Photoelectrochemical Selective Oxidation of Glycerol to Glyceraldehyde with Bi-Based Metal–Organic-Framework-Decorated WO3 Photoanode
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of WO3 and Bi-MOFs
2.3. Material Characterization
2.4. PEC Measurements
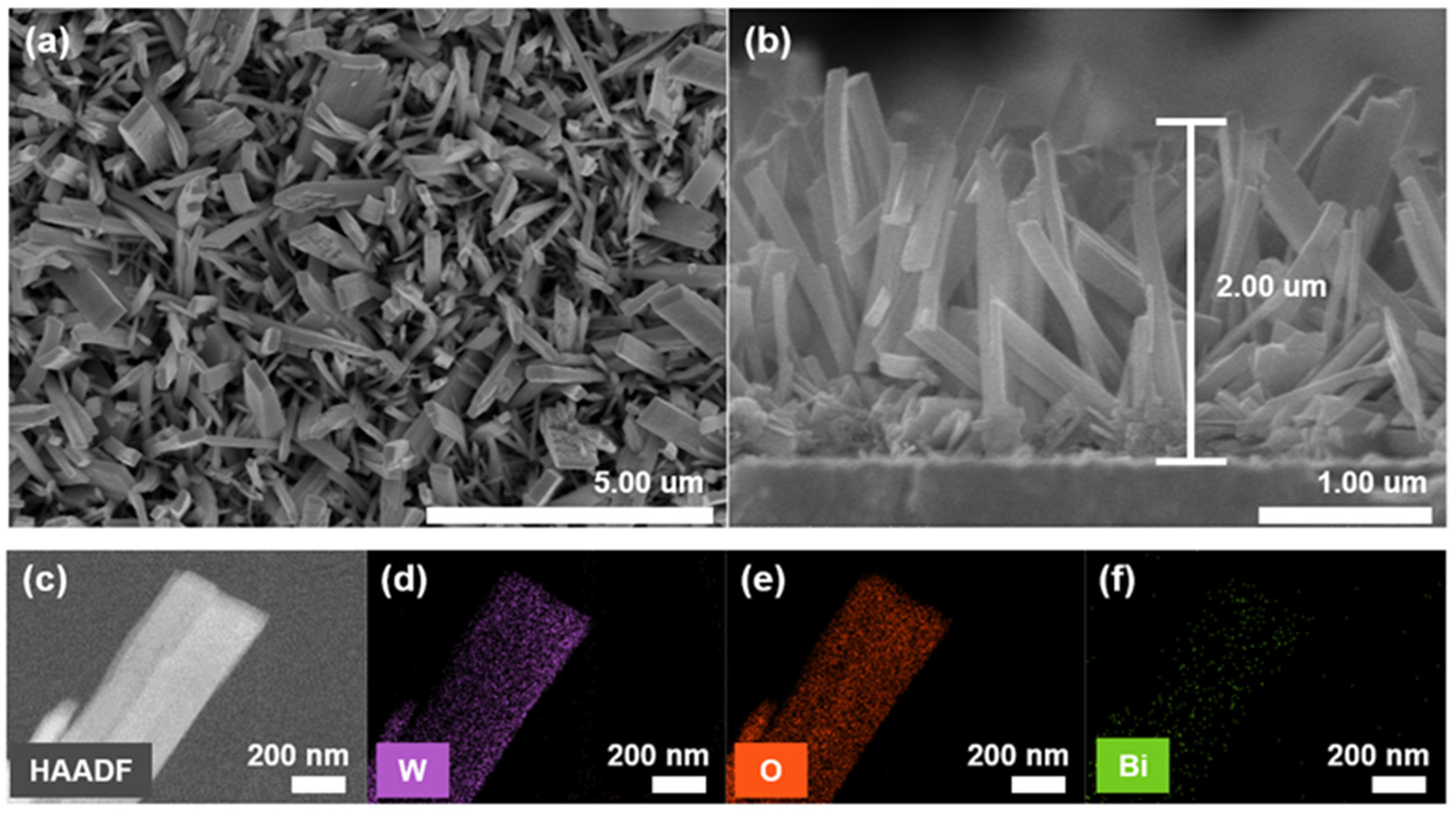
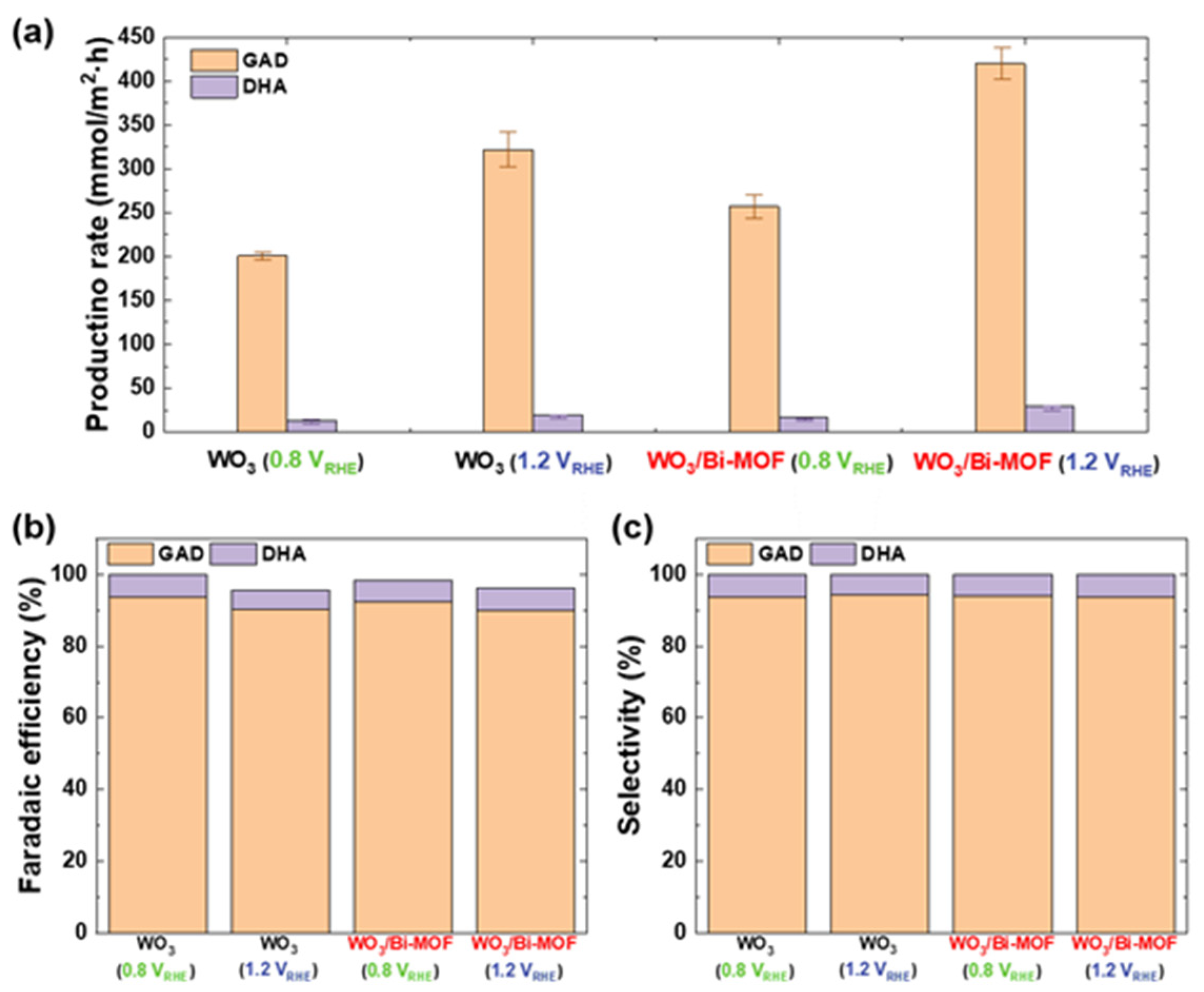
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 0003. [Google Scholar] [CrossRef]
- Voloshin, R.A.; Rodionova, M.V.; Zharmukhamedov, S.K.; Veziroglu, T.N.; Allakhverdiev, S.I. Review: Biofuel production from plant and algal biomass. Int. J. Hydrogen Energy 2016, 41, 17257–17273. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2020–2029; OECD/FAO: Rome, Italy, 2020. [Google Scholar]
- Zhou, C.C.; Beltramini, J.N.; Fan, Y.X.; Lu, G.M. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Yazdani, S.S.; Gonzalez, R. Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, X.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production from steam reforming of ethanol and glycerol over ceria-supported metal catalysts. Int. J. Hydrogen Energy 2007, 32, 2367–2373. [Google Scholar] [CrossRef]
- Kondarides, D.I.; Daskalakim, V.M.; Patsoura, A.; Verykios, X.E. Hydrogen Production by Photo-Induced Reforming of Biomass Components and Derivatives at Ambient Conditions. Catal. Lett. 2007, 122, 26–32. [Google Scholar] [CrossRef]
- Maris, E.; Davis, R. Hydrogenolysis of glycerol over carbon-supported Ru and Pt catalysts. J. Catal. 2007, 249, 328–337. [Google Scholar] [CrossRef]
- Chaminand, J.; Djakovitch, L.; Gallezot, P.; Marion, P.; Pinel, C. Glycerol hydrogenolysis on heterogeneous catalysts. Green Chem. 2004, 6, 359–361. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Bellière-Baca, V.; Rey, P.; Dumeignil, F. Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chem. 2010, 12, 2079–2098. [Google Scholar] [CrossRef]
- Yang, J.; Huang, L.; Yi, T.; Wang, X.; Gao, L.; Liu, W. Glycerin to Acrolein: Can Renewable Processes Challenge Traditional Processes? Chem. Eng. Technol. 2022, 45, 1326–1336. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Maestro-Madurga, B.; Pesquera-Rodríguez, A.; Ramírez-López, C.; Lorenzo-Ibarreta, L.; Torrecilla-Soria, J.; Villarán-Velasco, M.C. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: Catalyst screening and reaction optimization. Appl. Catal. A Gen. 2009, 366, 315–324. [Google Scholar] [CrossRef]
- Bancquart, S.; Vanhove, C.; Pouilloux, Y.; Barrault, J. Glycerol transesterification with methyl stearate over solid basic catalysts. Appl. Catal. A Gen. 2001, 218, 1–11. [Google Scholar] [CrossRef]
