Investigation of the Influence of Wound-Treatment-Relevant Buffer Systems on the Colloidal and Optical Properties of Gold Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of AuNPs Using Na3Cit and AA: Design-of-Experiments (DoE) Studies
2.2.2. Physicochemical and Optical Characterization of AuNPs
2.2.3. Effect of pH and Ion Concentration on the Physicochemical and Optical Properties of AuNPs
2.2.4. Simulation of the Local pH-Value Distribution near the Particle Surface
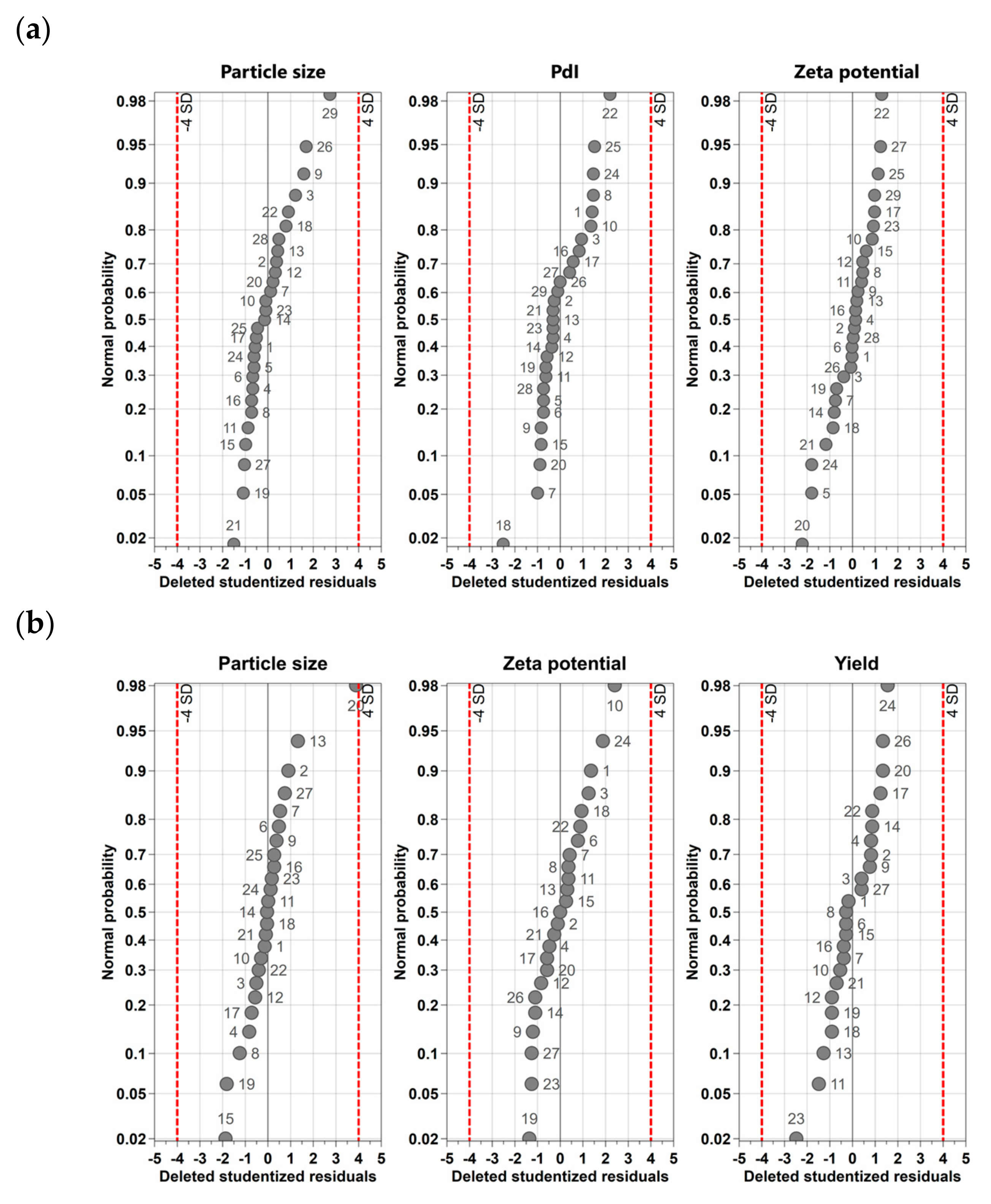
2.2.5. Statistics
3. Results
3.1. Design-of-Experiments (DoE) Studies
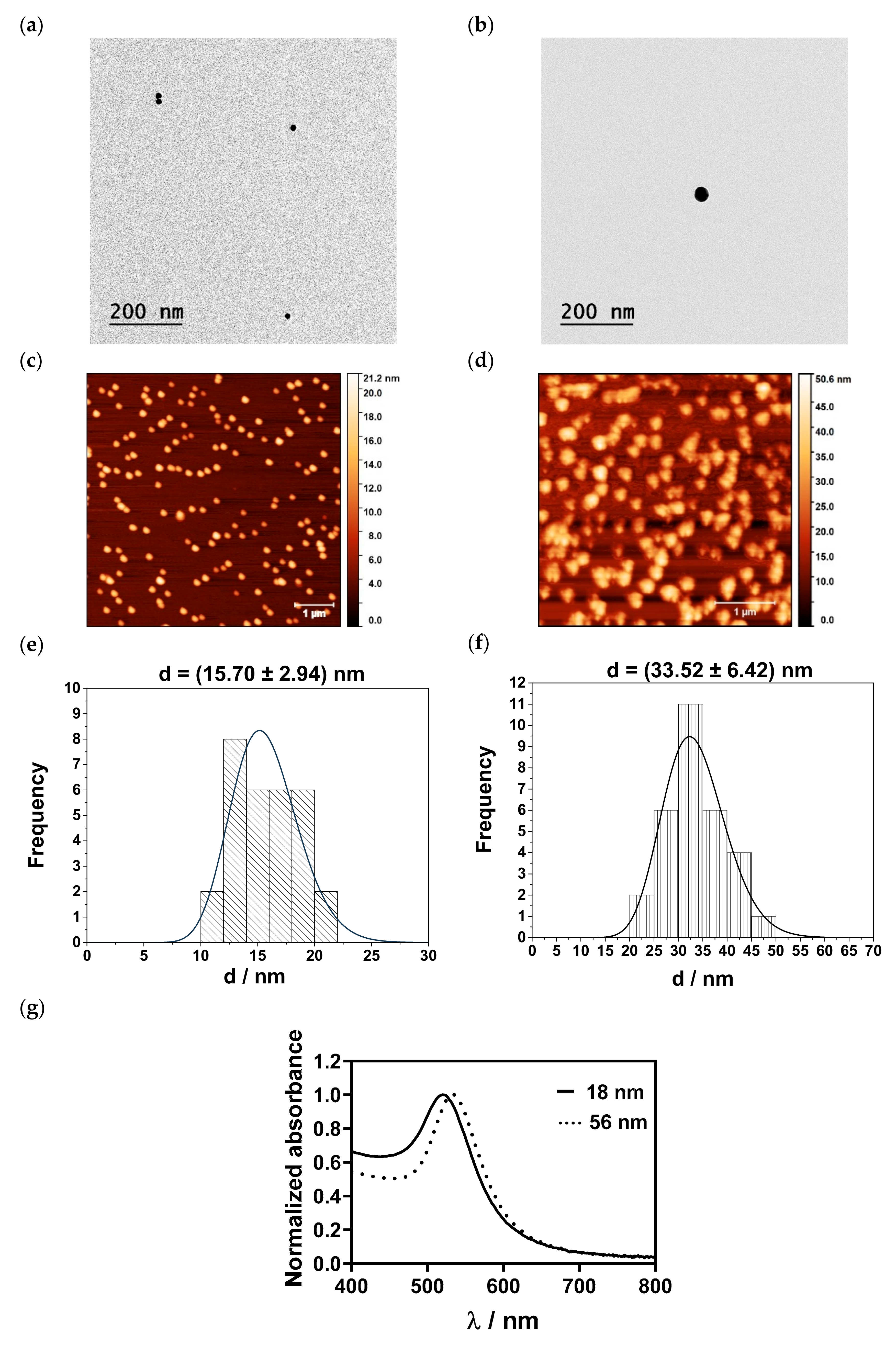
3.2. Physicochemical and Optical Characterization of AuNPs
3.3. Effect of the pH on the Physicochemical and Optical Properties of AuNPs
3.4. Simulation of the Local pH-Value Distribution near the Particle Surface
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, S.; Singhai, A.K. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. On streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2013, 6, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Proksch, E. Buffering Capacity. In pH of the Skin: Issues and Challenges; Surber, C., Abels, C., Maibach, H., Eds.; Karger: Basel, Switzerland, 2018; Volume 54, pp. 11–18. [Google Scholar] [CrossRef]
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Aly, R.; Shirley, C.; Cunico, B.; Maibach, H.I. Effect of prolonged occlusion on the microbial flora, pH, carbon dioxide and transepidermal water loss on human skin. J. Investig. Dermatol. 1978, 71, 378–381. [Google Scholar] [CrossRef]
- Khalilov, R. A comprehensive review of advanced nano-biomaterials in regenerative medicine and drug delivery. Adv. Biol. Earth Sci. 2023, 8, 5–18. [Google Scholar]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, E.R. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011, 14, 31–63. [Google Scholar] [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Akturk, O.; Kismet, K.; Yasti, A.C.; Kuru, S.; Duymus, M.E.; Kaya, F.; Caydere, M.; Hucumenoglu, S.; Keskin, D. Collagen/gold nanoparticle nanocomposites: A potential skin wound healing biomaterial. J. Biomater. Appl. 2016, 31, 283–301. [Google Scholar] [CrossRef]
