Effect of High-Energy Ball Milling, Capping Agents and Alkalizer on Capacitance of Nanostructured FeOOH Anodes
Abstract
1. Introduction
2. Materials and Methods
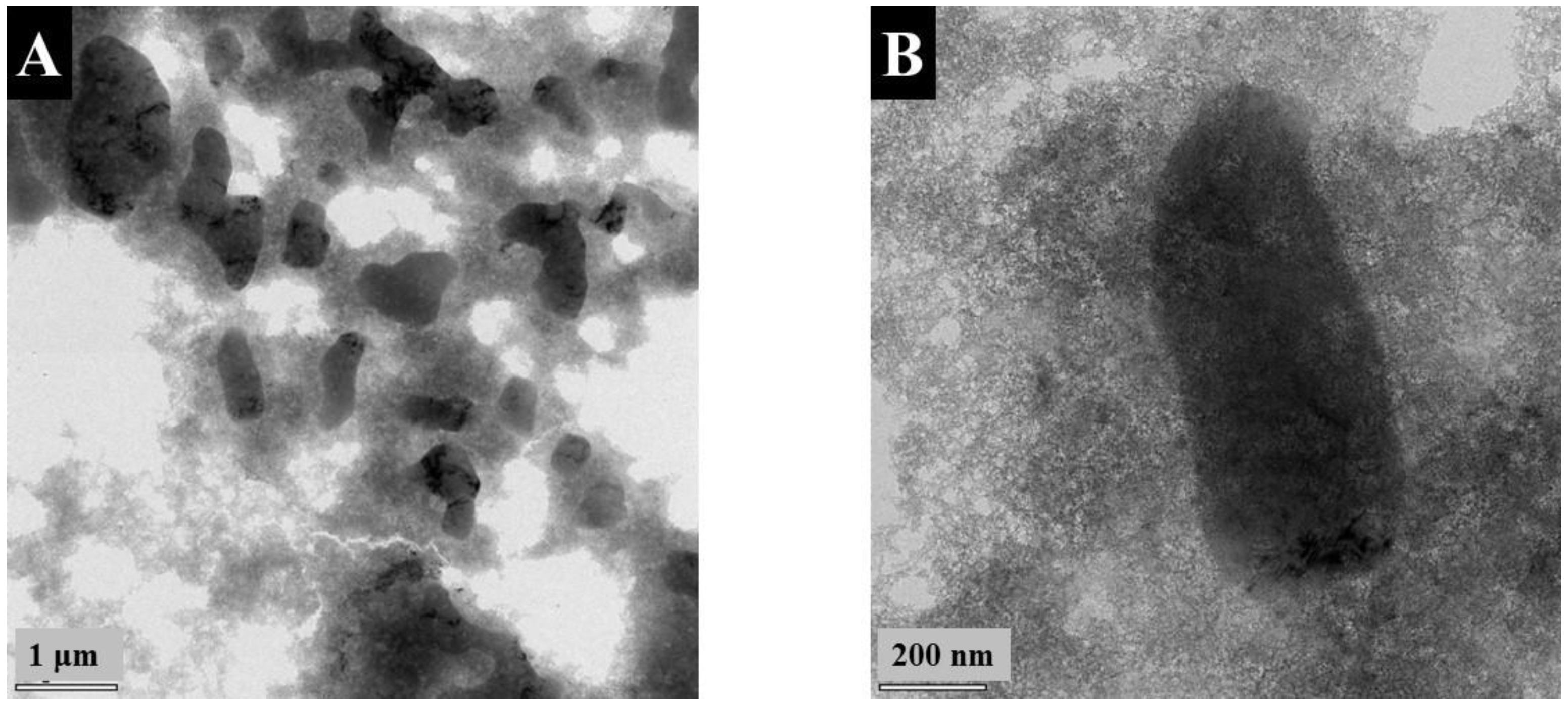
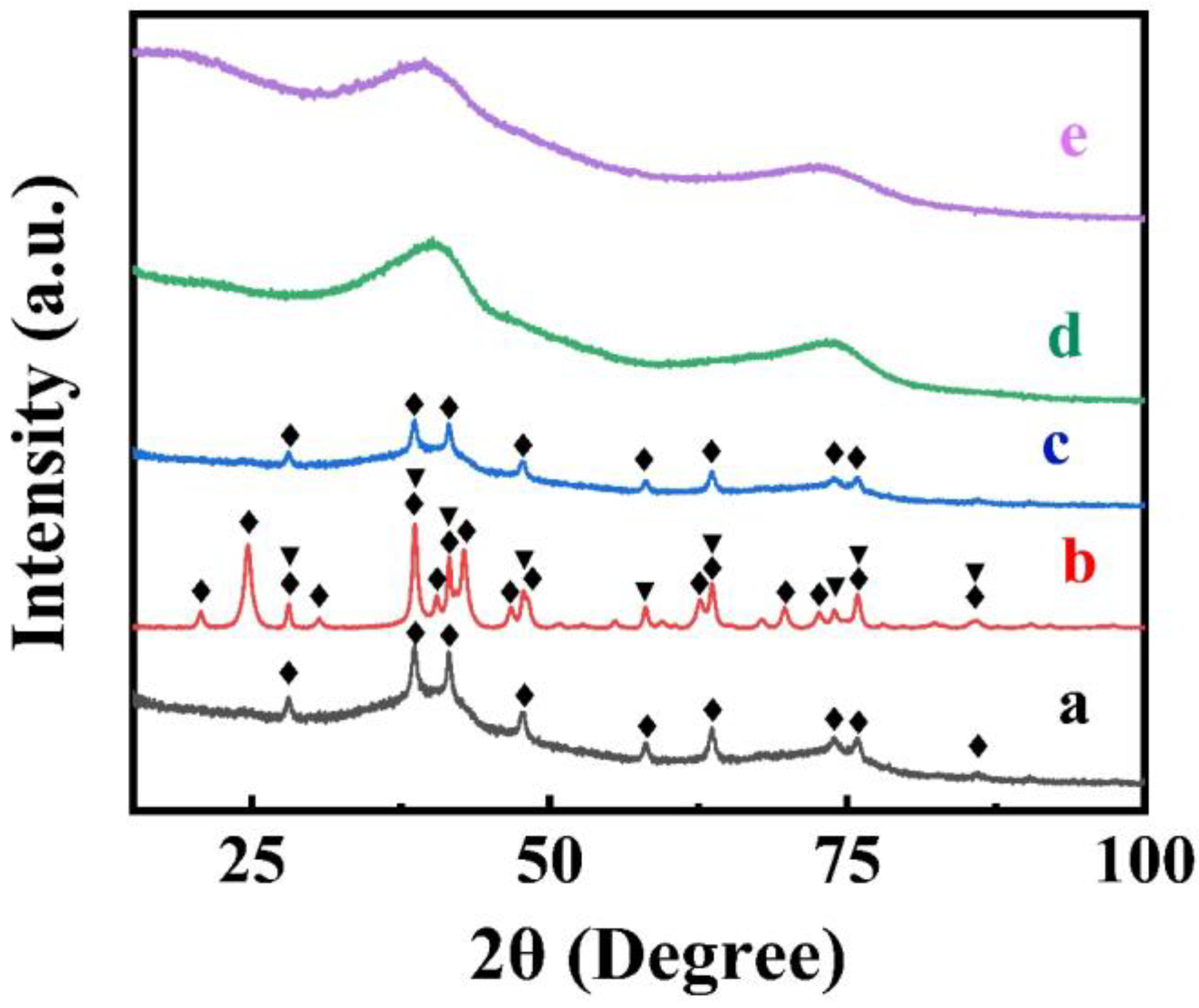
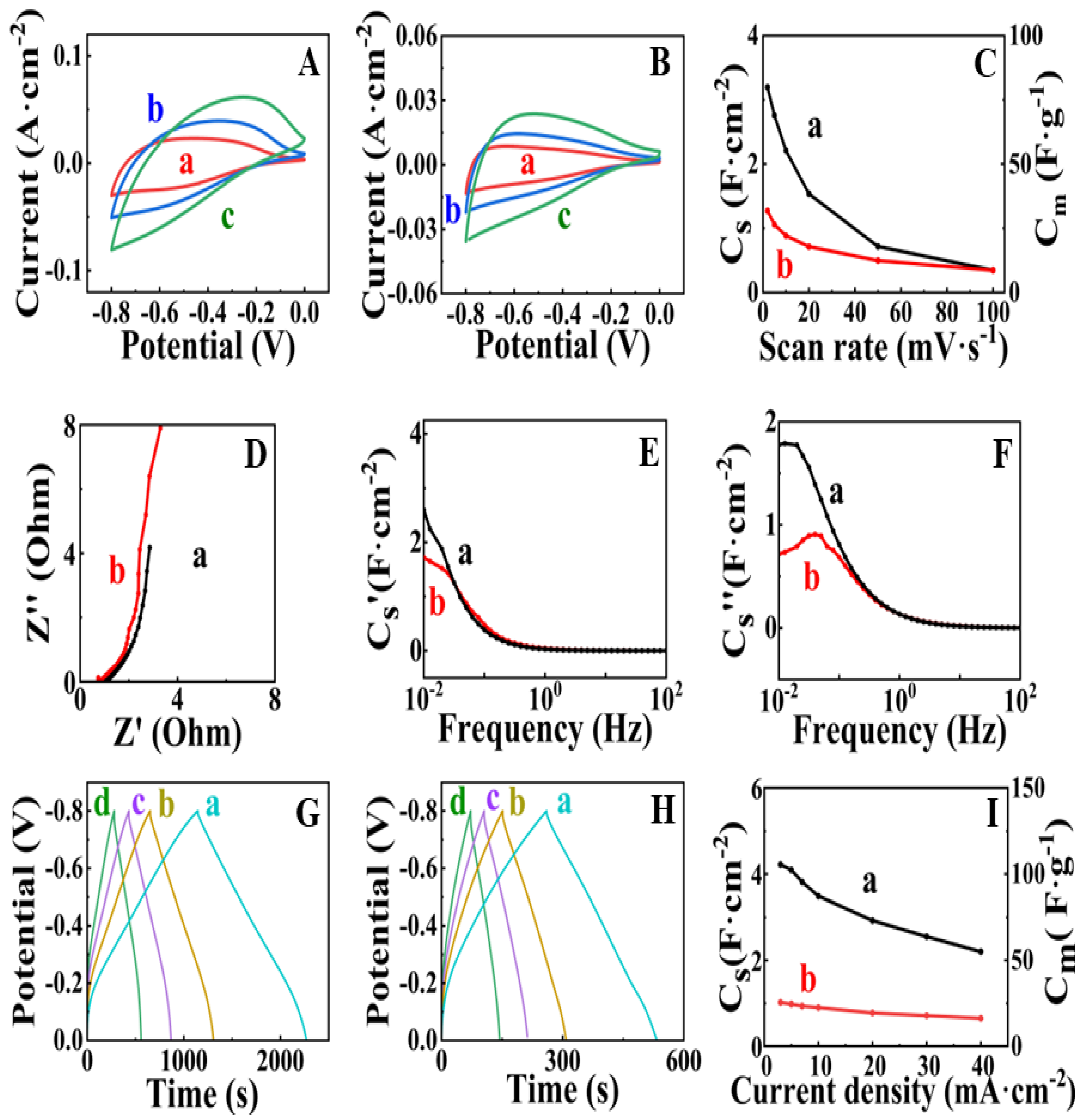
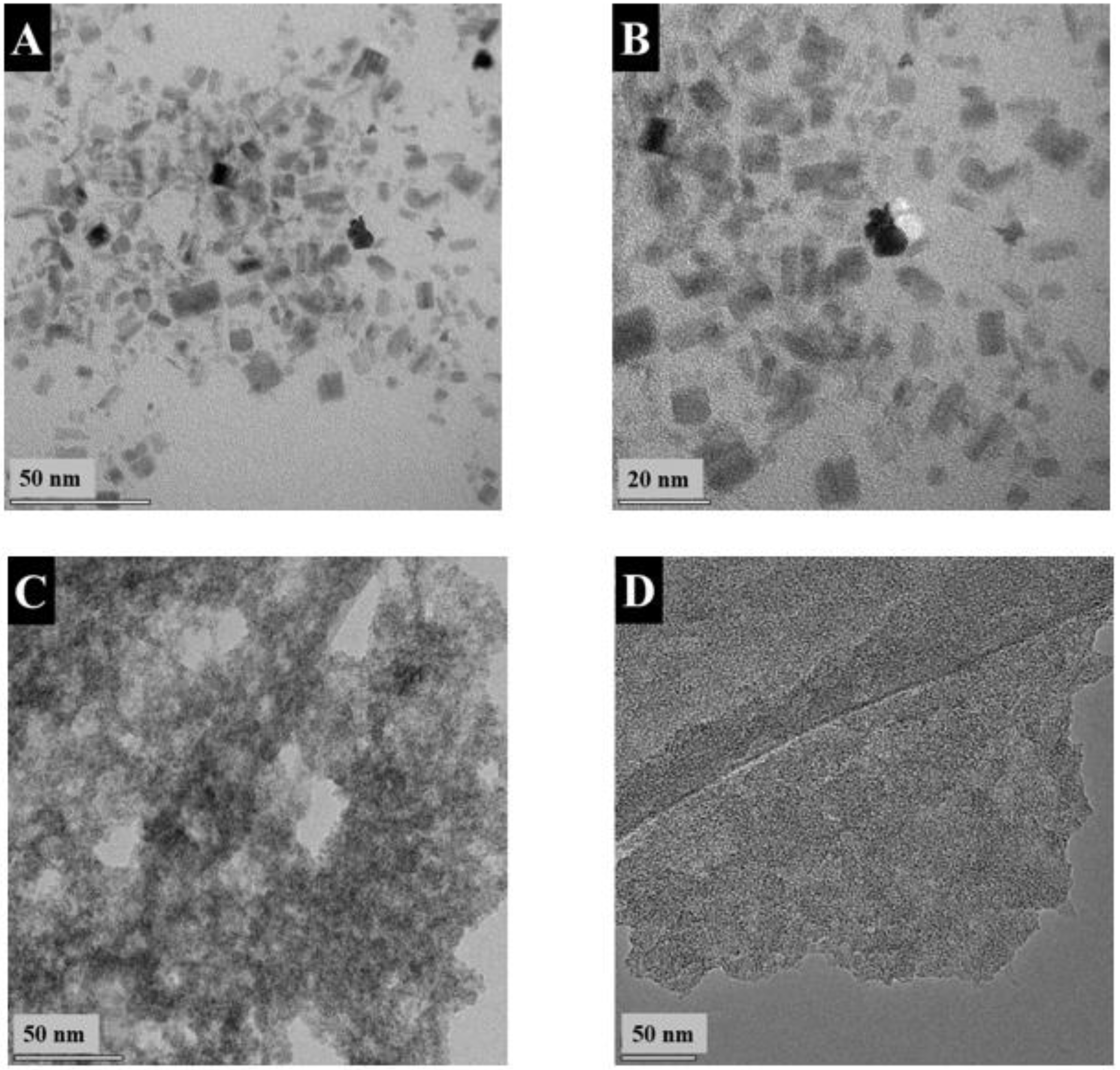
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mu, J.-J.; Liu, Z.-M.; Lai, Q.-S.; Wang, D.; Gao, X.-W.; Yang, D.-R.; Chen, H.; Luo, W.-B. An industrial pathway to emerging presodiation strategies for increasing the reversible ions in sodium-ion batteries and capacitors. Energy Mater. 2022, 2, 200043. [Google Scholar] [CrossRef]
- Le, P.; Minh Tam, H.; Anthony, P.O.M.; Hongxia, W. Revealing energy storage mechanism of CsPbBr3 perovskite for ultra-stable symmetric supercapacitors. Energy Mater. 2023, 3, 300012. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Asymmetric Supercapacitors Based on Activated-Carbon-Coated Carbon Nanotubes. ChemElectroChem 2015, 2, 396–403. [Google Scholar] [CrossRef]
- You, C.; Wu, W.; Yuan, W.; Han, P.; Zhang, Q.; Chen, X.; Yuan, X.; Liu, L.; Ye, J.; Fu, L. Brine Refrigerants for Low-cost, Safe Aqueous Supercapacitors with Ultra-long Stable Operation at Low Temperatures. Adv. Funct. Mater. 2023, 33, 2208206. [Google Scholar] [CrossRef]