- Klepáčová, K.; Mravec, D.; Kaszonyi, A.; Bajus, M. Etherification of glycerol and ethylene glycol by isobutylene. Appl. Catal. A Gen. 2007, 328, 1–13. [Google Scholar] [CrossRef]
- Melero, J.A.; Vicente, G.; Paniagua, M.; Morales, G.; Muñoz, P. Etherification of biodiesel-derived glycerol with ethanol for fuel formulation over sulfonic modified catalysts. Bioresour. Technol. 2012, 103, 142–151. [Google Scholar] [CrossRef]
- Martin, A.; Richter, M. Oligomerization of glycerol—A critical review. Eur. J. Lipid Sci. Technol. 2010, 113, 100–117. [Google Scholar] [CrossRef]
- Barrault, J.; Clacens, J.-M.; Pouilloux, Y. Selective Oligomerization of Glycerol over Mesoporous Catalysts. Top. Catal. 2004, 27, 137–142. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Nocito, F.; Pastore, C. A study on the carboxylation of glycerol to glycerol carbonate with carbon dioxide: The role of the catalyst, solvent and reaction conditions. J. Mol. Catal. A Chem. 2006, 257, 149–153. [Google Scholar] [CrossRef]
- Ezhova, N.N.; Korosteleva, I.G.; Kolesnichenko, N.V.; Kuz’min, A.E.; Khadzhiev, S.N.; Vasil’eva, M.A.; Voronina, Z.D. Glycerol carboxylation to glycerol carbonate in the presence of rhodium complexes with phosphine ligands. Pet. Chem. 2012, 52, 91–96. [Google Scholar] [CrossRef]
- Carrettin, S.; McMorn, P.; Johnston, P.; Griffin, K.; Hutchings, G.J. Selective oxidation of glycerol to glyceric acid using a gold catalyst in aqueous sodium hydroxide. Chem. Commun. 2002, 7, 696–697. [Google Scholar] [CrossRef] [PubMed]
- Carrettin, S.; McMorn, P.; Johnston, P.; Griffin, K.; Kiely, C.J.; Hutchings, G.J. Oxidation of glycerol using supported Pt, Pd and Au catalysts. Phys. Chem. Chem. Phys. 2003, 5, 1329–1336. [Google Scholar] [CrossRef]
- Mou, H.; Chang, Q.; Xie, Z.; Hwang, S.; Kattel, S.; Chen, J.G. Enhancing glycerol electrooxidation from synergistic interactions of platinum and transition metal carbides. Appl. Catal. B Environ. 2022, 316, 121648. [Google Scholar] [CrossRef]
- Huang, X.; Guo, Y.; Zou, Y.; Jiang, J. Electrochemical oxidation of glycerol to hydroxypyruvic acid on cobalt (oxy)hydroxide by high-valent cobalt redox centers. Appl. Catal. B Environ. 2022, 309, 121247. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, G.; Cao, S.; Chen, G.; Li, C.; Izquierdo, R.; Sun, S. Advanced semiconductor catalyst designs for the photocatalytic reduction of CO2. Mater. Rep. Energy, 2023; ahead of print. [Google Scholar] [CrossRef]
