Comparative Behavior of Viscose-Based Supercapacitor Electrodes Activated by KOH, H2O, and CO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Activated Carbon Fibers
2.2. Electrode Fabrication and Electrochemical Characterization
2.3. Structural Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Tian, F.; Lan, X.; Liu, Y.; Yang, W.; Zhang, J.; Yu, Y. Building P-doped Mo, S2/g-C3N4 layered heterojunction with a dual-internal electric field for efficient photocatalytic sterilization. Chem. Eng. J. 2022, 429, 132588. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, F.; Gao, M.; Yang, W.; Yu, Y. L-Cysteine capped Mo2C/Zn0.67Cd0.33S heterojunction with intimate covalent bonds enables efficient and stable H2-Releasing photocatalysis. Chem. Eng. J. 2021, 428, 132628. [Google Scholar] [CrossRef]
- United Nations, Paris Agreement. 2015. Available online: https://bit.ly/3gw (accessed on 27 November 2020).
- Directorate-General for Climate Action (European Commission), Going Climate-Neutral by 2050: A Strategic Long-Term Vision for a Prosperous, Modern, Competitive and Climate-Neutral EU Economy. 2019. Available online: https://bit.ly/3s (accessed on 27 November 2020).
- Enock, T.K.; King’ondu, C.K.; Pogrebnoi, A.; Jande, Y.A.C. Status of Biomass Derived Carbon Materials for Supercapacitor Application. Int. J. Electrochem. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Hao, L.; Li, X.; Zhi, L. Carbonaceous Electrode Materials for Supercapacitors. Adv. Mater. 2013, 25, 3899–3904. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.B.; Hayes, J.G. Cycle Testing of Supercapacitors for Long-Life Robust Applications. IEEE Trans. Power Electron. 2015, 30, 2505–2516. [Google Scholar] [CrossRef]