- Jin, W.-H.; Cao, G.-T.; Sun, J.-Y. Hybrid supercapacitor based on MnO2 and columned FeOOH using Li2SO4 electrolyte solution. J. Power Sources 2008, 175, 686–691. [Google Scholar] [CrossRef]
- Xia, Q.; Xu, M.; Xia, H.; Xie, J. Nanostructured iron oxide/hydroxide-based electrode materials for supercapacitors. ChemNanoMat 2016, 2, 588–600. [Google Scholar] [CrossRef]
- Chen, R.; Puri, I.K.; Zhitomirsky, I. High areal capacitance of FeOOH-carbon nanotube negative electrodes for asymmetric supercapacitors. Ceram. Int. 2018, 44, 18007–18015. [Google Scholar] [CrossRef]
- Chen, L.-F.; Yu, Z.-Y.; Wang, J.-J.; Li, Q.-X.; Tan, Z.-Q.; Zhu, Y.-W.; Yu, S.-H. Metal-like fluorine-doped β-FeOOH nanorods grown on carbon cloth for scalable high-performance supercapacitors. Nano Energy 2015, 11, 119–128. [Google Scholar] [CrossRef]
- Shen, B.; Guo, R.; Lang, J.; Liu, L.; Liu, L.; Yan, X. A high-temperature flexible supercapacitor based on pseudocapacitive behavior of FeOOH in an ionic liquid electrolyte. J. Mater. Chem. A 2016, 4, 8316–8327. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, M.; Shi, X.; Zeng, H.; Xia, H. Amorphous FeOOH quantum dots assembled mesoporous film anchored on graphene nanosheets with superior electrochemical performance for supercapacitors. Adv. Funct. Mater. 2016, 26, 919–930. [Google Scholar] [CrossRef]