- He, J.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Single-atom catalysts for high-efficiency photocatalytic and photoelectrochemical water splitting: Distinctive roles, unique fabrication methods and specific design strategies. J. Mater. Chem. A 2022, 10, 6835–6871. [Google Scholar] [CrossRef]
- Han, X.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Non-metal fluorine doping in Ruddlesden–Popper perovskite oxide enables high-efficiency photocatalytic water splitting for hydrogen production. Mater. Today Energy 2022, 23, 100896. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Enhancing the photocatalytic activity of Ruddlesden-Popper Sr2TiO4 for hydrogen evolution through synergistic silver doping and moderate reducing pretreatment. Mater. Today Energy 2022, 23, 100899. [Google Scholar] [CrossRef]
- Lu, X.; Xie, S.; Yang, H.; Tong, Y.; Ji, H. Photoelectrochemical hydrogen production from biomass derivatives and water. Chem. Soc. Rev. 2014, 43, 7581–7593. [Google Scholar] [CrossRef]
- Ibadurrohman, M.; Hellgardt, K. Photoelectrochemical performance of graphene-modified TiO2 photoanodes in the presence of glycerol as a hole scavenger. Int. J. Hydrogen Energy 2014, 39, 18204–18215. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; Raja, K.S.; Mahajan, V.K.; Misra, M. Efficient Photoelectrolysis of Water using TiO2 Nanotube Arrays by Minimizing Recombination Losses with Organic Additives. J. Phys. Chem. C 2008, 112, 11007–11012. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Chen, P.; Hou, Z. Selective oxidation of glycerol in a base-free aqueous solution: A short review. Chin. J. Catal. 2019, 40, 1020–1034. [Google Scholar] [CrossRef]
- Katryniok, B.; Kimura, H.; Skrzyńska, E.; Girardon, J.-S.; Fongarland, P.; Capron, M.; Ducoulombier, R.; Mimura, N.; Paul, S.; Dumeignil, F. Selective catalytic oxidation of glycerol: Perspectives for high value chemicals. Green Chem. 2011, 13, 1960–1979. [Google Scholar] [CrossRef]
- Skrzyńska, E.; Wondołowska-Grabowska, A.; Capron, M.; Dumeignil, F. Crude glycerol as a raw material for the liquid phase oxidation reaction. Appl. Catal. A Gen. 2014, 482, 245–257. [Google Scholar] [CrossRef]
- Dodekatos, G.; Schünemann, S.; Tüysüz, H. Recent Advances in Thermo-, Photo-, and Electrocatalytic Glycerol Oxidation. ACS Catal. 2018, 8, 6301–6333. [Google Scholar] [CrossRef]