- Hassel, A.W. Pervasive electrochemistry. J. Solid State Electrochem. 2020, 24, 2083–2085. [Google Scholar] [CrossRef]
- Stavropoulos, G.; Zabaniotou, A. Minimizing activated carbons production cost. Fuel Process. Technol. 2009, 90, 952–957. [Google Scholar] [CrossRef]
- Dos Reis, G.S.; Larsson, S.H.; De De Oliveira, H.P.; Thyrel, M.; Lima, E.C. Sustainable Biomass Activated Carbons as Electrodes for Battery and Supercapacitors—A Mini-Review. Nanomaterials 2020, 10, 1398. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [Green Version]
- Tay, T.; Ucar, S.; Karagöz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 2009, 165, 481–485. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, H.; Tao, X.; Liang, Y.; Yang, S.J.; Huang, J.; Yuan, T.; Titirici, M.; Zhang, Q. Recent progress on biomass-derived ecomaterials toward advanced rechargeable lithium batteries. EcoMat 2020, 2, e12019. [Google Scholar] [CrossRef]
- Pandolfo, A.; Hollenkamp, A. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Béguin, F.; Frackowiak, E. Supercapacitors: Materials, Systems, and Applications; Wiley: Weinheim, Germany, 2013. [Google Scholar]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Kawano, T.; Kubota, M.; Onyango, M.S.; Watanabe, F.; Matsuda, H. Preparation of activated carbon from petroleum coke by KOH chemical activation for adsorption heat pump. Appl. Therm. Eng. 2008, 28, 865–871. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F.; Molina-Sabio, M. Activated carbons from lignocellulosic materials by chemical and/or physical activation: An overview. Carbon 1992, 30, 1111–1118. [Google Scholar] [CrossRef]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Hydrothermal Carbonization of Abundant Renewable Natural Organic Chemicals for High-Performance Supercapacitor Electrodes. Adv. Energy Mater. 2011, 1, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Breitenbach, S.; Lumetzberger, A.; Hobisch, M.A.; Unterweger, C.; Spirk, S.; Stifter, D.; Fürst, C.; Hassel, A.W. Supercapacitor Electrodes from Viscose-Based Activated Carbon Fibers: Significant Yield and Performance Improvement Using Diammonium Hydrogen Phosphate as Impregnating Agent. C 2020, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Franck, H.-G.; Knop, A. Kohleveredlung: Chemie und Technologie; Springer: Berlin, Germany, 1979. [Google Scholar]
- Breitenbach, S.; Gavrilov, N.; Pašti, I.; Unterweger, C.; Duchoslav, J.; Stifter, D.; Hassel, A.W.; Fürst, C. Biomass-Derived Carbons as Versatile Materials for Energy-Related Applications: Capacitive Properties vs. Oxygen Reduction Reaction Catalysis. C 2021, 7, 55. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Jänes, A.; Eskusson, J.; Thomberg, T.; Lust, E. Supercapacitors Based on Propylene Carbonate with Small Addition of Different Sulfur Containing Organic Solvents. J. Electrochem. Soc. 2014, 161, A1284–A1290. [Google Scholar] [CrossRef]