- Liang, W.; Poon, R.; Zhitomirsky, I. Zn-doped FeOOH-polypyrrole electrodes for supercapacitors. Mater. Lett. 2019, 255, 126542. [Google Scholar] [CrossRef]
- Biswal, A.; Panda, P.K.; Acharya, A.N.; Tripathy, B.C.; Alenazey, F.; Jiang, Z.-T.; Sundaram, M.M. Tuning the morphology and redox behaviour by varying the concentration of Fe in a CoNiFe ternary oxide heterostructure for hybrid devices. New J. Chem. 2020, 44, 9921–9932. [Google Scholar] [CrossRef]
- Biswal, A.; Panda, P.K.; Acharya, A.N.; Mohapatra, S.; Swain, N.; Tripathy, B.C.; Jiang, Z.-T.; Minakshi Sundaram, M. Role of additives in electrochemical deposition of ternary metal oxide microspheres for supercapacitor applications. Acs Omega 2020, 5, 3405–3417. [Google Scholar] [CrossRef] [PubMed]
- Wickramaarachchi, K.; Minakshi, M.; Aravindh, S.A.; Dabare, R.; Gao, X.; Jiang, Z.-T.; Wong, K.W. Repurposing N-doped grape marc for the fabrication of supercapacitors with theoretical and machine learning models. Nanomaterials 2022, 12, 1847. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jing, C.; Fu, X.; Shen, M.; Cao, T.; Huo, W.; Liu, X.; Yao, H.-C.; Zhang, Y.; Yao, K.X. In-situ fabricating MnO2 and its derived FeOOH nanostructures on mesoporous carbon towards high-performance asymmetric supercapacitor. Appl. Surf. Sci. 2020, 503, 144123. [Google Scholar] [CrossRef]
