRef]
- Koohestani, B.; Mokhtari, P.; Yilmaz, E.; Mahdipour, F.; Darban, A.K. Geopolymerization mechanism of binder-free mine tailings by sodium silicate. Constr. Build. Mater. 2021, 268, 121217. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Fernandez-Jimenez, A.; Blanco, M.T.; Palomo, A. FTIR study of the sol-gel synthesis of cementitious gels: C-S-H and N-A-S-H. J. Sol Gel Sci. Technol. 2008, 45, 63–72. [Google Scholar] [CrossRef]
- Temuujin, J.; Van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Academic Press: New York, NY, USA, 2013; pp. 107–110. [Google Scholar]



| Portlandite (Space Group: P-3m1) | ||
|---|---|---|
| (hkl) | Experimental dhkl (nm) | Theoretical dhkl (nm) |
| (011) | 0.2617 | 0.2685 |
| (101) | 0.2617 | 0.2685 |
| Phase | PDF Code | Original AAM wt. % | AAM + 1%Nano wt. % | AAM + 2%Nano wt. % | AAM + 3%Nano wt. % |
|---|---|---|---|---|---|
| Quartz | 01-077-8621 | 38.91 | 37.24 | 34.76 | 31.94 |
| Albite | 04-017-1022 | 9.21 | 8.43 | 8.13 | 7.90 |
| Muscovite | 00-001-1098 | 6.32 | 5.86 | 4.64 | 3.10 |
| Portlandite | 00-001-1079 | - | 0.21 | 0.21 | 0.20 |
| Calcite | 00-024-0027 | - | 0.21 | 0.22 | 0.33 |
| Magnetite | 01-075-0449 | 0.33 | 0.21 | 0.21 | 0.20 |
| Zeolite | 01-076-0620 | 1.20 | 0.63 | 0.24 | - |
| C-S-H | 01-076-0618 | - | 0.47 | 0.57 | 0.61 |
| C-A-S-H | 00-001-1079 | 0.71 | 0.83 | 0.89 | 0.92 |
| % Amorphous | - | 43.07 | 45.90 | 50.13 | 54.72 |
| Element | Semiquantitative Chemical Composition (wt.%) | |||
|---|---|---|---|---|
| Original AAM | AAM + 1% Nano | AAM + 2% Nano | AAM + 3% Nano | |
| O | 36.77 | 37.89 | 37.40 | 39.05 |
| Na | 9.23 | 8.45 | 8.02 | 8.05 |
| Mg | 0.98 | 0.83 | 1.16 | 1.40 |
| Al | 6.76 | 6.51 | 6.83 | 7.53 |
| Si | 36.30 | 37.65 | 37.12 | 34.09 |
| K | 1.88 | 1.42 | 1.56 | 1.77 |
| Ca | 1.48 | 1.88 | 2.13 | 2.37 |
| Fe | 6.60 | 5.37 | 5.78 | 5.74 |
| AAM System (Pore Range 2–4 nm) | BET Surface Area (m2/g) | Average Pore Diameter (nm) | Total Pore Volume (cm3/g) (Pores of 2–4 nm) × 10−5 |
|---|---|---|---|
| Original AAM | 1.151 | 3.44 | 5.21 |
| AAM + Nano 1% | 1.119 | 3.31 | 5.17 |
| AAM + Nano 2% | 0.921 | 3.25 | 4.14 |
| AAM + Nano 3% | 0.918 | 3.23 | 4.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera-Mercado, Y.; Zhang, N.; Hedayat, A.; Figueroa, L.; Saucedo-Salazar, E.; Clements, C.; Bolaños Sosa, H.G.; Tupa, N.; Morales, I.Y.; Canahua Loza, R.S. Production and Incorporation of Calcium-Hydrolyzed Nanoparticles in Alkali-Activated Mine Tailings. Nanomaterials 2023, 13, 1875. https://doi.org/10.3390/nano13121875
Perera-Mercado Y, Zhang N, Hedayat A, Figueroa L, Saucedo-Salazar E, Clements C, Bolaños Sosa HG, Tupa N, Morales IY, Canahua Loza RS. Production and Incorporation of Calcium-Hydrolyzed Nanoparticles in Alkali-Activated Mine Tailings. Nanomaterials. 2023; 13(12):1875. https://doi.org/10.3390/nano13121875
Chicago/Turabian StylePerera-Mercado, Yibran, Nan Zhang, Ahmadreza Hedayat, Linda Figueroa, Esmeralda Saucedo-Salazar, Cara Clements, Héctor Gelber Bolaños Sosa, Néstor Tupa, Isaac Yanqui Morales, and Reynaldo Sabino Canahua Loza. 2023. "Production and Incorporation of Calcium-Hydrolyzed Nanoparticles in Alkali-Activated Mine Tailings" Nanomaterials 13, no. 12: 1875. https://doi.org/10.3390/nano13121875
APA StylePerera-Mercado, Y., Zhang, N., Hedayat, A., Figueroa, L., Saucedo-Salazar, E., Clements, C., Bolaños Sosa, H. G., Tupa, N., Morales, I. Y., & Canahua Loza, R. S. (2023). Production and Incorporation of Calcium-Hydrolyzed Nanoparticles in Alkali-Activated Mine Tailings. Nanomaterials, 13(12), 1875. https://doi.org/10.3390/nano13121875