- Jung, K.; Seifert, M.; Herrling, T.; Fuchs, J. UV-generated free radicals (FR) in skin: Their prevention by sunscreens and their induction by self-tanning agents. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2008, 69, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Vandelli, M.A.; Rivasi, F.; Guerra, P.; Forni, F.; Arletti, R. Gelatin microspheres crosslinked with D,L-glyceraldehyde as a potential drug delivery system: Preparation, characterisation, in vitro and in vivo studies. Int. J. Pharm. 2001, 215, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, J.-C.; Cai, W.; Ma, J.; Bin Yang, H.; Xiao, H.; Li, J.; Xiong, Y.; Huang, Y.; Liu, B. Selective photoelectrochemical oxidation of glycerol to high value-added dihydroxyacetone. Nat. Commun. 2019, 10, 1779. [Google Scholar] [CrossRef]
- Vo, T.-G.; Kao, C.-C.; Kuo, J.-L.; Chiu, C.-C.; Chiang, C.-Y. Unveiling the crystallographic facet dependence of the photoelectrochemical glycerol oxidation on bismuth vanadate. Appl. Catal. B Environ. 2020, 278, 119303. [Google Scholar] [CrossRef]
- Seadira, T.W.P.; Sadanandam, G.; Ntho, T.; Masuku, C.M.; Scurrell, M.S. Preparation and characterization of metals supported on nanostructured TiO2 hollow spheres for production of hydrogen via photocatalytic reforming of glycerol. Appl. Catal. B Environ. 2018, 222, 133–145. [Google Scholar] [CrossRef]
- Reddy, N.L.; Cheralathan, K.K.; Kumari, V.D.; Neppolian, B.; Venkatakrishnan, S.M. Photocatalytic Reforming of Biomass Derived Crude Glycerol in Water: A Sustainable Approach for Improved Hydrogen Generation Using Ni(OH)2 Decorated TiO2 Nanotubes under Solar Light Irradiation. ACS Sustain. Chem. Eng. 2018, 6, 3754–3764. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.; Jeong, S.Y.; Seo, S.; Kim, C.; Yoon, H.; Jang, H.W.; Lee, S. Surface-Modified Co-doped ZnO Photoanode for Photoelectrochemical Oxidation of Glycerol. Catal. Today 2021, 359, 43–49. [Google Scholar] [CrossRef]
- Kim, S.; An, E.; Oh, I.; Hwang, J.B.; Seo, S.; Jung, Y.; Park, J.-C.; Choi, H.; Choi, C.H.; Lee, S. CeO2 nanoarray decorated Ce-doped ZnO nanowire photoanode for efficient hydrogen production with glycerol as a sacrificial agent. Catal. Sci. Technol. 2022, 12, 5517–5523. [Google Scholar] [CrossRef]
- Yu, J.; Dappozze, F.; Martín-Gomez, J.; Hidalgo-Carrillo, J.; Marinas, A.; Vernoux, P.; Caravaca, A.; Guillard, C. Glyceraldehyde production by photocatalytic oxidation of glycerol on WO3-based materials. Appl. Catal. B Environ. 2021, 299, 120616. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, Y.; Zhu, Z.; Hou, Z. Selective oxidation of glycerol over different shaped WO3 supported Pt NPs. Mol. Catal. 2022, 523, 111545. [Google Scholar] [CrossRef]
