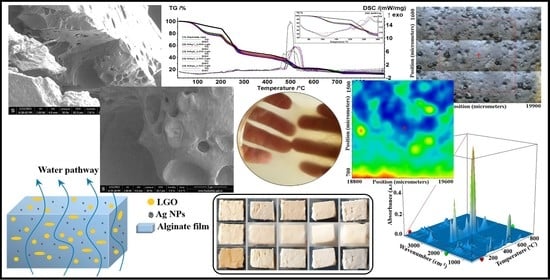
Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Silver Nanoparticles
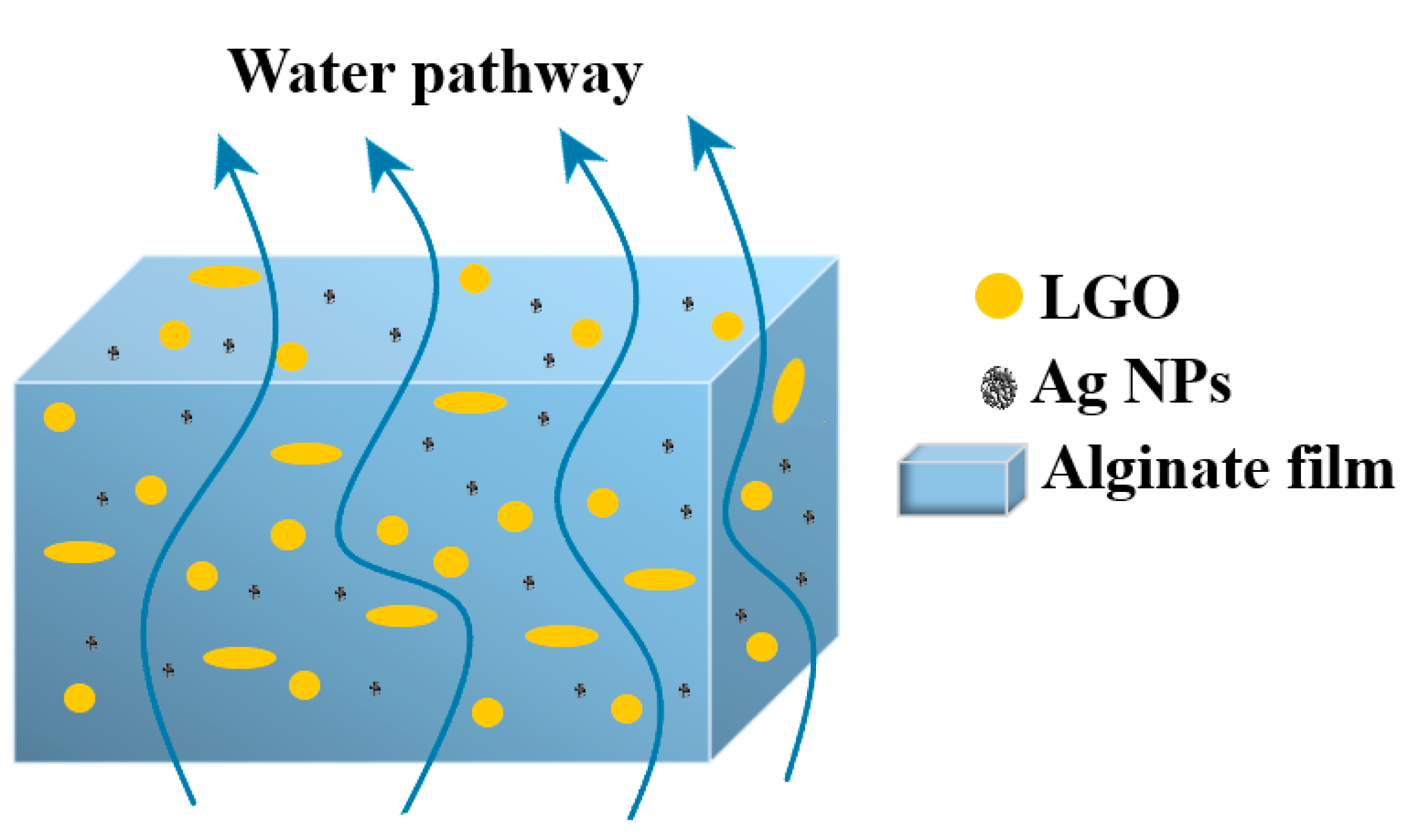
2.3. Synthesis of Alginate/Ag/LGO Films
2.4. Characterization of Alginate Films
3. Results and Discussion
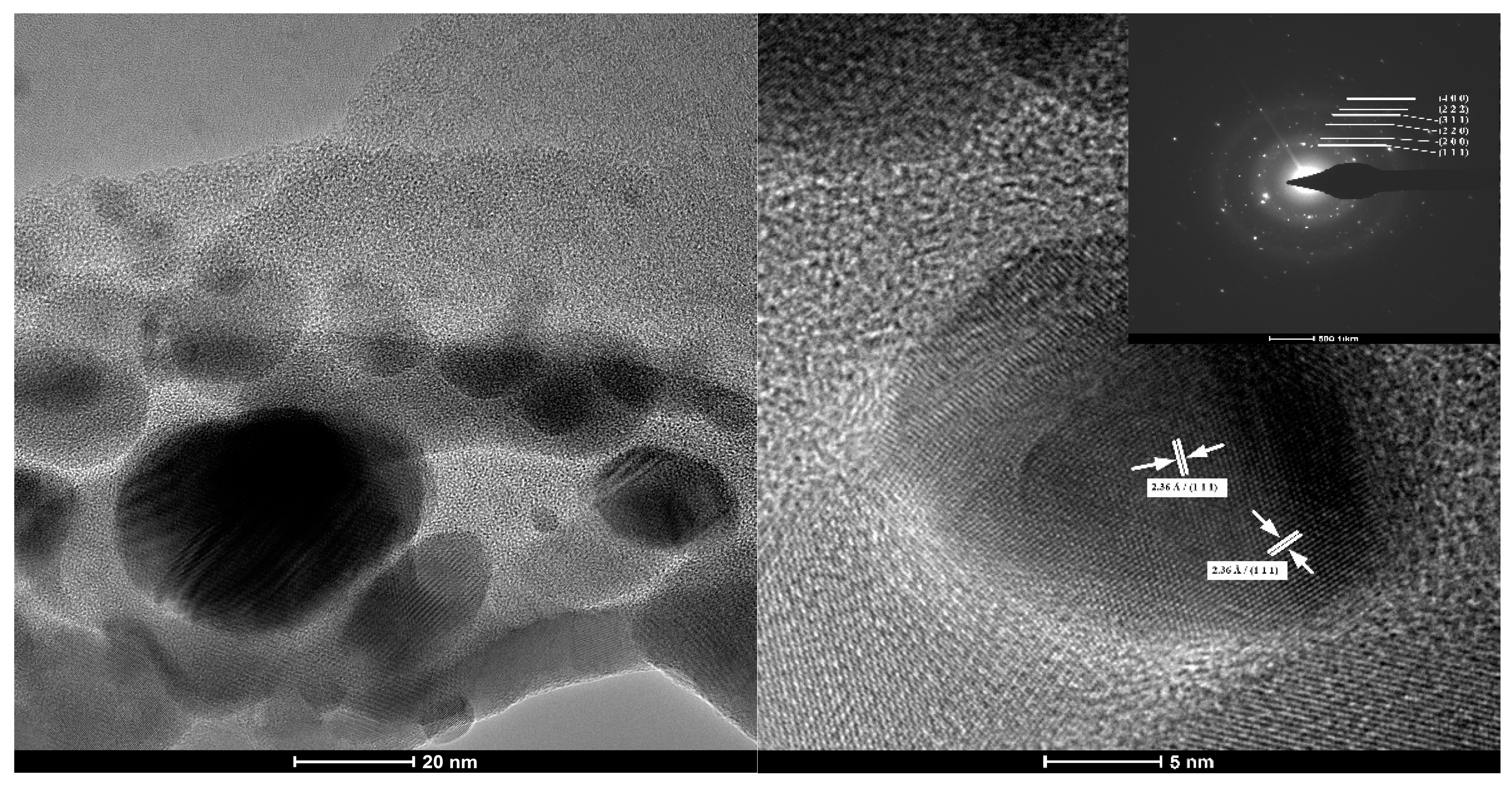
3.1. Transmision Electron Microscopy
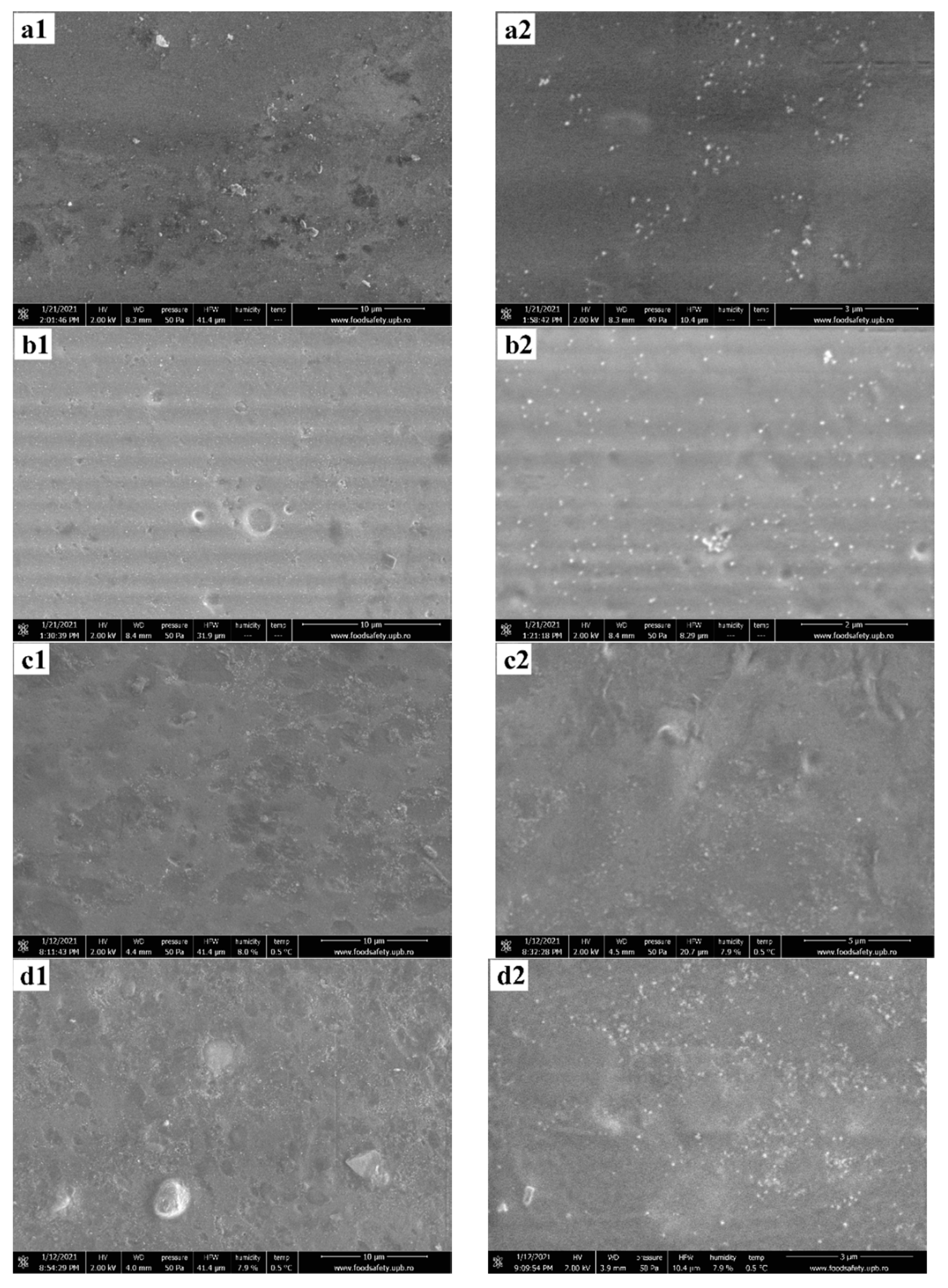
3.2. Environmental Scanning Electron Microscopy
3.3. FTIR Spectroscopy and Microscopy
3.3.1. FTIR Spectroscopy
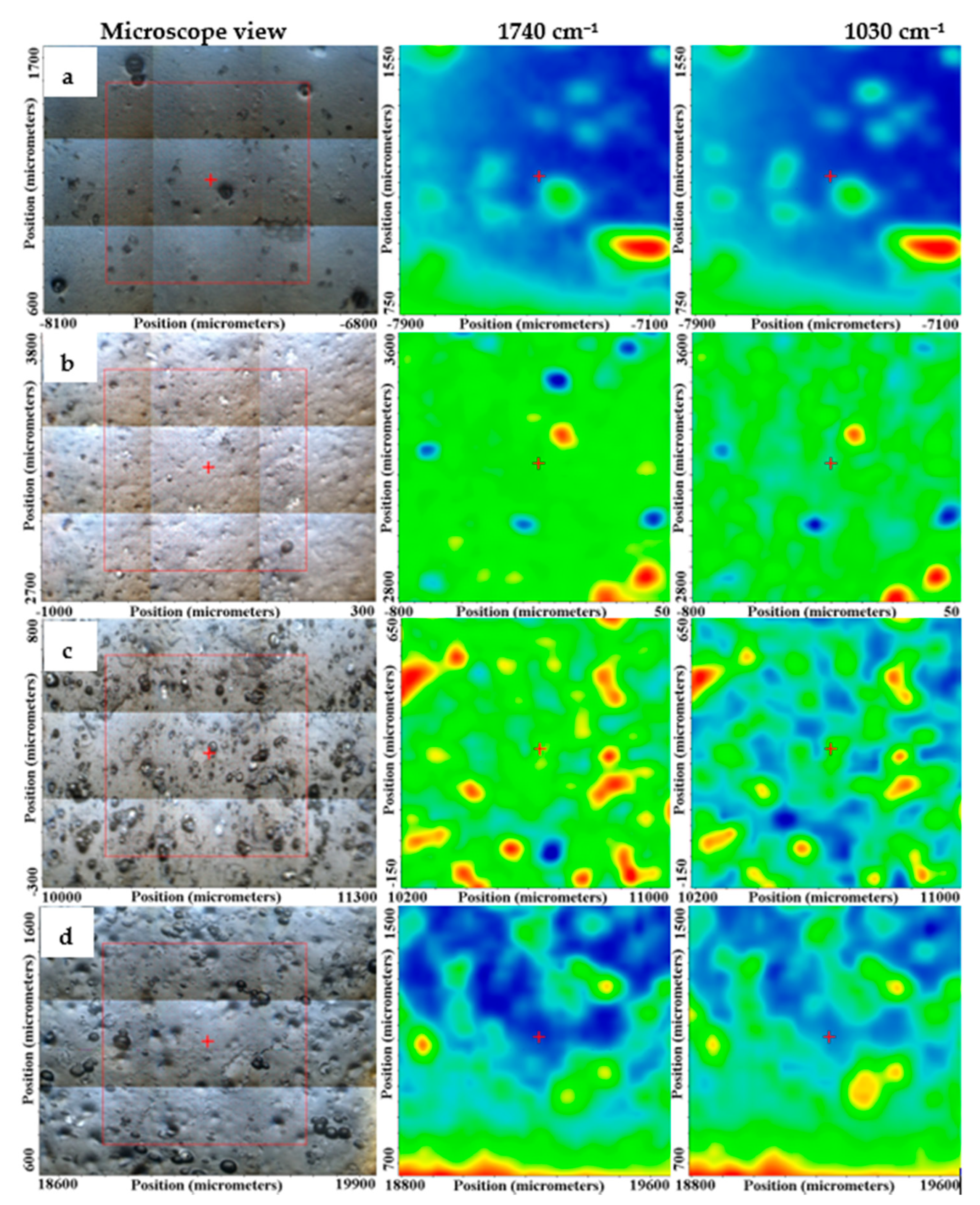
3.3.2. FTIR Microscopy
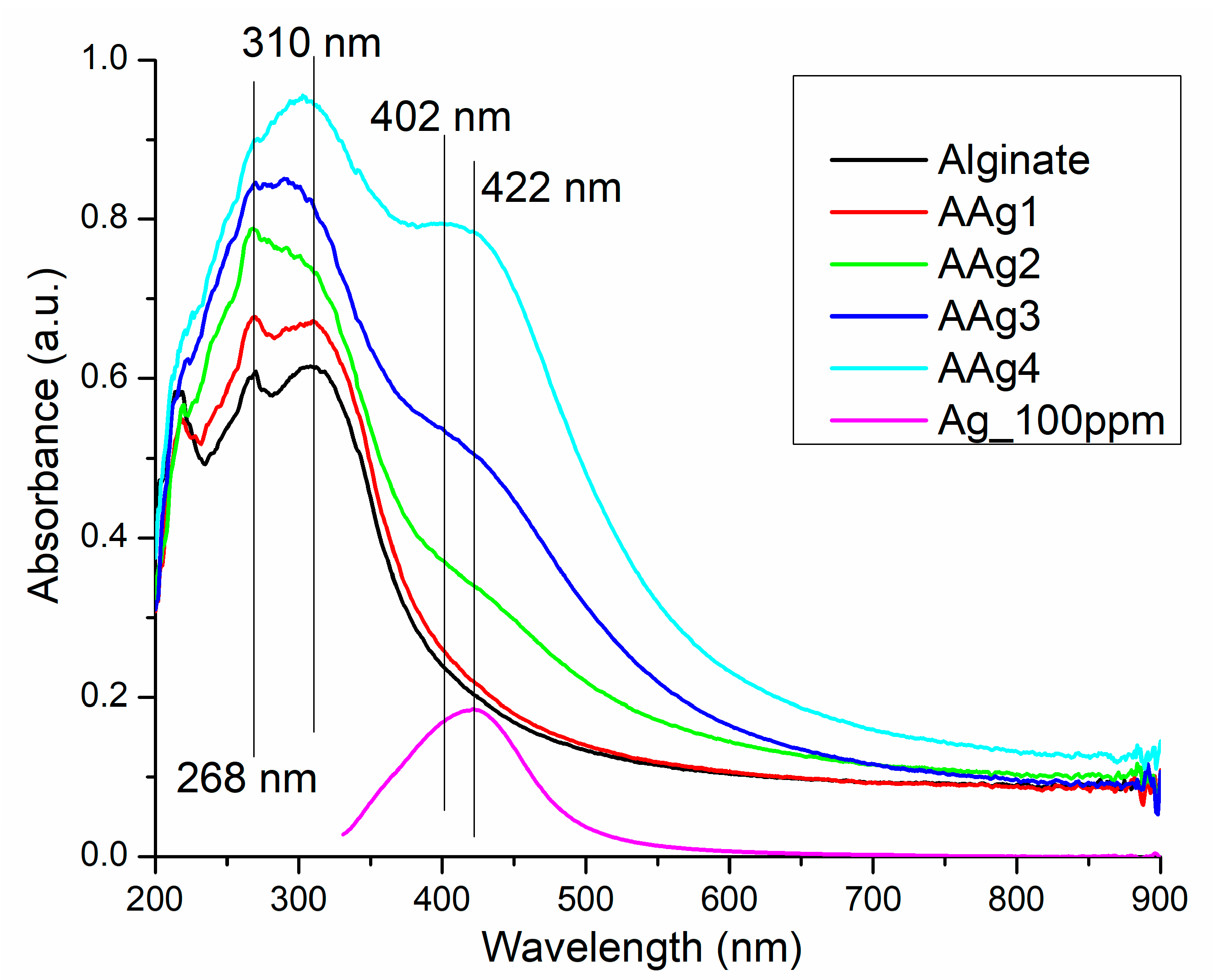
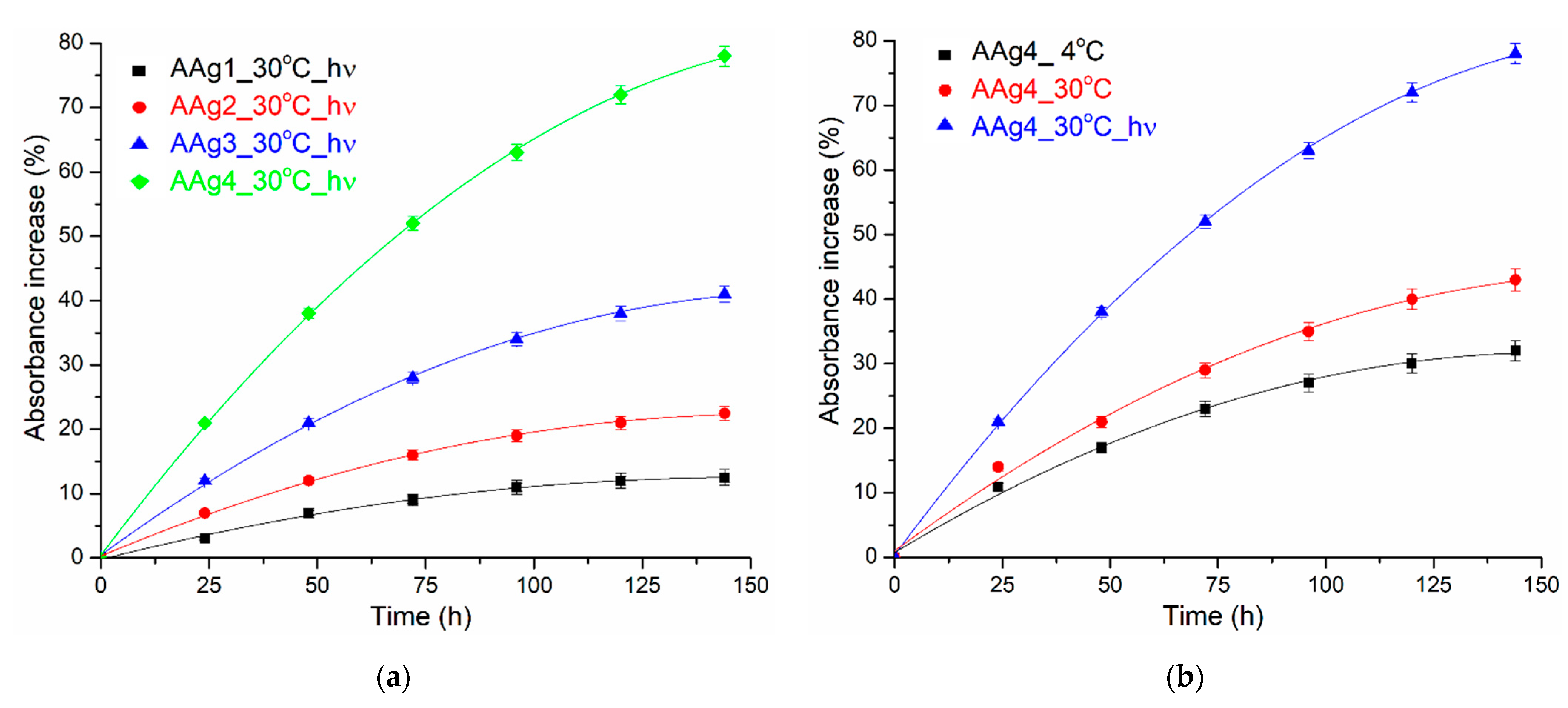
3.4. UV-Vis and Fluorescence Spectroscopy
UV-Vis Spectroscopy
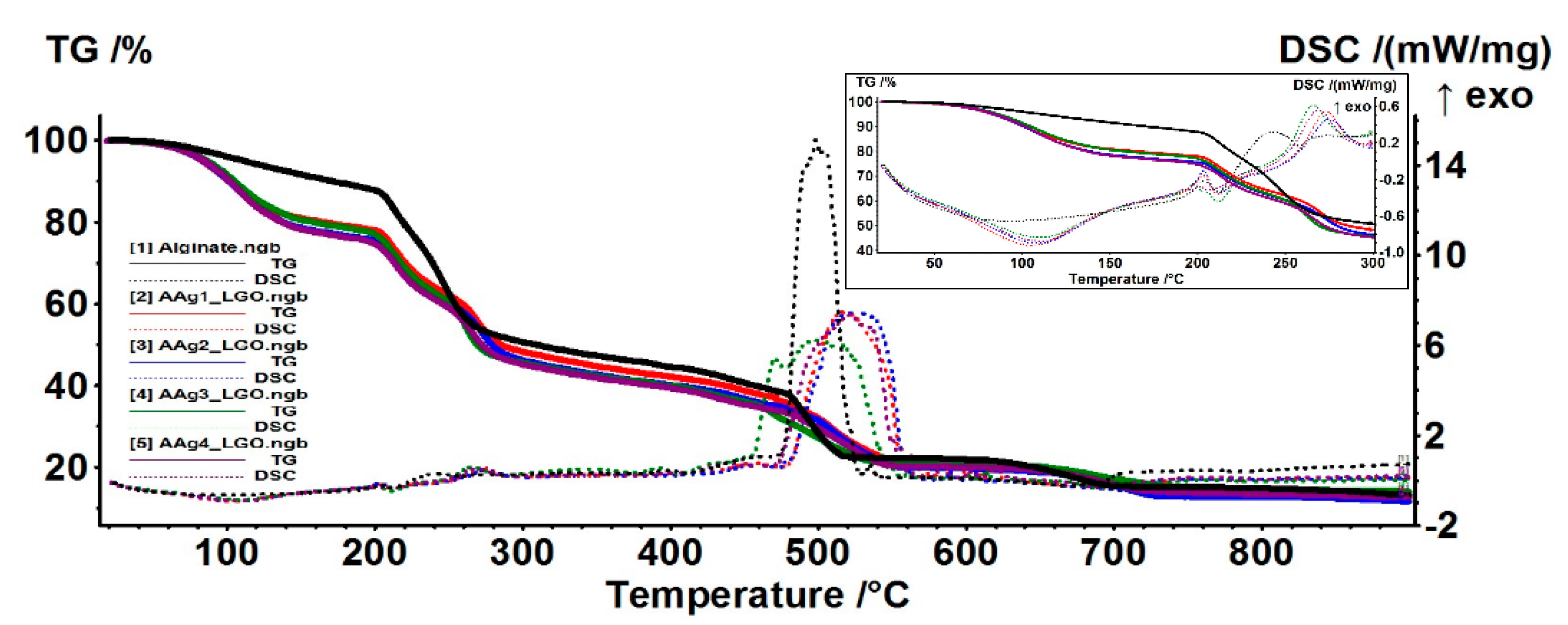
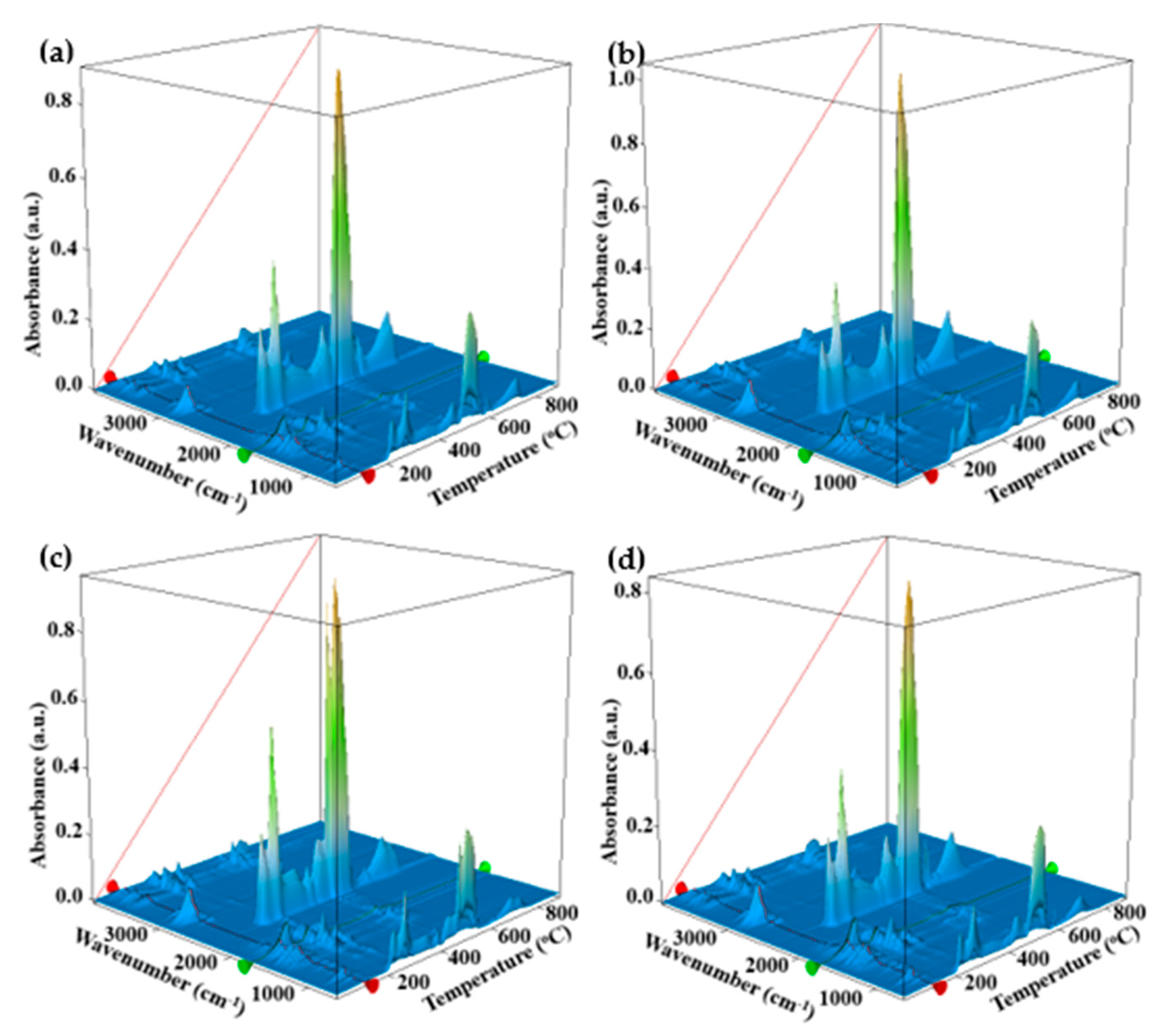
3.5. Thermal Analysis TG/DSC–FTIR
3.6. Water Vapor Permeability (WVP)
3.7. Swelling Study
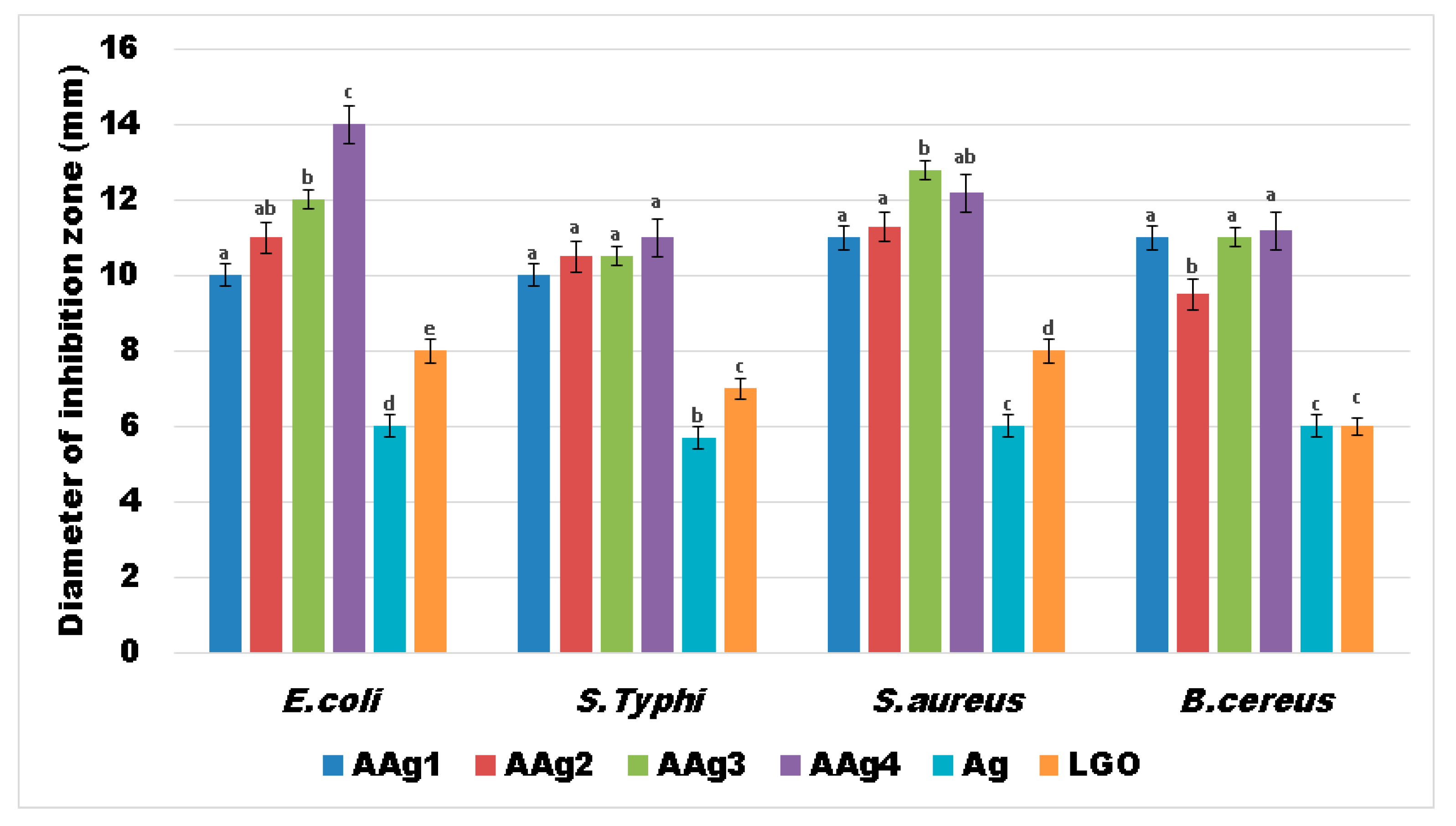
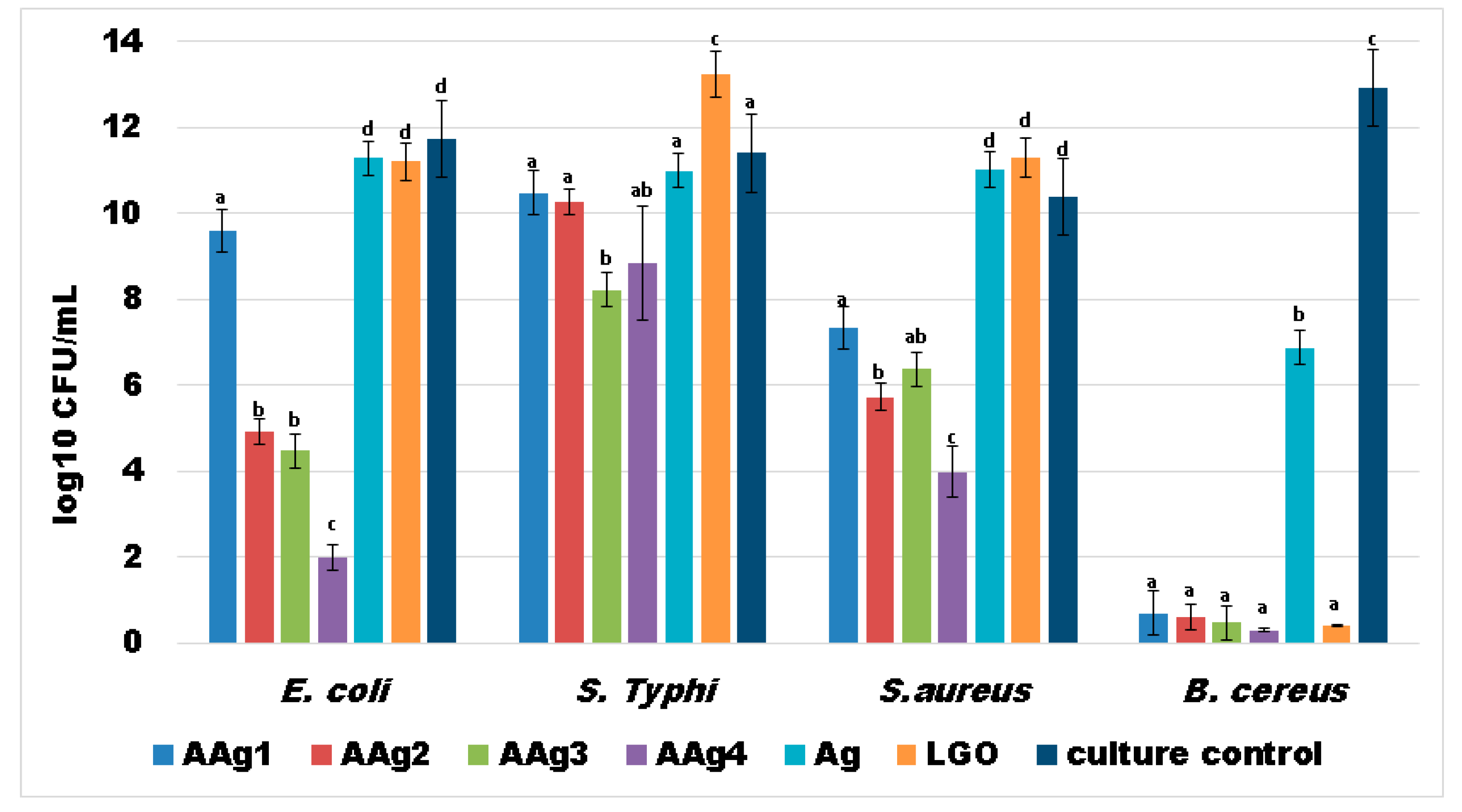
3.8. Antibacterial Activity
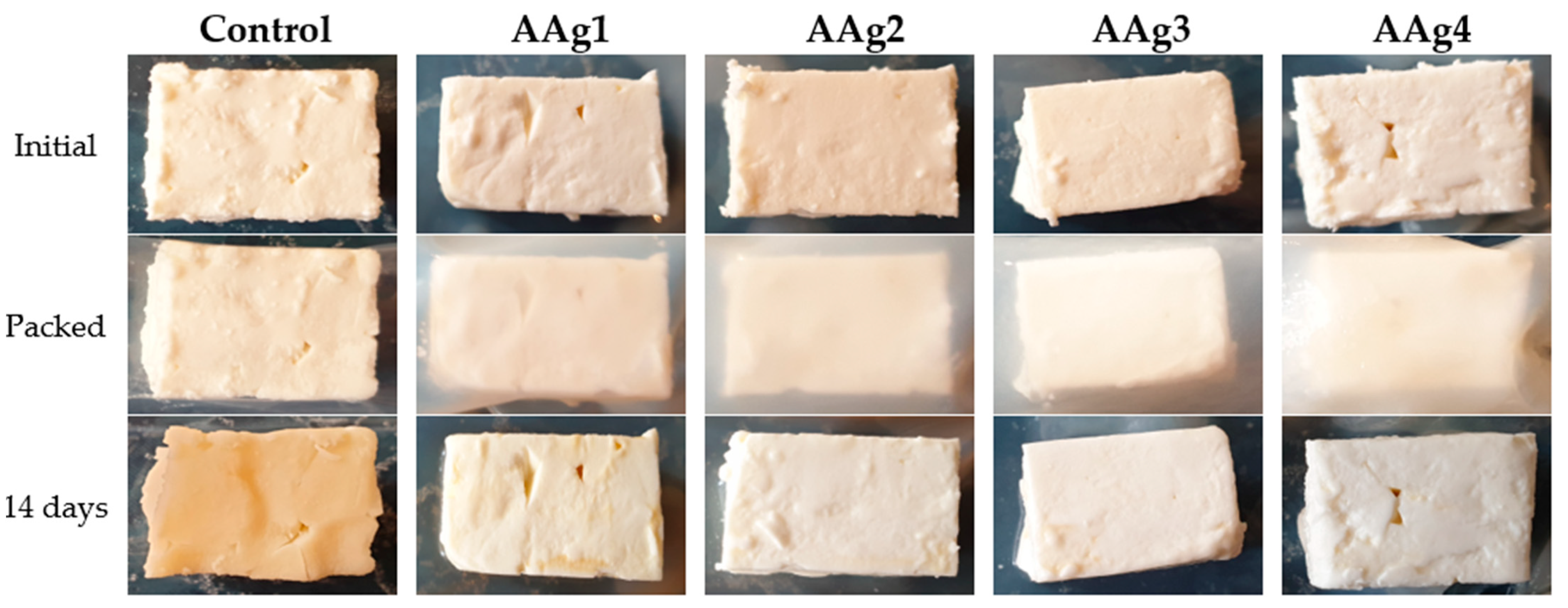
3.9. Evaluation of Potential Use of AAg1–AAg4 Films as Food Packaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Andronescu, E. Smart Food Packaging Designed by Nanotechnological and Drug Delivery Approaches. Coatings 2020, 10, 806. [Google Scholar] [CrossRef]
- Radulescu, M.; Ficai, D.; Oprea, O.; Ficai, A.; Andronescu, E.; Holban, A.M. Antimicrobial Chitosan based Formulations with Impact on Different Biomedical Applications. Curr. Pharm. Biotechnol. 2015, 16, 128–136. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Trusca, R.D.; Andronescu, E.; Holban, A. Biodegradable Alginate Films with ZnO Nanoparticles and Citronella Essential Oil—A Novel Antimicrobial Structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Lemnaru, G.-M.; Trusca, R.D.; Ilie, C.-I.; Tiplea, R.D.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Ditu, L.-M. Antibacterial Activity of Bacterial Cellulose Loaded with Bacitracin and Amoxicillin: In Vitro Studies. Molecules 2020, 25, 4069. [Google Scholar] [CrossRef] [PubMed]
- Wilpiszewska, K.; Antosik, A.K.; Schmidt, B.; Janik, J.; Rokicka, J. Hydrophilic Films Based on Carboxymethylated Derivatives of Starch and Cellulose. Polymers 2020, 12, 2447. [Google Scholar] [CrossRef]
- Li, S.B.; Yi, J.J.; Yu, X.M.; Wang, Z.Y.; Wang, L. Preparation and characterization of pullulan derivative/chitosan composite film for potential antimicrobial applications. Int. J. Biol. Macromol. 2020, 148, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Longano, D.; Costa, C.; Ditaranto, N.; Ancona, A.; Cioffi, N.; Scrocco, C.; Sabbatini, L.; Conto, F.; Del Nobile, M.A. A novel preservation technique applied to fiordilatte cheese. Innov. Food Sci. Emerg. Technol. 2013, 19, 158–165. [Google Scholar] [CrossRef]
- Gherasim, O.; Popescu, R.C.; Grumezescu, V.; Mogosanu, G.D.; Mogoanta, L.; Iordache, F.; Holban, A.M.; Vasile, B.S.; Birca, A.C.; Oprea, O.C.; et al. MAPLE Coatings Embedded with Essential Oil-Conjugated Magnetite for Anti-Biofilm Applications. Materials 2021, 14, 1612. [Google Scholar] [CrossRef]
- Dimulescu, I.A.; Nechifor, A.C.; Bardaca, C.; Oprea, O.; Pascu, D.; Totu, E.E.; Albu, P.C.; Nechifor, G.; Bungau, S.G. Accessible Silver-Iron Oxide Nanoparticles as a Nanomaterial for Supported Liquid Membranes. Nanomaterials 2021, 11, 1204. [Google Scholar] [CrossRef]