- Singh, N.; Tanwar, S.; Kumar, P.; Sharma, A.; Yadav, B. Advanced sustainable solid state energy storage devices based on FeOOH nanorod loaded carbon@ PANI electrode: GCD cycling and TEM correlation. J. Alloys Compd. 2023, 947, 169580. [Google Scholar] [CrossRef]
- Chen, R.; Puri, I.; Zhitomirsky, I. Polypyrrole-carbon nanotube-FeOOH composites for negative electrodes of asymmetric supercapacitors. J. Electrochem. Soc. 2019, 166, A935. [Google Scholar] [CrossRef]
- Sun, Q.; Yao, K.; Zhang, Y. MnO2-directed synthesis of NiFe-LDH@ FeOOH nanosheeet arrays for supercapacitor negative electrode. Chin. Chem. Lett. 2020, 31, 2343–2346. [Google Scholar] [CrossRef]
- O’Neill, L.; Johnston, C.; Grant, P.S. Enhancing the supercapacitor behaviour of novel Fe3O4/FeOOH nanowire hybrid electrodes in aqueous electrolytes. J. Power Sources 2015, 274, 907–915. [Google Scholar] [CrossRef]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The development of pseudocapacitor electrodes and devices with high active mass loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Capsoni, D.; Lucini, P.; Conti, D.M.; Bianchi, M.; Maraschi, F.; De Felice, B.; Bruni, G.; Abdolrahimi, M.; Peddis, D.; Parolini, M. Fe3O4-Halloysite Nanotube Composites as Sustainable Adsorbents: Efficiency in Ofloxacin Removal from Polluted Waters and Ecotoxicity. Nanomaterials 2022, 12, 4330. [Google Scholar] [CrossRef] [PubMed]