- Li, Y.; Mei, Q.; Liu, Z.; Hu, X.; Zhou, Z.; Huang, J.; Bai, B.; Liu, L.; Ding, F.; Wang, Q. Fluorine-doped iron oxyhydroxide cocatalyst: Promotion on the WO3 photoanode conducted photoelectrochemical water splitting. Appl. Catal. B Environ. 2022, 304, 120995. [Google Scholar] [CrossRef]
- Monllor-Satoca, D.; Borja, L.; Rodes, A.; Gómez, R.; Salvador, P. Photoelectrochemical behavior of nanostructured WO3 thin-film electrodes: The oxidation of formic acid. Chemphyschem 2006, 7, 2540–2551. [Google Scholar] [CrossRef]
- Iwai, T. Temperature Dependence of the Optical Absorption Edge of Tungsten Trioxide Single Crystal. Phys. Soc. Jpn. 1960, 15, 1596–1600. [Google Scholar] [CrossRef]
- Berak, J.M.; Sienko, M. Effect of oxygen-deficiency on electrical transport properties of tungsten trioxide crystals. J. Solid. State Chem. 1970, 2, 109–133. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, G.; Liu, W.; Xi, Y.; Golosov, D.A.; Zavadski, S.M.; Melnikov, S.N. 3D core-shell WO3@ α-Fe2O3 photoanode modified by ultrathin FeOOH layer for enhanced photoelectrochemical performances. J. Alloys Compd. 2020, 834, 154992. [Google Scholar] [CrossRef]
- Dong, P.; Hou, G.; Xi, X.; Shao, R.; Dong, F. WO3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications. Environ. Sci. Nano 2017, 4, 539–557. [Google Scholar] [CrossRef]
- Nagy, D.; Szilágyi, I.M.; Fan, X. Effect of the morphology and phases of WO3 nanocrystals on their photocatalytic efficiency. RSC Adv. 2016, 6, 33743–33754. [Google Scholar] [CrossRef]
- Gillet, M.; Aguir, K.; Lemire, C.; Gillet, E.; Schierbaum, K. The structure and electrical conductivity of vacuum-annealed WO3 thin films. Thin Solid Film. 2004, 467, 239–246. [Google Scholar] [CrossRef]
- Miseki, Y.; Kusama, H.; Sugihara, H.; Sayama, K. Cs-modified WO3 photocatalyst showing efficient solar energy conversion for O2 production and Fe (III) ion reduction under visible light. J. Phys. Chem. Lett. 2010, 1, 1196–1200. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef]
- Hwang, D.W.; Kim, J.; Park, T.J.; Lee, J.S. Mg-doped WO3 as a novel photocatalyst for visible light-induced water splitting. Catal. Lett. 2002, 80, 53–57. [Google Scholar] [CrossRef]
- Cole, B.; Marsen, B.; Miller, E.; Yan, Y.; To, B.; Jones, K.; Al-Jassim, M. Evaluation of Nitrogen Doping of Tungsten Oxide for Photoelectrochemical Water Splitting. J. Phys. Chem. C 2008, 112, 5213–5220. [Google Scholar] [CrossRef]