- Vasile, B.S.; Oprea, O.; Voicu, G.; Ficai, A.; Andronescu, E.; Teodorescu, A.; Holban, A. Synthesis and characterization of a novel controlled release zinc oxide/gentamicin-chitosan composite with potential applications in wounds care. Int. J. Pharm. 2014, 463, 161–169. [Google Scholar] [CrossRef]
- Gingasu, D.; Mindru, I.; Patron, L.; Ianculescu, A.; Vasile, E.; Marinescu, G.; Preda, S.; Diamandescu, L.; Oprea, O.; Popa, M.; et al. Synthesis and Characterization of Chitosan-Coated Cobalt Ferrite Nanoparticles and Their Antimicrobial Activity. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1932–1941. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Trusca, R.D.; Ilie, C.I.; Oprea, O.C.; Andronescu, E. Innovative Antimicrobial Chitosan/ZnO/Ag NPs/Citronella Essential Oil Nanocomposite-Potential Coating for Grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Duan, G.G.; Zhang, G.Y.; Yang, H.Q.; He, S.J.; Jiang, S.H. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Y.G.; Li, X.L.; Guo, X.L.; Li, W.X.; Chen, J.W.; Liu, Q.; Xu, Q.L.; Wang, Q.; Yang, H.; Shui, Y.R.; et al. Effects of Different TiO(2)Nanoparticles Concentrations on the Physical and Antibacterial Activities of Chitosan-Based Coating Film. Nanomaterials 2020, 10, 1365. [Google Scholar] [CrossRef]
- Viktorova, J.; Stupak, M.; Rehorova, K.; Dobiasova, S.; Hoang, L.; Hajslova, J.; Thanh, T.V.; Tri, L.V.; Tuan, N.V.; Ruml, T. Lemon Grass Essential Oil does not Modulate Cancer Cells Multidrug Resistance by Citral-Its Dominant and Strongly Antimicrobial Compound. Foods 2020, 9, 585. [Google Scholar] [CrossRef] [PubMed]
- Fleancu, M.C.; Olteanu, N.L.; Rogozea, A.E.; Crisciu, A.V.; Pincovschi, I.; Mihaly, M. Physical-chemical parameters promoting phase changes in non-ionic environmental-friendly microemulsions. Fluid Phase Equilib. 2013, 337, 18–25. [Google Scholar] [CrossRef]
- Sonseca, A.; Madani, S.; Rodriguez, G.; Hevilla, V.; Echeverria, C.; Fernandez-Garcia, M.; Munoz-Bonilla, A.; Charef, N.; Lopez, D. Multifunctional PLA Blends Containing Chitosan Mediated Silver Nanoparticles: Thermal, Mechanical, Antibacterial, and Degradation Properties. Nanomaterials 2020, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Vizzini, P.; Beltrame, E.; Zanet, V.; Vidic, J.; Manzano, M. Development and Evaluation of qPCR Detection Method and Zn-MgO/Alginate Active Packaging for Controlling Listeria monocytogenes Contamination in Cold-Smoked Salmon. Foods 2020, 9, 1353. [Google Scholar] [CrossRef]
- Kontominas, M.G. Use of Alginates as Food Packaging Materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef]
- Chen, K.Y.; Zeng, S.Y. Preparation and Characterization of Quaternized Chitosan Coated Alginate Microspheres for Blue Dextran Delivery. Polymers 2017, 9, 210. [Google Scholar] [CrossRef]
- Chang, Y.H.; Huang, C.F.; Hsu, W.J.; Chang, F.C. Removal of Hg2+ from aqueous solution using alginate gel containing chitosan. J. Appl. Polym. Sci. 2007, 104, 2896–2905. [Google Scholar] [CrossRef]
- Paduraru, A.; Ghitulica, C.; Trusca, R.; Surdu, V.A.; Neacsu, I.A.; Holban, A.M.; Birca, A.C.; Iordache, F.; Vasile, B.S. Antimicrobial Wound Dressings as Potential Materials for Skin Tissue Regeneration. Materials 2019, 12, 1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheorghita, R.; Amariei, S.; Norocel, L.; Gutt, G. New Edible Packaging Material with Function in Shelf Life Extension: Applications for the Meat and Cheese Industries. Foods 2020, 9, 562. [Google Scholar] [CrossRef]