- Naraginti, S.; Kumari, P.L.; das Sivakumar, R.K.A.; Patil, S.H.; Andhalkar, V.V. Amelioration of excision wounds by topical application of green synthesized, formulated silver and gold nanoparticles in albino Wistar rats. Mater. Sci. Eng. C 2016, 62, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.L.; Hsiung, T.M.; Chen, Y.Y.; Huang, Y.F.; Huang, C.C. Colorimetric detection of heavy metal ions using label-free gold nanoparticles and alkanethiols. J. Phys. Chem. C 2010, 114, 16329–16334. [Google Scholar] [CrossRef]
- Gu, H.; Ho, P.L.; Tong, E.; Wang, L.; Xu, B. Presenting Vancomycin on Nanoparticles to Enhance Antimicrobial Activities. Nano Lett. 2003, 3, 1261–1263. [Google Scholar] [CrossRef]
- Norman, S.; Stone, J.W.; Gole, A.; Murphy, C.; Sabo-Attwood, T.L. Targeted Photothermal Lysis of the Pathogenic Bacteria, Pseudomonas aeruginosa, with Gold Nanorods. Nano Lett. 2008, 8, 302–306. [Google Scholar] [CrossRef]
- Gil-Tomás, J.; Tubby, S.; Parkin, I.P.; Narband, N.; Dekker, L.; Nair, S.P.; Wilson, M.; Street, C. Lethal photosensitisation of Staphylococcus aureus using a toluidine blue O–tiopronin–gold nanoparticle conjugate. J. Mater. Chem. 2007, 17, 3739–3746. [Google Scholar] [CrossRef]
- Joshi, P.; Chakraborti, S.; Ramirez-Vick, J.E.; Ansari, Z.A.; Shanker, V.; Chakrabarti, P.; Singh, S.P. The anticancer activity of chloroquine-gold nanoparticles against MCF-7 breast cancer cells. Colloids Surf. B Biointerfaces 2012, 95, 195–200. [Google Scholar] [CrossRef]
- Schwert, G.W.; Eisenberg, M.A. The kinetics of the amidase and esterase activities of trypsin. J. Biol. Chem. 1949, 179, 665–672. [Google Scholar] [CrossRef]
- Han, K.; Zhu, J.Y.; Wang, S.B.; Li, Z.H.; Cheng, S.X.; Zhang, X.Z. Tumor targeted gold nanoparticles for FRET-based tumor imaging and light responsive on-demand drug release. J. Mater Chem. B. 2015, 3, 8065–8069. [Google Scholar] [CrossRef]
- Guo, B.; Zebda, R.; Drake, S.J.; Sayes, C.M. Synergistic effect of co-exposure to carbon black and Fe2O3 nanoparticles on oxidative stress in cultured lung epithelial cells. Part. Fiber Toxicol. 2009, 6, 4. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Al-Jamal, K.T.; Bomans, P.H.; Frederik, P.M.; Kostarelos, K. Functionalized-quantum-dot-liposome hybrids as multimodal nanoparticles for cancer. Small 2008, 4, 1406–1415. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Auinger, M.; Katsounaros, I.; Meier, J.C.; Klemm, S.O.; Ulrich Biedermann, P.; Topalov, A.A.; Rohwerdera, M.; Mayrhofer, K.J.J. Near-surface ion distribution and buffer effects during electrochemical reactions. Phys. Chem. Chem. Phys. 2011, 13, 16384–16394. [Google Scholar] [CrossRef] [PubMed]