- Seabold, J.A.; Choi, K.S. Effect of a cobalt-based oxygen evolution catalyst on the stability and the selectivity of photo-oxidation reactions of a WO3 photoanode. Abstr. Pap. Am. Chem. Soc. 2011, 23, 1105–1112. [Google Scholar] [CrossRef]
- Cai, L.; Zhao, J.; Li, H.; Park, J.; Cho, I.S.; Han, H.S.; Zheng, X. One-Step Hydrothermal Deposition of Ni:FeOOH onto Photoanodes for Enhanced Water Oxidation. ACS Energy Lett. 2016, 1, 624–632. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Deng, H.; Grunder, S.; Cordova, K.E.; Valente, C.; Furukawa, H.; Hmadeh, M.; Gándara, F.; Whalley, A.C.; Liu, Z.; Asahina, S.; et al. Large-pore apertures in a series of metal-organic frameworks. Science 2012, 336, 1018–1023. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Liao, P.Q.; Shen, J.Q.; Zhang, J.P. Metal–organic frameworks for electrocatalysis. Coord. Chem. Rev. 2018, 373, 22–48. [Google Scholar] [CrossRef]
- Greathouse, J.A.; Allendorf, M.D. The interaction of water with MOF-5 simulated by molecular dynamics. J. Am. Chem. Soc. 2006, 128, 10678–10679. [Google Scholar] [CrossRef]
- Kaye, S.S.; Dailly, A.; Yaghi, O.M.; Long, J.R. Impact of preparation and handling on the hydrogen storage properties of Zn4O(1,4-benzenedicarboxylate)3 (MOF-5). J. Am. Chem. Soc. 2007, 129, 14176–14177. [Google Scholar] [CrossRef]
- Low, J.J.; Benin, A.I.; Jakubczak, P.; Abrahamian, J.F.; Faheem, S.A.; Willis, R.R. Virtual high throughput screening confirmed experimentally: Porous coordination polymer hydration. J. Am. Chem. Soc. 2009, 131, 15834–15842. [Google Scholar] [CrossRef]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef]
- Nemiwal, M.; Gosu, V.; Zhang, T.C.; Kumar, D. Metal organic frameworks as electrocatalysts: Hydrogen evolution reactions and overall water splitting. Int. J. Hydrogen Energy 2021, 46, 10216–10238. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Z.; Zeng, G.; Zhang, C.; Xiao, R.; Huang, D.; Lai, C.; Cheng, M.; Wang, W.; Xiong, W.; et al. Recent progress on metal-organic frameworks based-and derived-photocatalysts for water splitting. Chem. Eng. J. 2020, 383, 123196. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Feng, Y.; Guan, B.; Lou, X.W.D.; Paik, U. Carbon coated porous nickel phosphides nanoplates for highly efficient oxygen evolution reaction. Energy Environ. Sci. 2016, 9, 1246–1250. [Google Scholar] [CrossRef]
- Yang, Y.; Lun, Z.; Xia, G.; Zheng, F.; He, M.; Chen, Q. Non-precious alloy encapsulated in nitrogen-doped graphene layers derived from MOFs as an active and durable hydrogen evolution reaction catalyst. Energy Environ. Sci. 2015, 8, 3563–3571. [Google Scholar] [CrossRef]