- Gheorghita, R.; Gutt, G.; Amariei, S. The Use of Edible Films Based on Sodium Alginate in Meat Product Packaging: An Eco-Friendly Alternative to Conventional Plastic Materials. Coatings 2020, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Gago, C.; Antao, R.; Dores, C.; Guerreiro, A.; Miguel, M.G.; Faleiro, M.L.; Figueiredo, A.C.; Antunes, M.D. The Effect of Nanocoatings Enriched with Essential Oils on ‘Rocha’ Pear Long Storage. Foods 2020, 9, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, A.; Khafagy, R.M.; Elhaes, H.; Ibrahim, M.A. Molecular properties of polyvinyl alcohol/sodium alginate composite. Biointerface Res. Appl. Chem. 2020, 10, 4734–4739. [Google Scholar]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef]
- Fahmy, A.; Khafagy, R.M.; Elhaes, H.; Ibrahim, M.A. Molecular Modeling Analyses of Polyvinyl Alcohol/ Sodium Alginate/ZnO Composite. Egypt. J. Chem. 2021, 64, 1149–1166. [Google Scholar]
- Lan, W.T.; Li, S.Y.; Shama, S.; Zhao, Y.Q.; Sameen, D.E.; He, L.; Liu, Y.W. Investigation of Ultrasonic Treatment on Physicochemical, Structural and Morphological Properties of Sodium Alginate/AgNPs/Apple Polyphenol Films and Its Preservation Effect on Strawberry. Polymers 2020, 12, 2096. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Hu, X.W.; Wang, M.H. Physical and bioactivities of biopolymeric films incorporated with cellulose, sodium alginate and copper oxide nanoparticles for food packaging application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, H.Q.; Yang, S.Z.; Zeng, J.R.; Wu, Z.Q. Sodium Alginate-Based Green Packaging Films Functionalized by Guava Leaf Extracts and Their Bioactivities. Materials 2019, 12, 2923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammoudi, N.; Cherif, H.Z.; Borsali, F.; Benmansour, K.; Meghezzi, A. Preparation of active antimicrobial and antifungal alginate-montmorillonite/lemon essential oil nanocomposite films. Mater. Technol. 2020, 35, 383–394. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Shavisi, N. Effects of sodium alginate coating containing Mentha spicata essential oil and cellulose nanoparticles on extending the shelf life of raw silver carp (Hypophthalmichthys molitrix) fillets. Food Sci. Biotechnol. 2019, 28, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Corbo, F.; Agrimi, G.; Ditaranto, N.; Cioffi, N.; Perna, F.; Quivelli, A.; Stefano, E.; Lunetti, P.; Muscella, A.; et al. Oxidized Alginate Dopamine Conjugate: In Vitro Characterization for Nose-to-Brain Delivery Application. Materials 2021, 14, 3495. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Longano, D.; Bonerba, E.; Tantillo, G.; Torsi, L.; Sabbatini, L.; Cioffi, N.; Ditaranto, N. Electrochemical Preparation of Synergistic Nanoantimicrobials. Molecules 2020, 25, 49. [Google Scholar] [CrossRef] [Green Version]
- Tsirigotis-Maniecka, M. Alginate-, Carboxymethyl Cellulose-, and kappa-Carrageenan-Based Microparticles as Storage Vehicles for Cranberry Extract. Molecules 2020, 25, 3998. [Google Scholar] [CrossRef]