- Arbi, H.M.; Koyyada, G.; Anil Kumar, Y.; Kumar Kulurumotlakatla, D.; Kim, J.H.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I.M. Hierarchically Developed Ni(OH)2@MgCo2O4 Nanosheet Composites for Boosting Supercapacitor Performance. Nanomaterials 2023, 13, 1414. [Google Scholar] [CrossRef] [PubMed]
- Mineo, G.; Bruno, E.; Mirabella, S. Advances in WO3-Based Supercapacitors: State-of-the-Art Research and Future Perspectives. Nanomaterials 2023, 13, 1418. [Google Scholar] [CrossRef]
- Mo, X.; Xu, G.; Kang, X.; Yin, H.; Cui, X.; Zhao, Y.; Zhang, J.; Tang, J.; Wang, F. A Facile Microwave Hydrothermal Synthesis of ZnFe2O4/rGO Nanocomposites for Supercapacitor Electrodes. Nanomaterials 2023, 13, 1034. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Ma, Y.; Cai, Z.; Yun, M.; Han, J.; Tong, Z.; Wang, M.; Suhr, J.; Xiao, L.; Jia, S.; et al. Mesoporous Cobalt Oxide (CoOx) Nanowires with Different Aspect Ratios for High Performance Hybrid Supercapacitors. Nanomaterials 2023, 13, 749. [Google Scholar] [CrossRef]
- Li, J.; Yang, Q.M.; Zhitomirsky, I. Nickel foam-based manganese dioxide–carbon nanotube composite electrodes for electrochemical supercapacitors. J. Power Sources 2008, 185, 1569–1574. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Fabrication of Polypyrrole-Coated Carbon Nanotubes Using Oxidant–Surfactant Nanocrystals for Supercapacitor Electrodes with High Mass Loading and Enhanced Performance. ACS Appl. Mater. Interfaces 2013, 5, 13161–13170. [Google Scholar] [CrossRef]