- Fernández, J.; Arulepp, M.; Leis, J.; Stoeckli, F.; Centeno, T.A. EDLC performance of carbide-derived carbons in aprotic and acidic electrolytes. Electrochim. Acta 2008, 53, 7111–7116. [Google Scholar] [CrossRef] [Green Version]
- Pell, W.G.; Conway, B.E. Analysis of power limitations at porous supercapacitor electrodes under cyclic voltammetry modulation and dc charge. J. Power Sources 2001, 96, 57–67. [Google Scholar] [CrossRef]
- Helseth, L. Comparison of methods for finding the capacitance of a supercapacitor. J. Energy Storage 2021, 35, 102304. [Google Scholar] [CrossRef]
- Romanos, J.; Beckner, M.; Rash, T.; Firlej, L.; Kuchta, B.; Yu, P.; Suppes, G.; Wexler, C.; Pfeifer, P. Nanospace engineering of KOH activated carbon. Nanotechnology 2011, 23, 015401. [Google Scholar] [CrossRef]
- Ma, W.; Xie, L.; Dai, L.; Sun, G.; Chen, J.; Su, F.; Cao, Y.; Lei, H.; Kong, Q.; Chen, C.-M. Influence of phosphorus doping on surface chemistry and capacitive behaviors of porous carbon electrode. Electrochim. Acta 2018, 266, 420–430. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251, 2283. [Google Scholar] [CrossRef] [PubMed]


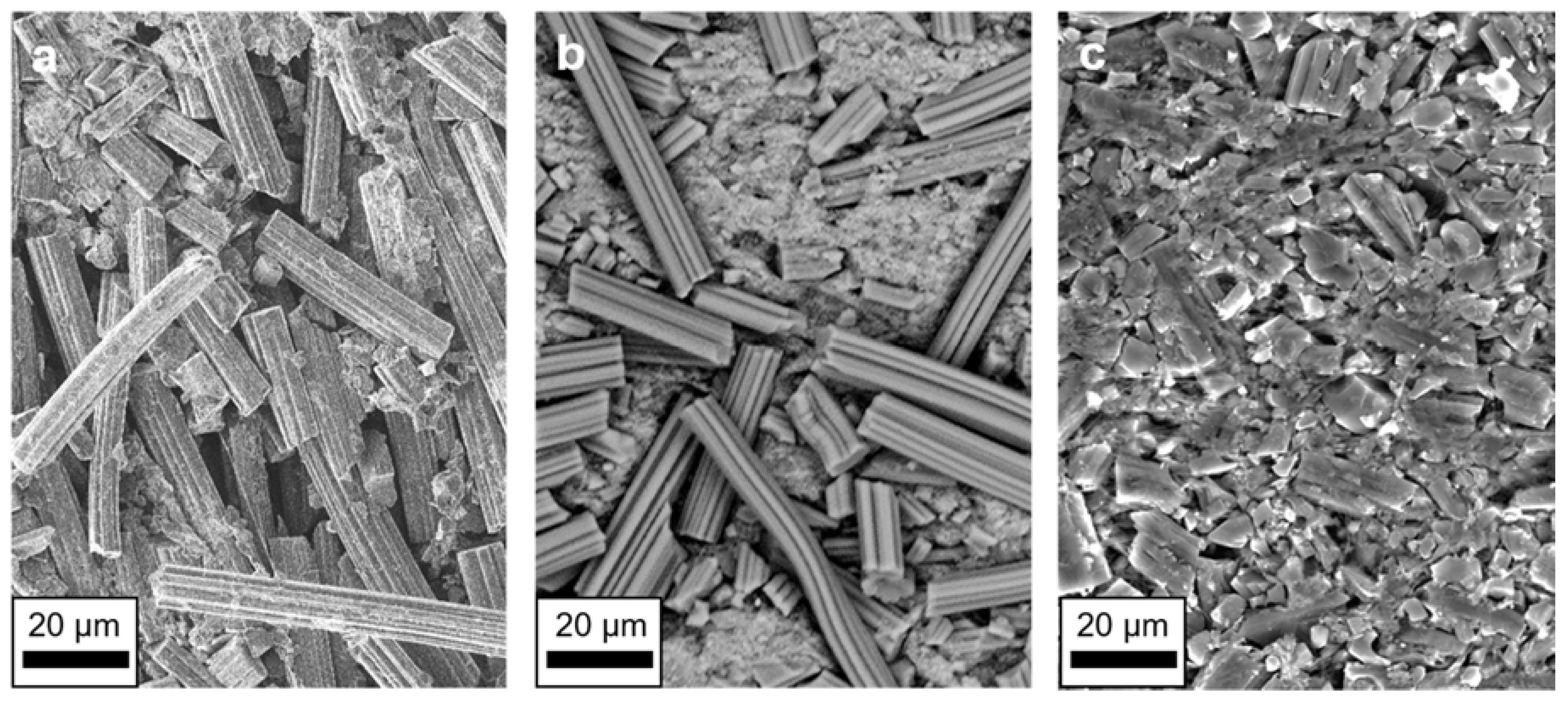
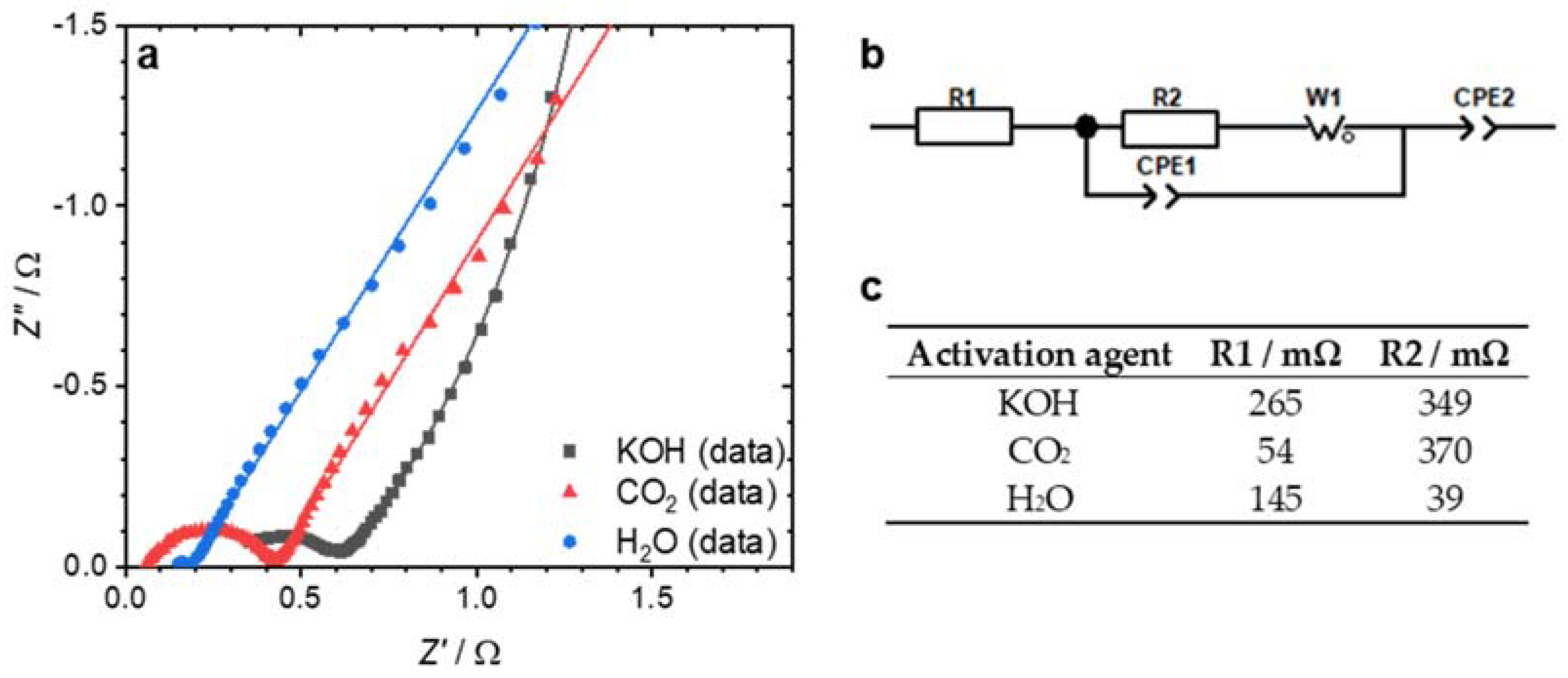
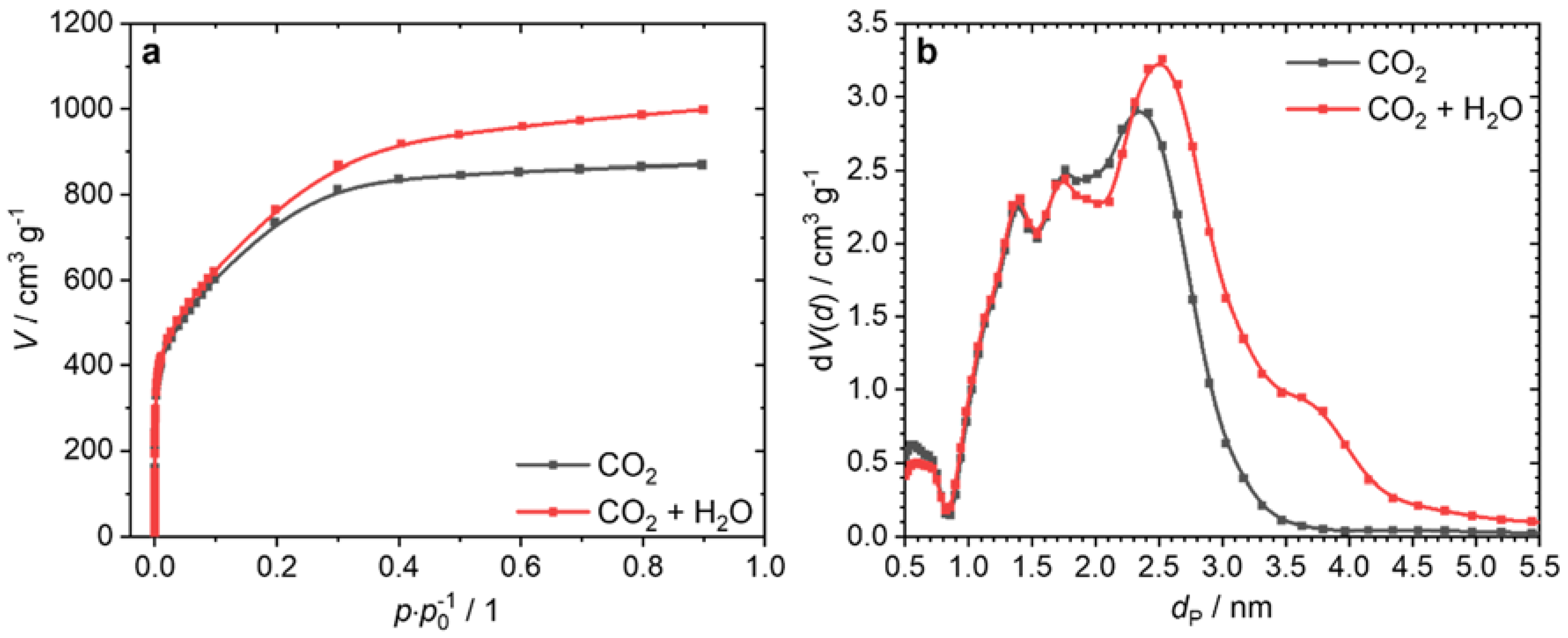
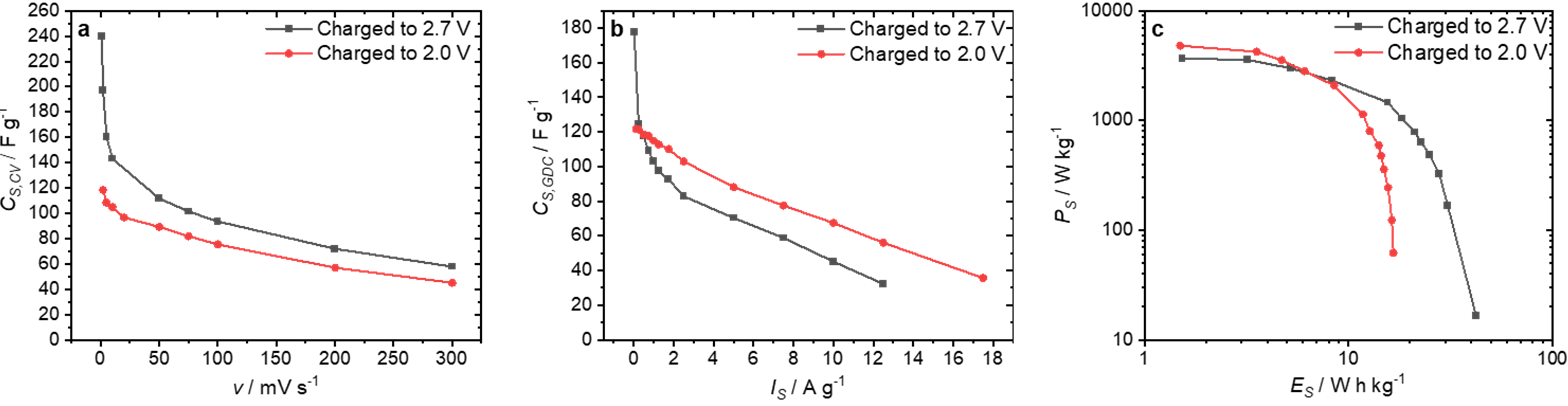
| Activation Agent | SBET/m2 g−1 | Vtot/cm3 g−1 | Total Yield/% |