- Pica, A.; Guran, C.; Andronescu, E.; Oprea, O.; Ficai, D.; Ficai, A. Antimicrobial performances of some film forming materials based on silver nanoparticles. J. Optoelectron. Adv. Mater. 2012, 14, 863–868. [Google Scholar]
- Nedelcu, I.A.; Ficai, A.; Sonmez, M.; Ficai, D.; Oprea, O.; Andronescu, E. Silver Based Materials for Biomedical Applications. Curr. Org. Chem. 2014, 18, 173–184. [Google Scholar] [CrossRef]
- Vasile, B.S.; Birca, A.C.; Musat, M.C.; Holban, A.M. Wound Dressings Coated with Silver Nanoparticles and Essential Oils for The Management of Wound Infections. Materials 2020, 13, 1682. [Google Scholar] [CrossRef] [Green Version]
- Khalir, W.K.A.W.M.; Shameli, K.; Jazayeri, S.D.; Othman, N.A.; Jusoh, N.W.C.; Hassan, N.M. In-Situ Biofabrication of Silver Nanoparticles inCeiba pentandraNatural Fiber UsingEntada spiralisExtract with Their Antibacterial and Catalytic Dye Reduction Properties. Nanomaterials 2020, 10, 1104. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Cotorcea, S.; Bungau, C.; Albu, P.C.; Pascu, D.; Oprea, O.; Grosu, A.R.; Pirtac, A.; Nechifor, G. Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles-Cellulose Derivatives for Air Odor Correction. Membranes 2021, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Kukushkina, E.A.; Hossain, S.I.; Sportelli, M.C.; Ditaranto, N.; Picca, R.A.; Cioffi, N. Ag-Based Synergistic Antimicrobial Composites. A Critical Review. Nanomaterials 2021, 11, 1687. [Google Scholar] [CrossRef]
- Istrati, D.; Lacatusu, I.; Bordei, N.; Badea, G.; Oprea, O.; Stefan, L.M.; Stan, R.; Badea, N.; Meghea, A. Phyto-mediated nanostructured carriers based on dual vegetable actives involved in the prevention of cellular damage. Mater. Sci. Eng. C 2016, 64, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Lacatusu, I.; Arsenie, L.V.; Badea, G.; Popa, O.; Oprea, O.; Badea, N. New cosmetic formulations with broad photoprotective and antioxidative activities designed by amaranth and pumpkin seed oils nanocarriers. Ind. Crops Prod. 2018, 123, 424–433. [Google Scholar] [CrossRef]
- Mihaly, M.; Lacatusu, I.; Meghea, A. Sulphonephtalein chromophore as molecular probe in micelle systems. Rev. Chim. 2007, 58, 929–932. [Google Scholar]
- Niculae, G.; Badea, N.; Meghea, A.; Oprea, O.; Lacatusu, I. Coencapsulation of Butyl-Methoxydibenzoylmethane and Octocrylene into Lipid Nanocarriers: UV Performance, Photostability and in vitro Release. Photochem. Photobiol. 2013, 89, 1085–1094. [Google Scholar] [CrossRef]
- Francikowski, J.; Baran, B.; Cup, M.; Janiec, J.; Krzyzowski, M. Commercially Available Essential Oil Formulas as Repellents Against the Stored-Product Pest Alphitobius diaperinus. Insects 2019, 10, 96. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.J.; Wang, J.Q. Constituents of the Essential Oils of Garlic and Citronella and Their Vapor-phase Inhibition Mechanism against S. aureus. Food Sci. Technol. Res. 2019, 25, 65–74. [Google Scholar] [CrossRef]
- Munteanu, S.B.; Vasile, C. Vegetable Additives in Food Packaging Polymeric Materials. Polymers 2020, 12, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdes, A.; Garcia-Serna, E.; Martinez-Abad, A.; Vilaplana, F.; Jimenez, A.; Garrigos, M.C. Gelatin-Based Antimicrobial Films Incorporating Pomegranate (Punica granatum L.) Seed Juice by-Product. Molecules 2020, 25, 166. [Google Scholar] [CrossRef] [Green Version]