- Zhang, C.; Zhitomirsky, I. Influence of High Energy Ball Milling and Dispersant on Capacitive Properties of Fe2O3—Carbon Nanotube Composites. J. Compos. Sci. 2022, 6, 177. [Google Scholar] [CrossRef]
- Ata, M.; Liu, Y.; Zhitomirsky, I. A review of new methods of surface chemical modification, dispersion and electrophoretic deposition of metal oxide particles. Rsc Adv. 2014, 4, 22716–22732. [Google Scholar] [CrossRef]
- Preisler, P.W.; Berger, L.; Hill, E.S. Oxidation—Reduction Potentials and Ionization Constants of the Reversible Series: Hexahydroxybenzene—Tetrahydroxyquinone—Rhodizonic Acid. J. Am. Chem. Soc. 1947, 69, 326–329. [Google Scholar] [CrossRef]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Uchikoshi, T.; Ozawa, K.; Sakka, Y. Effect of polyethylenimine on the dispersion and electrophoretic deposition of nano-sized titania aqueous suspensions. J. Eur. Ceram. Soc. 2006, 26, 1555–1560. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, F.; Suzuki, T.S.; Sakka, Y. Role of the initial degree of ionization of polyethylenimine in the dispersion of silicon carbide nanoparticles. J. Am. Ceram. Soc. 2003, 86, 189–191. [Google Scholar] [CrossRef]