- Kim, S.; Pena, T.A.D.; Seo, S.; Choi, H.; Park, J.; Lee, J.H.; Woo, J.; Choi, C.H.; Lee, S. Co-catalytic effects of Bi-based metal-organic framework on BiVO4 photoanodes for photoelectrochemical water oxidation. Appl. Surf. Sci. 2021, 563, 150357. [Google Scholar] [CrossRef]
- Wang, G.; Sun, Q.; Liu, Y.; Huang, B.; Dai, Y.; Zhang, X.; Qin, X. A bismuth-based metal-organic framework as an efficient visible-light-driven photocatalyst. Chemistry 2015, 21, 2364–2367. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Wang, F.; Qi, K.; Zhang, H.; Cui, X.; Zheng, W. Bi-metal-organic frameworks type II heterostructures for enhanced photocatalytic styrene oxidation. Nanoscale 2019, 11, 7554–7559. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Karthik, M.; Prabhakaran, S. Facile and one step synthesis of WO3 nanorods and nanosheets as an efficient photocatalyst and humidity sensing material. Vacuum 2018, 155, 224–232. [Google Scholar] [CrossRef]
- Katrib, A.; Hemming, F.; Wehrer, P.; Hilaire, L.; Maire, G. The multi-surface structure and catalytic properties of partially reduced WO3, WO2 and WC + O2 or W + O2 as characterized by XPS. J. Electron. Spectrosc. Relat. Phenom. 1995, 76, 195–200. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Nguyen, T.D.; Van Nguyen, T. Microwave-Assisted Solvothermal Synthesis and Photocatalytic Activity of Bismuth(III) Based Metal–Organic Framework. Top. Catal. 2020, 63, 1109–1120. [Google Scholar] [CrossRef]
- Huang, L.-W.; Vo, T.-G.; Chiang, C.-Y. Converting glycerol aqueous solution to hydrogen energy and dihydroxyacetone by the BiVO4 photoelectrochemical cell. Electrochim. Acta 2019, 322, 134725. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S. Synthesis and electrocatalytic activity evaluation of nanoflower shaped Ni-Pd on alcohol oxidation reaction. J. Electrochem. Soc. 2014, 161, F1300. [Google Scholar] [CrossRef]
- Du, W.; Mackenzie, K.E.; Milano, D.F.; Deskins, N.A.; Su, D.; Teng, X. Palladium–tin alloyed catalysts for the ethanol oxidation reaction in an alkaline medium. ACS Catal. 2012, 2, 287–297. [Google Scholar] [CrossRef]
- Houache, M.S.E.; Hughes, K.; Baranova, E.A. Study on catalyst selection for electrochemical valorization of glycerol. Sustain. Energy Fuels 2019, 3, 1892–1915. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Zhang, B.; Yan, D.; Xiang, X. Mediating the Oxidizing Capability of Surface-Bound Hydroxyl Radicals Produced by Photoelectrochemical Water Oxidation to Convert Glycerol into Dihydroxyacetone. Acs Catal. 2022, 12, 6946–6957. [Google Scholar] [CrossRef]