- Becerril, R.; Nerin, C.; Silva, F. Encapsulation Systems for Antimicrobial Food Packaging Components: An Update. Molecules 2020, 25, 1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou Baker, D.H.; Al-Moghazy, M.; ElSayed, A.A.A. The in vitro cytotoxicity, antioxidant and antibacterial potential of Satureja hortensis L. essential oil cultivated in Egypt. Bioorg. Chem. 2020, 95, 103559. [Google Scholar] [CrossRef] [PubMed]
- Sutili, F.J.; Kreutz, L.C.; Flores, F.C.; da Silva, C.D.; Kirsten, K.S.; Voloski, A.P.D.; Frandoloso, R.; Pinheiro, C.G.; Heinzmann, B.M.; Baldisserotto, B. Effect of dietary supplementation with citral-loaded nanostructured systems on innate immune responses and gut microbiota of silver catfish (Rhamdia quelen). J. Funct. Foods 2019, 60, 103454. [Google Scholar] [CrossRef]
- Bazargani-Gilani, B.; Pajohi-Alamoti, M. The effects of incorporated resveratrol in edible coating based on sodium alginate on the refrigerated trout (Oncorhynchus mykiss) fillets’ sensorial and physicochemical features. Food Sci. Biotechnol. 2020, 29, 207–216. [Google Scholar] [CrossRef]
- Rao, B.M.; Jesmi, D.; Viji, P. Chilled Storage of Pangasianodon hypophthalmus Fillets Coated with Plant Oil Incorporated Alginate Gels: Effect of Clove Leaf, Clove Bud, Rosemary and Thyme Oils. J. Aquat. Food Prod. Technol. 2017, 26, 744–755. [Google Scholar] [CrossRef]
- Lichanporn, I.; Techavuthiporn, C.; Wongs-Aree, C. Effect of Silver Particle-longkong Peel Extract Coating on Postharvest Decay and Browning in Longkong Fruit. Hortic. J. 2020, 89, 328–336. [Google Scholar] [CrossRef]
- Popescu, E.L.; Balasoiu, M.; Cristea, O.M.; Stoica, A.E.; Oprea, O.C.; Vasile, B.S.; Grumezescu, A.M.; Bancescu, G.; Busuioc, C.J.; Mogosanu, G.D.; et al. Study of antimicrobial effects of functionalized silver nanoparticles. Rom. J. Morphol. Embryol. 2019, 60, 939–946. [Google Scholar] [PubMed]
- Tymczewska, A.; Furtado, B.U.; Nowaczyk, J.; Hrynkiewicz, K.; Szydłowska-Czerniak, A. Development and Characterization of Active Gelatin Films Loaded with Rapeseed Meal Extracts. Materials 2021, 14, 2869. [Google Scholar] [CrossRef]
- Li, W.; Zheng, K.; Chen, H.; Feng, S.; Wang, W.; Qin, C. Influence of Nano Titanium Dioxide and Clove Oil on Chitosan–Starch Film Characteristics. Polymers 2019, 11, 1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socaciu, M.I.; Fogarasi, M.; Semeniuc, C.A.; Socaci, S.A.; Rotar, M.A.; Muresan, V.; Pop, O.L.; Vodnar, D.C. Formulation and Characterization of Antimicrobial Edible Films Based on Whey Protein Isolate and Tarragon Essential Oil. Polymers 2020, 12, 1748. [Google Scholar] [CrossRef]
- Mohammed, H.B.; Rayyif, S.M.I.; Curutiu, C.; Birca, A.C.; Oprea, O.C.; Grumezescu, A.M.; Ditu, L.M.; Gheorghe, I.; Chifiriuc, M.C.; Mihaescu, G.; et al. Eugenol-Functionalized Magnetite Nanoparticles Modulate Virulence and Persistence in Pseudomonas aeruginosa Clinical Strains. Molecules 2021, 26, 2189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Li, H.; Wang, Y.; Wang, Y.S.; Yan, J. Preparation of Ag NPs and Its Multifunctional Finishing for Cotton Fabric. Polymers 2021, 13, 1338. [Google Scholar] [CrossRef] [PubMed]
- Thwala, M.; Klaine, S.; Musee, N. Exposure Media and Nanoparticle Size Influence on the Fate, Bioaccumulation, and Toxicity of Silver Nanoparticles to Higher Plant Salvinia minima. Molecules 2021, 26, 2305. [Google Scholar] [CrossRef]