- Dietrich, A.; Neubrand, A. Effects of particle size and molecular weight of polyethylenimine on properties of nanoparticulate silicon dispersions. J. Am. Ceram. Soc. 2001, 84, 806–812. [Google Scholar] [CrossRef]
- Laarz, E.; Bergström, L. Dispersing WC–Co powders in aqueous media with polyethylenimine. Int. J. Refract. Met. Hard Mater. 2000, 18, 281–286. [Google Scholar] [CrossRef]
- Rubianes, M.D.; Rivas, G.A. Dispersion of multi-wall carbon nanotubes in polyethylenimine: A new alternative for preparing electrochemical sensors. Electrochem. Commun. 2007, 9, 480–484. [Google Scholar] [CrossRef]
- Li, J.; Zhitomirsky, I. Cathodic electrophoretic deposition of manganese dioxide films. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 248–253. [Google Scholar] [CrossRef]
- Milne, J.; Silva, R.M.; Zhitomirsky, I. Surface modification and dispersion of ceramic particles using liquid-liquid extraction method for application in supercapacitor electrodes. J. Eur. Ceram. Soc. 2019, 39, 3450–3455. [Google Scholar] [CrossRef]
- Milne, J.; Silva, R.; Wang, Z.; Zhitomirsky, I. Phase transfer of oxide particles using hydroxamic acid derivatives and application for supercapacitors. Ceram. Int. 2019, 45, 2498–2503. [Google Scholar] [CrossRef]

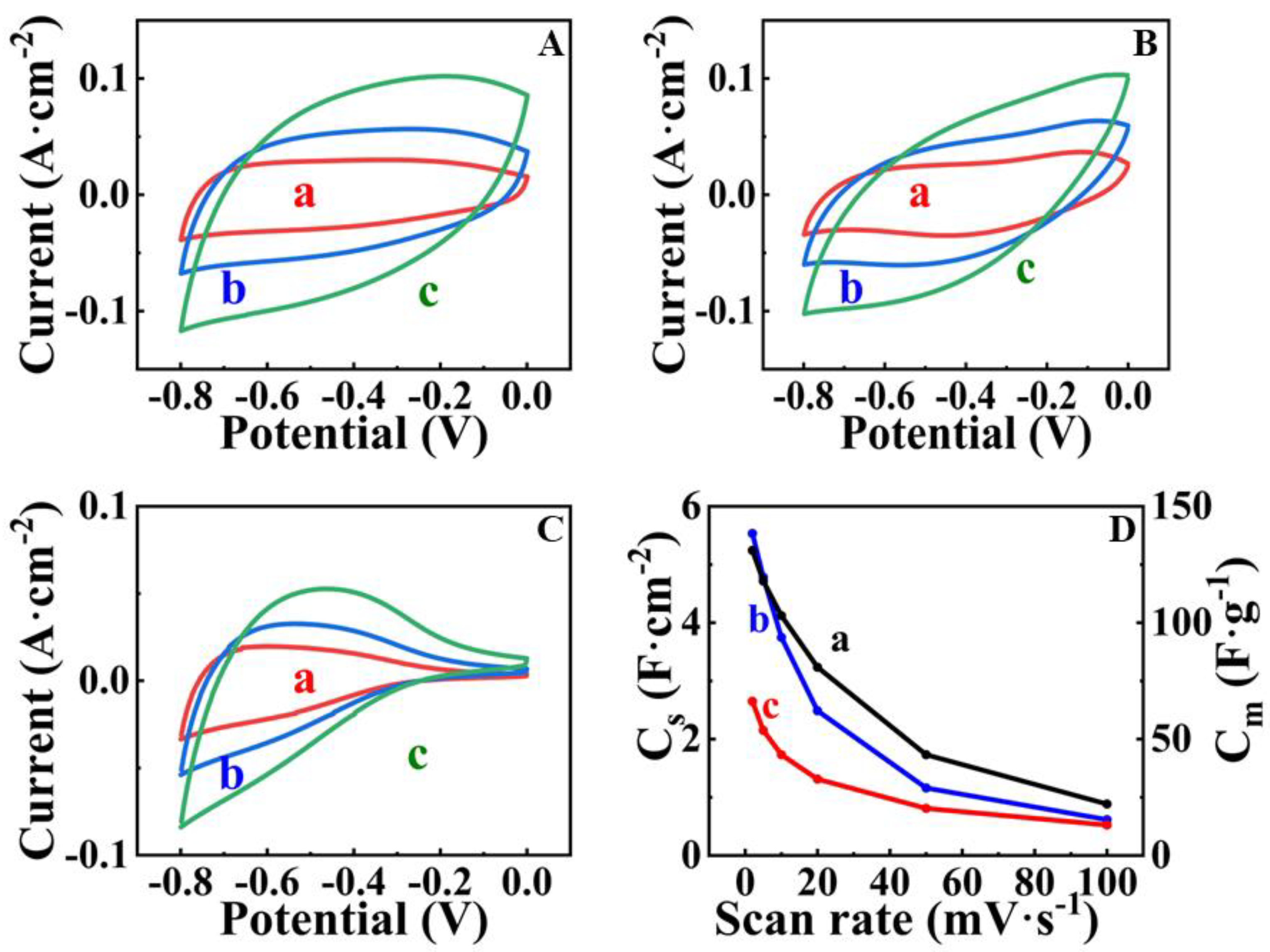
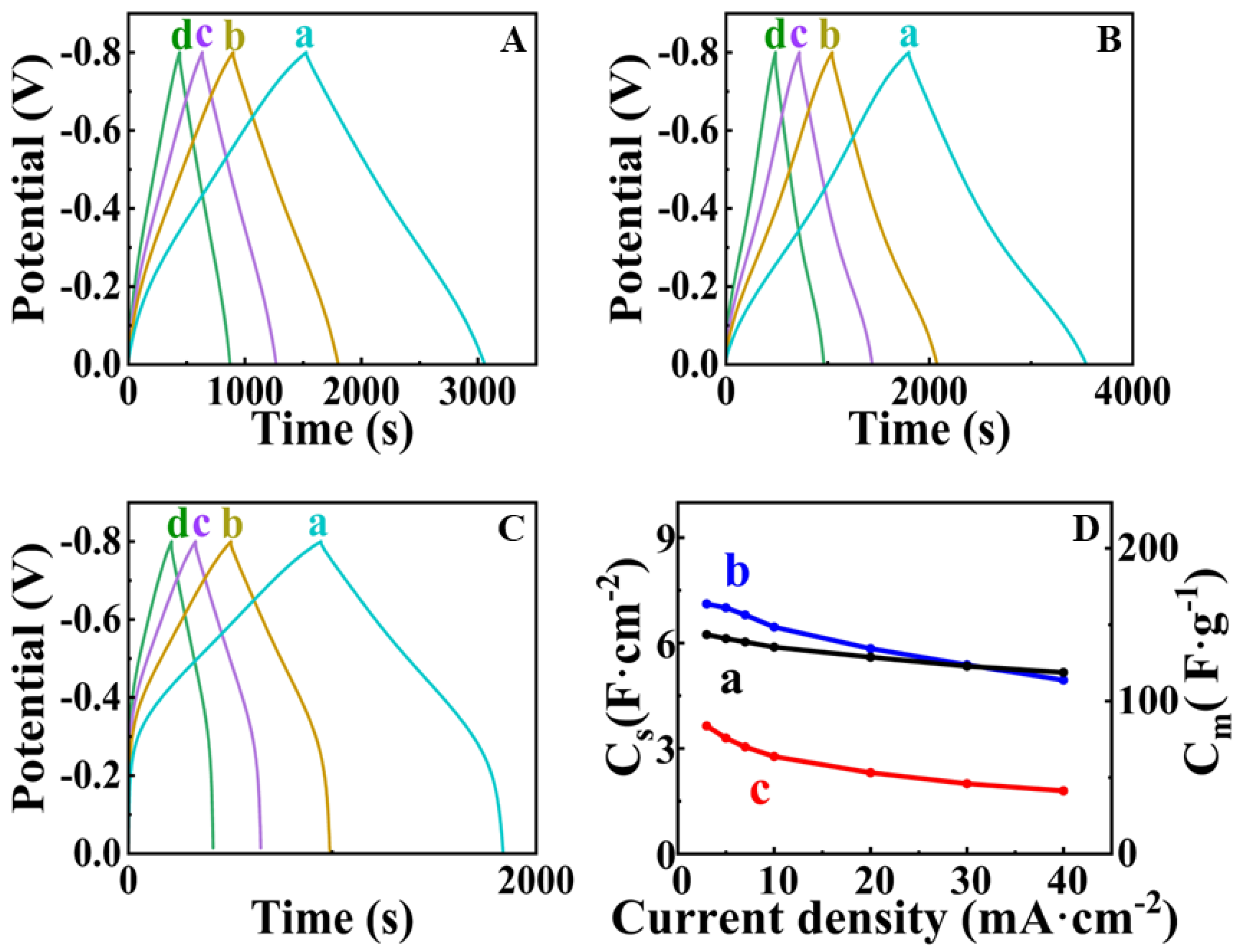