- Stepan, T.; Tete, L.; Laundry-Mottiar, L.; Romanovskaia, E.; Hedberg, Y.S.; Danninger, H.; Auinger, M. Effect of nanoparticle size on the near-surface pH-distribution in aqueous and carbonate buffered solutions. Electrochim. Acta 2022, 409, 139923–139932. [Google Scholar] [CrossRef]
- Baber, R.; Mazzei, L.; Kim Thanh, N.T.; Gavriilidis, A. An engineering approach to synthesis of gold and silver nanoparticles by controlling hydrodynamics and mixing based on a coaxial flow reactor. Nanoscale 2017, 9, 14149–14161. [Google Scholar] [CrossRef]
- Damilos, S.; Alissandratos, I.; Panariello, L.; Radhakrishnan, A.N.P.; Cao, E.; Wu, G.; Besenhard, M.O.; Kulkarni, A.A.; Makatsoris, C.; Gavriilidis, A. Continuous citrate-capped gold nanoparticle synthesis in a two-phase flow reactors. J. Flow Chem. 2021, 11, 553–567. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, S.; Li, A.; Chen, L.; Lu, J.; Geng, X.; Xie, M.; Liang, X.; Wan, Y.; Yang, P. Continuous high-flux synthesis of gold nanoparticles with controllable sizes: A simple microfluidic system. Appl. Nanosci. 2020, 10, 661–669. [Google Scholar] [CrossRef]
- Turkevich, J.; Cooper, P.H.J. A study of the nucleation and growth process in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 55, 55–75. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 5–7. [Google Scholar] [CrossRef]
- Niidome, Y.; Nishioka, K.; Kawasaki, H.; Yamada, S. Rapid synthesis of gold nanorods by the combination of chemical reduction and photoirradiation processes; morphological changes depending on the growing processes. Chem. Comm. 2003, 18, 2376–2377. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gandhi, K.S.; Kumar, R. Modeling of formation of gold nanoparticles by citrate method. Ind. Eng. Chem. Res. 2007, 46, 3128–3136. [Google Scholar] [CrossRef]
- Pal, A.; Esumi, K.; Pal, T. Preparation of nanosized gold particles in a biopolymer using UV photoactivation. J. Colloid Interface Sci. 2005, 288, 396–401. [Google Scholar] [CrossRef]
- Wangoo, N.; Bhasin, K.K.; Mehta, S.K.; Suri, C.R. Synthesis and capping of water-dispersed gold nanoparticles by an amino acid: Bioconjugation and binding studies. J. Colloid Interface Sci. 2008, 323, 247–254. [Google Scholar] [CrossRef]
- Sau, T.K.; Murphy, C.J. Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J. Am. Chem. Soc. 2004, 126, 9–10. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef]