- Lamb, D.; Shaw, R.A. Visualizing Vapor Pressure: A Mechanical Demonstration of Liquid Vapor Phase Equilibrium. Bull. Am. Meteorol. Soc. 2016, 97, 1355–1362. [Google Scholar] [CrossRef]
- Zook, J.M.; Halter, M.D.; Cleveland, D.; Long, S.E. Disentangling the effects of polymer coatings on silver nanoparticle agglomeration, dissolution, and toxicity to determine mechanisms of nanotoxicity. J. Nanopart. Res. 2012, 14, 1165. [Google Scholar] [CrossRef]
- Go, E.J.; Song, K.B. Effect of java citronella essential oil addition on the physicochemical properties of Gelidium corneum-chitosan composite films. Food Sci. Biotechnol. 2020, 29, 909–915. [Google Scholar] [CrossRef]
- Wang, M.S.; Li, H.B.; Li, Y.H.; Mo, F.; Li, Z.; Chai, R.; Wang, H.X. Dispersibility and Size Control of Silver Nanoparticles with Anti-Algal Potential Based on Coupling Effects of Polyvinylpyrrolidone and Sodium Tripolyphosphate. Nanomaterials 2020, 10, 1042. [Google Scholar] [CrossRef]
- Fratoddi, I.; Battocchio, C.; Iucci, G.; Catone, D.; Cartoni, A.; Paladini, A.; O’Keeffe, P.; Nappini, S.; Cerra, S.; Venditti, I. Silver Nanoparticles Functionalized by Fluorescein Isothiocyanate or Rhodamine B Isothiocyanate: Fluorescent and Plasmonic Materials. Appl. Sci. 2021, 11, 2472. [Google Scholar] [CrossRef]
- Marinescu, L.; Ficai, D.; Oprea, O.; Marin, A.; Ficai, A.; Andronescu, E.; Holban, A.M. Optimized Synthesis Approaches of Metal Nanoparticles with Antimicrobial Applications. J. Nanomater. 2020, 2020, 6651207. [Google Scholar] [CrossRef]
- Nicosia, A.; Abbadessa, A.; Vento, F.; Mazzaglia, A.; Mineo, P.G. Silver Nanoparticles Decorated with PEGylated Porphyrins as Potential Theranostic and Sensing Agents. Materials 2021, 14, 2764. [Google Scholar] [CrossRef]
- Panda, S.K.; Chakraborti, S.; Basu, R.N. Size and shape dependences of the colloidal silver nanoparticles on the light sources in photo-mediated citrate reduction technique. Bull. Mater. Sci. 2018, 41, 90. [Google Scholar] [CrossRef] [Green Version]
- Kanmani, P.; Lim, S.T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multidrug resistant pathogens. Process Biochem. 2013, 48, 1099–1106. [Google Scholar] [CrossRef]
- Dou, Y.; Huang, X.; Zhang, B.N.; He, M.; Yin, G.Q.; Cui, Y.D. Preparation and characterization of a dialdehyde starch crosslinked feather keratin film for food packaging application. RSC Adv. 2015, 5, 27168–27174. [Google Scholar] [CrossRef]
- Yin, Y.D.; Li, Z.Y.; Zhong, Z.Y.; Gates, B.; Xia, Y.N.; Venkateswaran, S. Synthesis and characterization of stable aqueous dispersions of silver nanoparticles through the Tollens process. J. Mater. Chem. 2002, 12, 522–527. [Google Scholar] [CrossRef]
- Pinto, V.V.; Ferreira, M.J.; Silva, R.; Santos, H.A.; Silva, F.; Pereira, C.M. Long time effect on the stability of silver nanoparticles in aqueous medium: Effect of the synthesis and storage conditions. Coll. Surfaces A Physicochem. Eng. Asp. 2010, 364, 19–25. [Google Scholar] [CrossRef]
- Korshed, P.; Li, L.; Ngo, D.T.; Wang, T. Effect of Storage Conditions on the Long-Term Stability of Bactericidal Effects for Laser Generated Silver Nanoparticles. Nanomaterials 2018, 8, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.P.; Zhang, J.Z.; Fang, Y. Photoluminescence from colloidal silver nanoparticles. J. Lumin. 2008, 128, 1635–1640. [Google Scholar] [CrossRef]