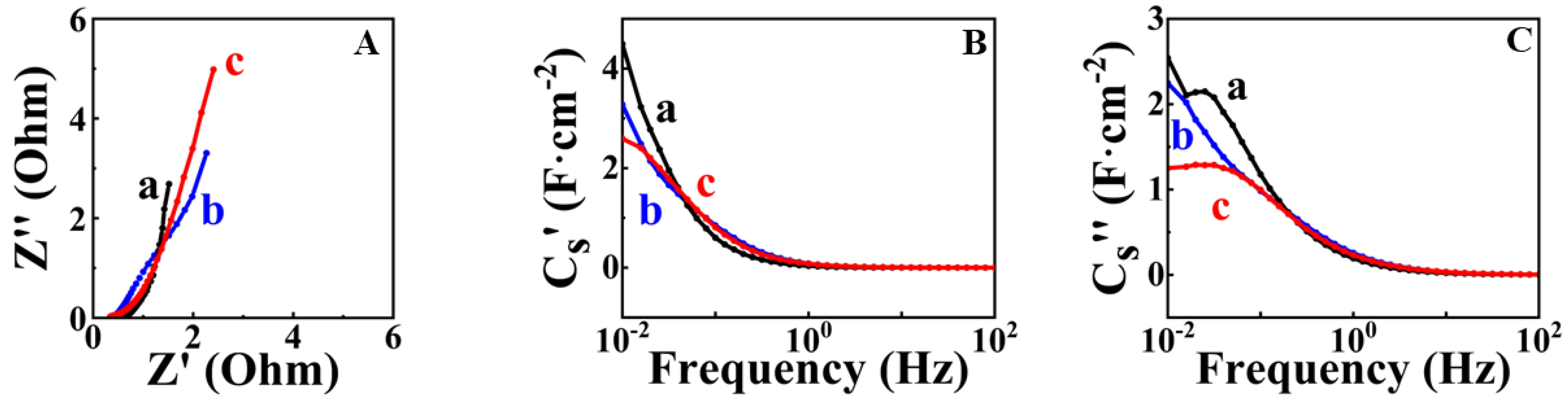
| Potential Range, V vs. SCE | Mass Loading, mg cm−2 | CS, F cm−2 | Reference |
|---|---|---|---|
| −0.8–0 | 36 | 2.4 | [38] |
| −0.8–0 | 39.6 | 3.3 | [39] |
| −0.8–+0.1 | 36 | 3.5 | [17] |
| −0.8–0 | 37 | 5.86 | [7] |
| −0.8–0 | 40 | 4.5 | [11] |
| −0.8–0 | 40 (FAM-PEI) | 3.35 | This work |
| −0.8–0 | 40 (FAM-THB) | 5.74 | This work |
| −0.8–0 | 40 (FAM-GC) | 6.54 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhitomirsky, I. Effect of High-Energy Ball Milling, Capping Agents and Alkalizer on Capacitance of Nanostructured FeOOH Anodes. Nanomaterials 2023, 13, 1693. https://doi.org/10.3390/nano13101693
Zhang C, Zhitomirsky I. Effect of High-Energy Ball Milling, Capping Agents and Alkalizer on Capacitance of Nanostructured FeOOH Anodes. Nanomaterials. 2023; 13(10):1693. https://doi.org/10.3390/nano13101693
Chicago/Turabian StyleZhang, Chengwei, and Igor Zhitomirsky. 2023. "Effect of High-Energy Ball Milling, Capping Agents and Alkalizer on Capacitance of Nanostructured FeOOH Anodes" Nanomaterials 13, no. 10: 1693. https://doi.org/10.3390/nano13101693
APA StyleZhang, C., & Zhitomirsky, I. (2023). Effect of High-Energy Ball Milling, Capping Agents and Alkalizer on Capacitance of Nanostructured FeOOH Anodes. Nanomaterials, 13(10), 1693. https://doi.org/10.3390/nano13101693