|---|---|---|---|
| KOH | 2553 | 1.10 | 2.1 |
| CO2 | 2737 | 1.35 | 9.2 |
| H2O | 2516 | 1.27 | 23.9 |
| Activation Agent | C/at.% | O/at.% | P/at.% | N/at.% |
|---|---|---|---|---|
| KOH | 97.4 ± 0.2 | 2.6 ± 0.2 | 0.0 | 0.0 |
| CO2 | 88.3 ± 0.7 | 8.0 ± 0.4 | 2.9 ± 0.2 | 0.9 ± 0.2 |
| H2O | 98.6 ± 0.3 | 1.4 ± 0.3 | 0.0 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breitenbach, S.; Duchoslav, J.; Mardare, A.I.; Unterweger, C.; Stifter, D.; Hassel, A.W.; Fürst, C. Comparative Behavior of Viscose-Based Supercapacitor Electrodes Activated by KOH, H2O, and CO2. Nanomaterials 2022, 12, 677. https://doi.org/10.3390/nano12040677
Breitenbach S, Duchoslav J, Mardare AI, Unterweger C, Stifter D, Hassel AW, Fürst C. Comparative Behavior of Viscose-Based Supercapacitor Electrodes Activated by KOH, H2O, and CO2. Nanomaterials. 2022; 12(4):677. https://doi.org/10.3390/nano12040677
Chicago/Turabian StyleBreitenbach, Stefan, Jiri Duchoslav, Andrei Ionut Mardare, Christoph Unterweger, David Stifter, Achim Walter Hassel, and Christian Fürst. 2022. "Comparative Behavior of Viscose-Based Supercapacitor Electrodes Activated by KOH, H2O, and CO2" Nanomaterials 12, no. 4: 677. https://doi.org/10.3390/nano12040677
APA StyleBreitenbach, S., Duchoslav, J., Mardare, A. I., Unterweger, C., Stifter, D., Hassel, A. W., & Fürst, C. (2022). Comparative Behavior of Viscose-Based Supercapacitor Electrodes Activated by KOH, H2O, and CO2. Nanomaterials, 12(4), 677. https://doi.org/10.3390/nano12040677