- Wang, J.; Liu, N.; Su, Q.; Lv, Y.; Yang, C.; Zhan, H. Green Synthesis of Gold Nanoparticles and Study of Their Inhibitory Effect on Bulk Cancer Cells and Cancer Stem Cells in Breast Carcinoma. Nanomaterials 2022, 12, 3324. [Google Scholar] [CrossRef]
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of precision gold nanoparticles using Turkevich method. Kona 2020, 37, 224–232. [Google Scholar] [CrossRef]
- Malassis, L.; Dreyfus, R.; Murphy, R.J.; Hough, L.A.; Donnio, B.; Murray, C.B. One-step green synthesis of gold and silver nanoparticles with ascorbic acid and their versatile surface post-functionalization. RSC Adv. 2016, 6, 33092–33100. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Woźniak, P.; Banzer, P.; Leuchs, G. Selective switching of individual multipole resonances in single dielectric nanoparticles. Laser Photonics Rev. 2015, 9, 231–240. [Google Scholar] [CrossRef]
- Banzer, P.; Peschel, U.; Quabis, S.; Leuchs, G. On the experimental investigation of the electric and magnetic response of a single nano-structure. Opt. Express 2010, 18, 10905–10923. [Google Scholar] [CrossRef] [PubMed]
- Eigen, M. Methods for investigation of ionic reactions in aqueous solutions with half-times as short as 10−9 sec. Application to neutralization and hydrolysis reactions. Discuss. Faraday Soc. 1954, 17, 194–205. [Google Scholar] [CrossRef]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size control of gold nanocrystals in citrate reduction: The third role of citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef] [PubMed]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: Size focusing versus Ostwald ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- Hussain, M.H.; Abu Bakar, N.F.; Mustapa, A.N.; Low, K.F.; Othman, N.H.; Adam, F. Synthesis of various size gold nanoparticles by chemical reduction method with different solvent polarity. Nanoscale Res. Lett. 2020, 15, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, D.; Cai, Y.; Ji, X.; Xie, R.; Yang, W. Tuning the size of gold nanoparticles in the citrate reduction by chloride ions. Nanoscale 2012, 4, 5071–5076. [Google Scholar] [CrossRef]
- Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple size-controlled synthesis of Au nanoparticles and their size dependent catalytic activity. Sci. Rep. 2018, 8, 4589–4599. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.; Daneshkhah, A.; Diwate, A.; Patel, H.; Smith, J.; Reul, O.; Cheng, R.; Izadian, A.; Hajrasouliha, A.R. Model for gold nanoparticle synthesis: Effect of pH and reaction time. ACS Omega 2021, 6, 16847–16853. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; Zhao, M.; Yang, T.H.; Gilroy, K.D.; da Silva, A.G.M.; Camargo, P.H.C.; Xia, Y. Synthesis of colloidal metal nanocrystals: A Comprehensive review on the reductants. Chem. Eur. J. 2018, 24, 16944–16963. [Google Scholar] [CrossRef]