- Luo, L.; Chen, W.; Xu, S.M.; Yang, J.; Li, M.; Zhou, H.; Xu, M.; Shao, M.; Kong, X.; Li, Z.; et al. Selective Photoelectrocatalytic Glycerol Oxidation to Dihydroxyacetone via Enhanced Middle Hydroxyl Adsorption over a Bi2O3-Incorporated Catalyst. J. Am. Chem. Soc. 2022, 144, 7720–7730. [Google Scholar] [CrossRef] [PubMed]
- Çetinkaya, S.; Khamidov, G.; Özcan, L.; Palmisano, L.; Yurdakal, S. Selective photoelectrocatalytic oxidation of glycerol by nanotube, nanobelt and nanosponge structured TiO2 on Ti plates. J. Environ. Chem. Eng. 2022, 10, 107210. [Google Scholar] [CrossRef]
- de Escobar, C.C.; Lansarin, M.A.; Santos, J.H.Z.D.; Brandestini, M.D. Molecularly imprinted photocatalyst for glyceraldehyde production. J. Sol-Gel Sci. Technol. 2018, 88, 220–226. [Google Scholar] [CrossRef]
- Ouyang, J.; Liu, X.; Wang, B.H.; Pan, J.B.; Shen, S.; Chen, L.; Au, C.T.; Yin, S.F. WO3 Photoanode with Predominant Exposure of {202} Facets for Enhanced Selective Oxidation of Glycerol to Glyceraldehyde. ACS Appl. Mater. Interfaces, 2022; ahead of print. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.; Kim, S.; Choi, H.; Kim, Y.; Hwang, J.B.; Lee, D.; Kim, Y.; Park, J.-C.; Kim, D.-Y.; Lee, S. Photoelectrochemical Selective Oxidation of Glycerol to Glyceraldehyde with Bi-Based Metal–Organic-Framework-Decorated WO3 Photoanode. Nanomaterials 2023, 13, 1690. https://doi.org/10.3390/nano13101690
Jung Y, Kim S, Choi H, Kim Y, Hwang JB, Lee D, Kim Y, Park J-C, Kim D-Y, Lee S. Photoelectrochemical Selective Oxidation of Glycerol to Glyceraldehyde with Bi-Based Metal–Organic-Framework-Decorated WO3 Photoanode. Nanomaterials. 2023; 13(10):1690. https://doi.org/10.3390/nano13101690
Chicago/Turabian StyleJung, Yoonsung, Seungkyu Kim, Hojoong Choi, Yunseul Kim, Jun Beom Hwang, Donghyeon Lee, Yejoon Kim, Jun-Cheol Park, Dong-Yu Kim, and Sanghan Lee. 2023. "Photoelectrochemical Selective Oxidation of Glycerol to Glyceraldehyde with Bi-Based Metal–Organic-Framework-Decorated WO3 Photoanode" Nanomaterials 13, no. 10: 1690. https://doi.org/10.3390/nano13101690
APA StyleJung, Y., Kim, S., Choi, H., Kim, Y., Hwang, J. B., Lee, D., Kim, Y., Park, J.-C., Kim, D.-Y., & Lee, S. (2023). Photoelectrochemical Selective Oxidation of Glycerol to Glyceraldehyde with Bi-Based Metal–Organic-Framework-Decorated WO3 Photoanode. Nanomaterials, 13(10), 1690. https://doi.org/10.3390/nano13101690





