Effect of Artemisinin-Loaded Mesoporous Cerium-Doped Calcium Silicate Nanopowder on Cell Proliferation of Human Periodontal Ligament Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Mesoporous Ce-Doped Nanopowders (Ce-NPs)
2.2. Characterization of MSNs
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. X-ray Fluorescence Spectroscopy (XRF)
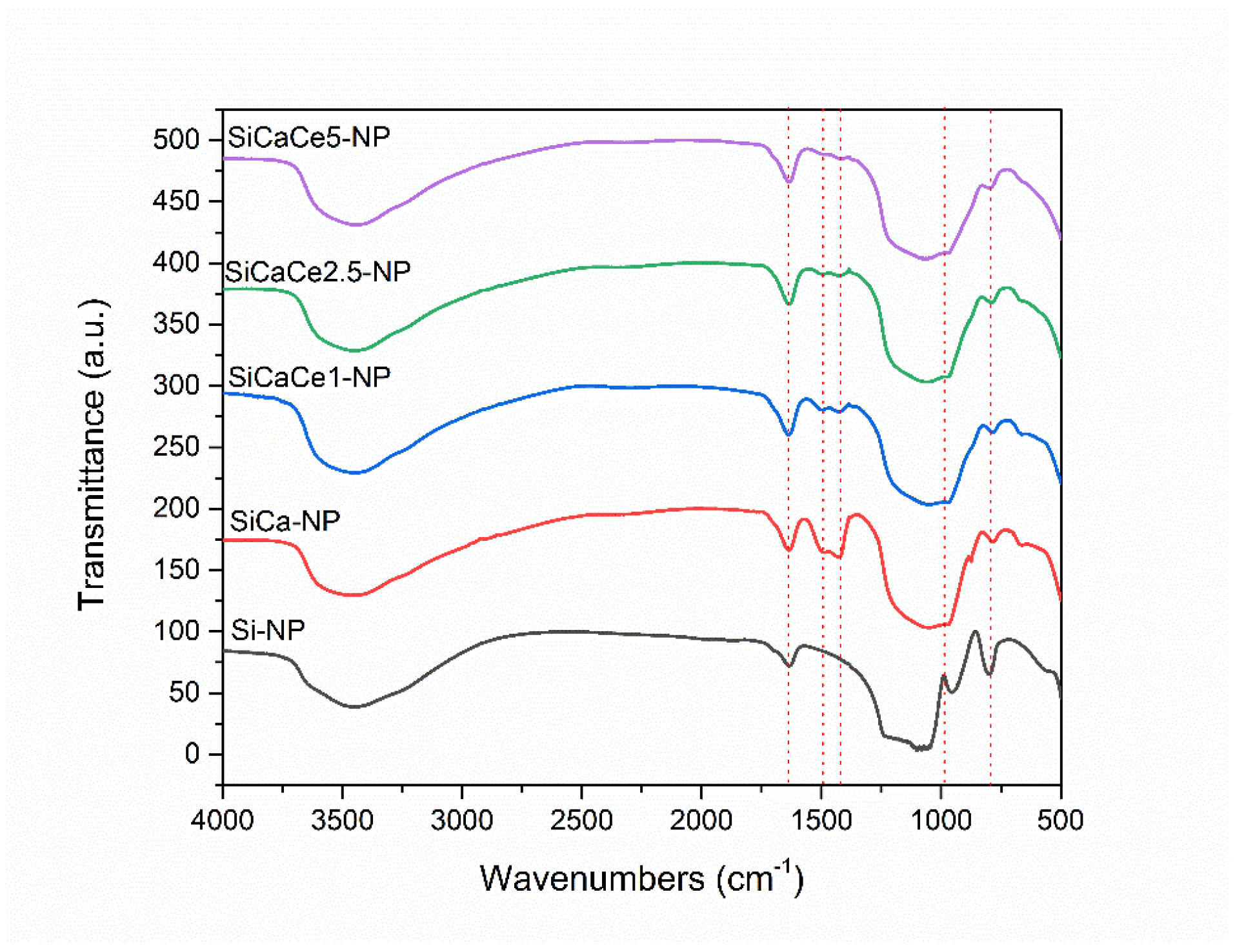
2.2.3. Fourier Transform Infrared Spectroscopy (FT-IR)
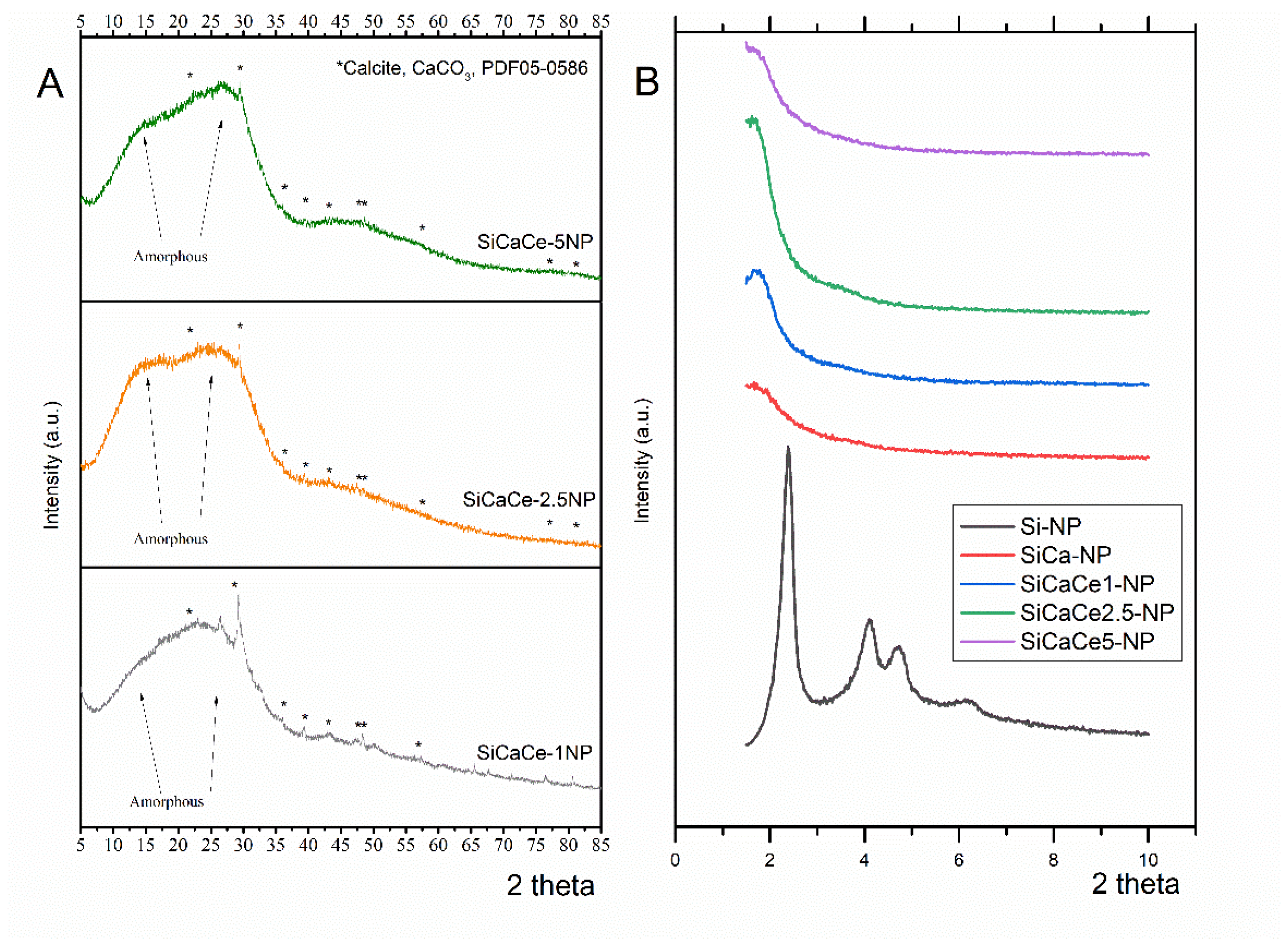
2.2.4. X-ray Diffraction Analysis (XRD)
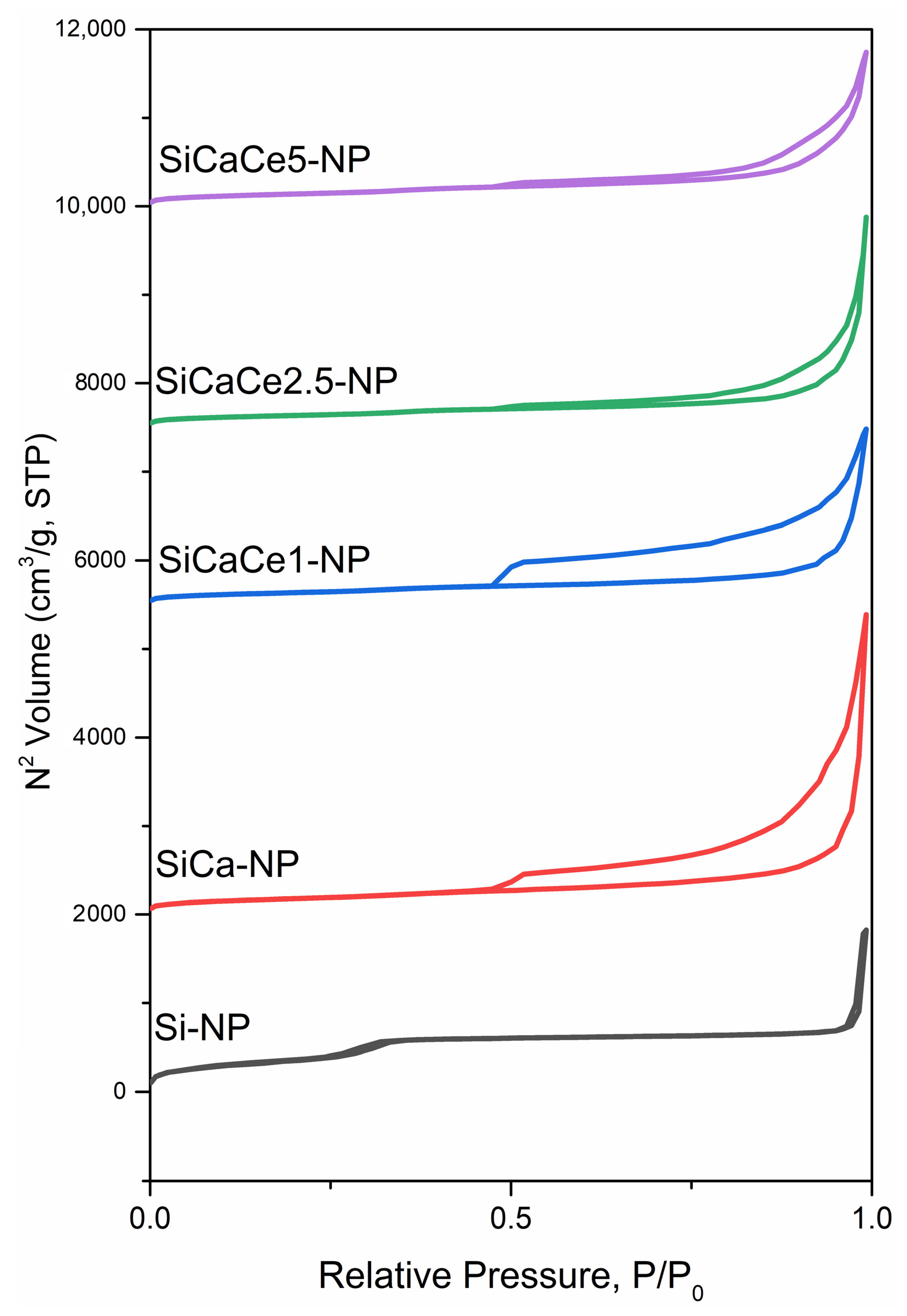