- Wuithschick, M.; Birnbaum, A.; Witte, S.; Sztucki, M.; Vainio, U.; Pinna, N.; Rademann, K.; Emmerling, F.; Kraehnert, R.; Polte, J. Turkevich in new robes: Key questions answered for the most common gold nanoparticle synthesis. ACS Nano 2015, 9, 7052–7071. [Google Scholar] [CrossRef]
- Ou, J.; Zhou, Z.; Chen, Z.; Tan, H. Optical diagnostic based on functionalized gold nanoparticles. Int. J. Mol. Sci. 2019, 20, 4346. [Google Scholar] [CrossRef] [PubMed]
- Mie, G. Contributions to the optics of turbid media, particularly of colloidal metal solutions. Ann. Phys. 1908, 25, 377–445. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Neuss, S.; Leifert, S.A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, Z.; Ma, J.; Wang, X.; Zhang, Y.; Wang, W.; Yuana, Z. The systematic evaluation of size-dependent toxicity and multi-time biodistribution of gold nanoparticles. Colloids Surf. B Biointerfaces 2018, 167, 260–266. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Wang, C.; Li, H.C.; Wang, T.; Liao, C.Y.; Cui, L.; Zhou, Q.F.; Yan, B.; Jiang, G.B. Impact of silver nanoparticles on human cells: Effect of particle size. Nanotoxicology 2010, 4, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.O.; Grabinski, C.M.; Schrand, A.M.; Murdock, A.M.R.C.; Wang, W.; Gu, B.; Schlager, J.J.; Hussain, S.M. Toxicity of amorphous silica nanoparticles in mouse keratinocytes. J. Nanopart. Res. 2009, 11, 15–24. [Google Scholar] [CrossRef]
- Lin, X.; Li, J.; Ma, S.; Liu, G.; Yang, K.; Tong, M.; Lin, D. Toxicity of TiO2 nanoparticles to escherichia coli: Effects of particle size, crystal phase and water chemistry. PLoS ONE 2014, 9, e110247. [Google Scholar] [CrossRef]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef]
- Bian, S.W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef]
- Pamies, R.; Ginés Hernández Cifre, J.; Fernández Espín, V.; Collado-González, M.; Díaz Baños, F.G.; García de la Torre, J. Aggregation behaviour of gold nanoparticles in saline aqueous media. J. Nanopart. Res. 2014, 16, 2376–2386. [Google Scholar] [CrossRef]