- Jia, K.; Wang, P.; Yuan, L.T.; Zhou, X.F.; Chen, W.J.; Liu, X.B. Facile synthesis of luminescent silver nanoparticles and fluorescence interactions with blue-emitting polyarylene ether nitrile. J. Mater. Chem. C 2015, 3, 3522–3529. [Google Scholar] [CrossRef]
- Liu, C.H.; Yang, X.P.; Yuan, H.Y.; Zhou, Z.D.; Xiao, D. Preparation of silver nanoparticle and its application to the determination of ct-DNA. Sensors 2007, 7, 708–718. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Zeng, J.; Tao, J.; Johnson, M.C.; Schmidt-Krey, I.; Blubaugh, L.; Zhu, Y.M.; Gu, Z.Z.; Xia, Y.N. Kinetically Controlled Overgrowth of Ag or Au on Pd Nanocrystal Seeds: From Hybrid Dimers to Nonconcentric and Concentric Bimetallic Nanocrystals. J. Am. Chem. Soc. 2012, 134, 15822–15831. [Google Scholar] [CrossRef]
- Kumar, K.V.A.; Sajna, M.S.; Thomas, V.; Joseph, C.; Unnikrishnan, N.V. Plasmonic and Energy Studies of Ag Nanoparticles in Silica-Titania Hosts. Plasmonics 2014, 9, 631–636. [Google Scholar] [CrossRef]
- Naidu, D.S.; John, M.J. Effect of Clay Nanofillers on the Mechanical and Water Vapor Permeability Properties of Xylan-Alginate Films. Polymers 2020, 12, 2279. [Google Scholar] [CrossRef]
- Jost, V.; Kobsik, K.; Schmid, M.; Noller, K. Influence of plasticiser on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydr. Polym. 2014, 110, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Cofelice, M.; Cuomo, F.; Chiralt, A. Alginate Films Encapsulating Lemongrass Essential Oil as Affected by Spray Calcium Application. Coll. Interfaces 2019, 3, 58. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Song, K.K.; Zhang, X.R.; Sun, Y.; Sui, Y.; Chen, Y.F.; Jia, Z.Y.; Sun, H.H.; Sun, Z.; Xia, X.D. Antimicrobial Activity and Possible Mechanism of Action of Citral against Cronobacter sakazakii. PLoS ONE 2016, 11, e0159006. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.J.; Liu, G.Z.; Li, J.G.; Chen, J.; Li, L.N.; Li, Z.; Zhang, X.L.; Zhang, S.M.; Thorne, R.F.; Zhang, S.Z. Antimicrobial Activity of Lemongrass Essential Oil (Cymbopogon flexuosus) and Its Active Component Citral Against Dual-Species Biofilms of Staphylococcus aureus and Candida Species. Front. Cell. Infect. Microbiol. 2020, 10, 603858. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.L.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, M.; Pohl, P. Synthesis of Biogenic Silver Nanoparticles (AgCl-NPs) Using a Pulicaria vulgaris Gaertn. Aerial Part Extract and Their Application as Antibacterial, Antifungal and Antioxidant Agents. Nanomaterials 2020, 10, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, F.; AlOmar, S.Y.; Albalawi, F.; Arshi, N.; Dwivedi, S.; Kumar, S.; Shaalan, N.M.; Ahmad, N. Microwave Mediated Fast Synthesis of Silver Nanoparticles and Investigation of Their Antibacterial Activities for Gram-Positive and Gram-Negative Microorganisms. Crystals 2021, 11, 666. [Google Scholar] [CrossRef]
- Naik, M.I.; Fomda, B.A.; Jaykumar, E.; Bhat, J.A. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacterias. Asian Pac. J. Trop. Med. 2010, 3, 535–538. [Google Scholar] [CrossRef] [Green Version]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nottagh, S.; Hesari, J.; Peighambardoust, S.H.; Rezaei-Mokarram, R.; Jafarizadeh-Malmiri, H. Effectiveness of edible coating based on chitosan and Natamycin on biological, physico-chemical and organoleptic attributes of Iranian ultra-filtrated cheese. Biologia 2020, 75, 605–611. [Google Scholar] [CrossRef]



| Sample Code | Alginate (g in 100 mL Water) | Ag NPs (mL of 100 ppm Solution) | Glycerol (mL Solution) | LGO (mL) |
|---|---|---|---|---|
| A | 3.00 | 0 | 2 | 0 |
| AAg1 | 3.00 | 5.0 | 2 | 1.0 |
| AAg2 | 3.00 | 10.0 | 2 | 1.0 |
| AAg3 | 3.00 | 25.0 | 2 | 1.0 |
| AAg4 | 3.00 | 50.0 | 2 | 1.0 |
| Sample/Assignment | A | AAg1 | AAg2 | AAg3 | AAg4 |
|---|---|---|---|---|---|
| υasC-O-C | 1031 | 1028 | 1027 | 1026 | 1027 |
| υsCOO- | 1416 | 1408 | 1407 | 1409 | 1410 |
| υasCOO- | 1596 | 1602 | 1600 | 1602 | 1604 |
| C = O group of LGO [67] | 1740 | 1738 | 1738 | 1739 | |
| υC-H (sat) | 2921 | 2930 | 2925 | 2921 | 2921 |
| υO-H | 3278 | 3277 | 3270 | 3284 | 3287 |
| Sample | Alginate | AAg1 | AAg2 | AAg3 | AAg4 |
|---|---|---|---|---|---|
| Thickness (mm) | 0.22 ± 0.01 | 0.21 ± 0.02 | 0.27 ± 0.02 | 0.30 ± 0.03 | 0.35 ± 0.02 |
| Opacity | 0.48 ± 0.02 a | 0.51 ± 0.05 a | 0.54 ± 0.04 a | 0.55 ± 0.06 a | 0.67 ± 0.04 b |
| Film Code | WVP (10−10 g/Pa∙m∙s) |
|---|---|
| A | 5.718 ± 0.011 a |
| AAg1 | 2.753 ± 0.042 b |
| AAg2 | 2.706 ± 0.035 b |
| AAg3 | 2.696 ± 0.024 b |
| AAg4 | 2.691 ± 0.054 b |
| Sample | Water PBS | |||||
|---|---|---|---|---|---|---|
| 0.25 h | 0.5 h | 1 h | 2 h | 3 h | 24 h | |
| A | 42.54% | 61.22% | 86.14% | 101.83% | 104.69% | 102.76% |
| 81.99% | 182.05% | 374.36% | 595.63% | 659.28% | 741.75% | |
| AAg1 | 61.12% | 84.92% | 103.67% | 108.38% | 105.53% | 100.43% |
| 113.94% | 276.24% | 451.33% | 556.02% | 596.58% | 564.89% | |
| AAg2 | 82.97% | 95.59% | 108.65% | 114.35% | 119.33% | 121.06% |
| 167.42% | 347.91% | 489.04% | 589.77% | 623.66% | 704.31% | |
| AAg3 | 110.13% | 123.90% | 130.79% | 126.41% | 128.91% | 128.53% |
| 231.88% | 454.65% | 580.89% | 647.52% | 691.74% | 675.24% | |
| AAg4 | 136.47% | 151.97% | 162.99% | 167.90% | 169.77% | 168.81% |
| 295.55% | 549.36% | 696.36% | 719.23% | 857.85% | 799.79% | |
| Sample/ Weight Loss (%) | A | AAg1 | AAg2 | AAg3 | AAg4 |
|---|---|---|---|---|---|
| 14 days | 39.32 ± 0.51 a | 2.39 ± 0.21 b | 2.31 ± 0.17 b | 2.61 ± 0.26 b | 2.55 ± 0.24 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motelica, L.; Ficai, D.; Oprea, O.-C.; Ficai, A.; Ene, V.-L.; Vasile, B.-S.; Andronescu, E.; Holban, A.-M. Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese. Nanomaterials 2021, 11, 2377. https://doi.org/10.3390/nano11092377
Motelica L, Ficai D, Oprea O-C, Ficai A, Ene V-L, Vasile B-S, Andronescu E, Holban A-M. Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese. Nanomaterials. 2021; 11(9):2377. https://doi.org/10.3390/nano11092377
Chicago/Turabian StyleMotelica, Ludmila, Denisa Ficai, Ovidiu-Cristian Oprea, Anton Ficai, Vladimir-Lucian Ene, Bogdan-Stefan Vasile, Ecaterina Andronescu, and Alina-Maria Holban. 2021. "Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese" Nanomaterials 11, no. 9: 2377. https://doi.org/10.3390/nano11092377
APA StyleMotelica, L., Ficai, D., Oprea, O.-C., Ficai, A., Ene, V.-L., Vasile, B.-S., Andronescu, E., & Holban, A.-M. (2021). Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese. Nanomaterials, 11(9), 2377. https://doi.org/10.3390/nano11092377