- Edwards, S.A.; Williams, D.R. Double layers and interparticle forces in colloid science and biology: Analytic results for the effect of ionic dispersion forces. Phys. Rev. Lett. 2004, 92, 248303. [Google Scholar] [CrossRef] [PubMed]
- Boström, M.; Williams, D.R.; Ninham, B.W. Specific ion effects: Why DLVO theory fails for biology and colloid systems. Phys. Rev. Lett. 2001, 87, 168103. [Google Scholar] [CrossRef] [PubMed]
- Csapó, E.; Sebők, D.; Makrai Babić, J.; Šupljika, F.; Bohus, G.; Dékány, I.; Kallay, N.; Preočanin, T. Surface and structural properties of gold nanoparticles and their biofunctionalized derivatives in aqueous electrolytes solution. J. Dispers. Sci. Technol. 2014, 35, 815–825. [Google Scholar] [CrossRef]
- Sangwan, S.; Seth, R. Synthesis, characterization and stability of gold nanoparticles (AuNPs) in different buffer systems. J. Clust. Sci. 2022, 33, 749–764. [Google Scholar] [CrossRef]
- Park, J.W.; Shumaker-Parry, J.S. Structural study of citrate layers on gold nanoparticles: Role of intermolecular interactions in stabilizing nanoparticles. J. Am. Chem. Soc. 2014, 136, 1907–1921. [Google Scholar] [CrossRef]
- Zümreoglu-Karan, B. A rationale on the role of intermediate Au(III)–vitamin C complexation in the production of gold nanoparticles. J. Nanopart. Res. 2009, 11, 1099–1105. [Google Scholar] [CrossRef]
- Grys, D.B.; de Nijs, B.; Salmon, A.R.; Huang, J.; Wang, W.; Chen, W.H.; Scherman, O.A.; Baumberg, J.J. Citrate coordination and bridging of gold nanoparticles: The role of gold adatoms in AuNP aging. ACS Nano 2020, 14, 8689–8696. [Google Scholar] [CrossRef]
- Aubard, J.; Bagnasco, E.; Pantigny, J.; Ruasse, M.F.; Levi, G.; Wentrup-Byrne, E. An ion-exchange reaction as measured by surface-enhanced raman spectroscopy on silver colloids. J. Phys. Chem. 1995, 99, 7075–7081. [Google Scholar] [CrossRef]
- Afshinnia, K.; Baalousha, M. Effect of phosphate buffer on aggregation kinetics of citrate-coated silver nanoparticles induced by monovalent and divalent electrolytes. Sci. Total Environ. 2017, 581–582, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Moudgil, L.; Singh, B.; Kaushal, A.; Mittal, A.; Saini, G.S.S.; Tripathi, S.K.; Singhe, G.; Kaura, A. Understanding the mechanism of replacement of citrate from the surface of gold nanoparticles by amino acids: A theoretical and experimental investigation and their biological application. RSC Adv. 2016, 6, 17373–17383. [Google Scholar] [CrossRef]
- Park, J.W.; Shumaker-Par, J.S. Strong resistance of citrate anions on metal nanoparticles to desorption under thiol functionalization. ACS Nano 2015, 9, 1665–1682. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.J.J.; Yang, J.; Chong, K.; Ma, Q.; Li, M.; Zhang, F.; Moon, W.J.; Zhang, G.; Liu, J. Good’s buffers have various affinities to gold nanoparticles regulating fluorescent and colorimetric DNA sensing. Chem. Sci. 2020, 11, 6795–6804. [Google Scholar] [CrossRef]
- Perera, G.S.; Athukorale, S.A.; Perez, F.; Pittman, C.U., Jr.; Zhang, D. Facile displacement of citrate residues from gold nanoparticle surfaces. J. Colloid Interface Sci. 2018, 511, 335–343. [Google Scholar] [CrossRef]
- White, P.; Hjortkjaer, J. Preparation and characterisation of a stable silver colloid for SER(R)S spectroscopy. J. Raman Spectrosc. 2014, 45, 32–40. [Google Scholar] [CrossRef]
- Li, S.; Lui, K.H.; Tsoi, T.H.; Lo, W.S.; Li, X.; Hu, X.; Tai, W.C.S.C.; Hung, H.L.; Gu, Y.J.; Wong, W.T. pH-responsive targeted gold nanoparticles for in vivo photoacoustic imaging of tumor microenvironments. Nanoscale Adv. 2019, 1, 554–564. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selmani, A.; Jeitler, R.; Auinger, M.; Tetyczka, C.; Banzer, P.; Kantor, B.; Leitinger, G.; Roblegg, E. Investigation of the Influence of Wound-Treatment-Relevant Buffer Systems on the Colloidal and Optical Properties of Gold Nanoparticles. Nanomaterials 2023, 13, 1878. https://doi.org/10.3390/nano13121878
Selmani A, Jeitler R, Auinger M, Tetyczka C, Banzer P, Kantor B, Leitinger G, Roblegg E. Investigation of the Influence of Wound-Treatment-Relevant Buffer Systems on the Colloidal and Optical Properties of Gold Nanoparticles. Nanomaterials. 2023; 13(12):1878. https://doi.org/10.3390/nano13121878
Chicago/Turabian StyleSelmani, Atiđa, Ramona Jeitler, Michael Auinger, Carolin Tetyczka, Peter Banzer, Brian Kantor, Gerd Leitinger, and Eva Roblegg. 2023. "Investigation of the Influence of Wound-Treatment-Relevant Buffer Systems on the Colloidal and Optical Properties of Gold Nanoparticles" Nanomaterials 13, no. 12: 1878. https://doi.org/10.3390/nano13121878
APA StyleSelmani, A., Jeitler, R., Auinger, M., Tetyczka, C., Banzer, P., Kantor, B., Leitinger, G., & Roblegg, E. (2023). Investigation of the Influence of Wound-Treatment-Relevant Buffer Systems on the Colloidal and Optical Properties of Gold Nanoparticles. Nanomaterials, 13(12), 1878. https://doi.org/10.3390/nano13121878