2.2.5. N2 Porosimetry
2.2.6. Apatite Forming Ability
2.3. Preparation of ART-Loaded NPs and UHPLC/HRMS Analysis of ART Concentration
2.4. Biological Evaluation of MSNs
2.4.1. Blood Sample Collection
2.4.2. Hemocompatibility Assay
2.4.3. Isolation of Human Periodontal Ligament Fibroblasts (hPDLFs)
2.4.4. Cytotoxicity Assay
2.4.5. Analysis of ROS Levels
3. Results and Discussion
3.1. Characterization of MSNs
3.1.1. Scanning Electron Microscopy (SEM)
3.1.2. X-ray Fluorescence Spectroscopy (XRF)
3.1.3. Fourier Transform Infrared Spectroscopy (FT-IR)
3.1.4. X-ray Diffraction Analysis
3.1.5. N2 Porosimetry
3.1.6. Apatite Forming Ability
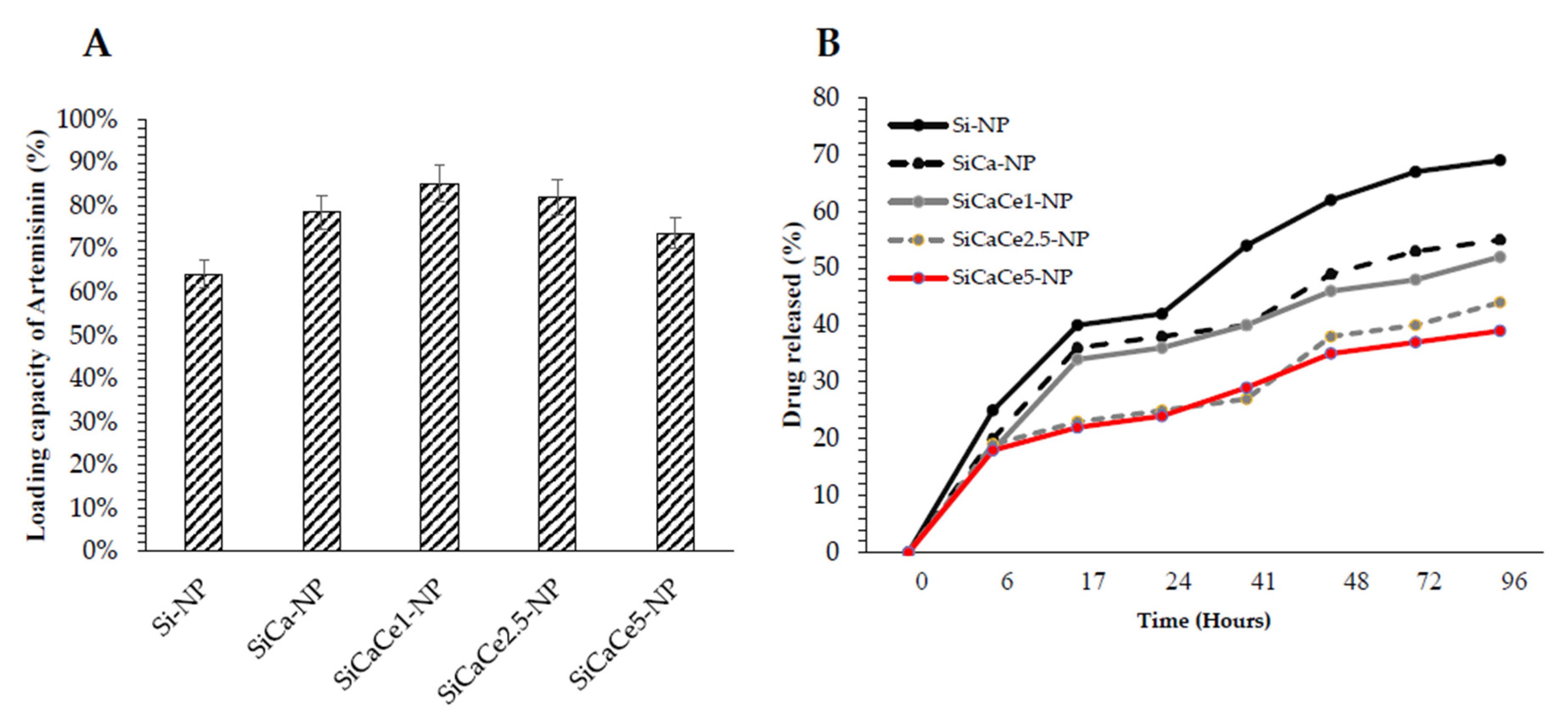
3.2. Artemisinin Loading and Release
3.3. Biological Behavior of NPs and ART-Loaded NPs
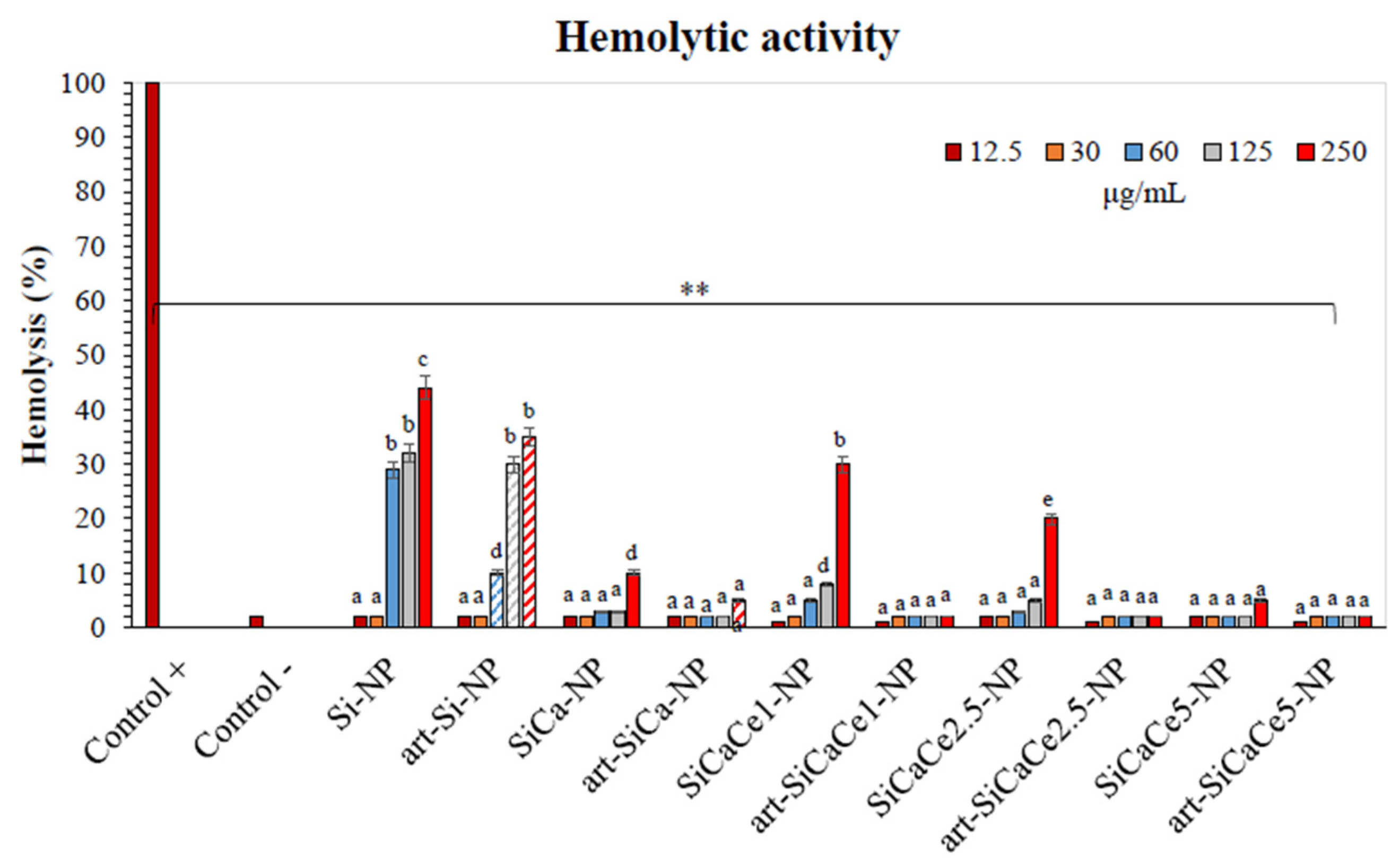
3.3.1. Hemocompatibility Assay
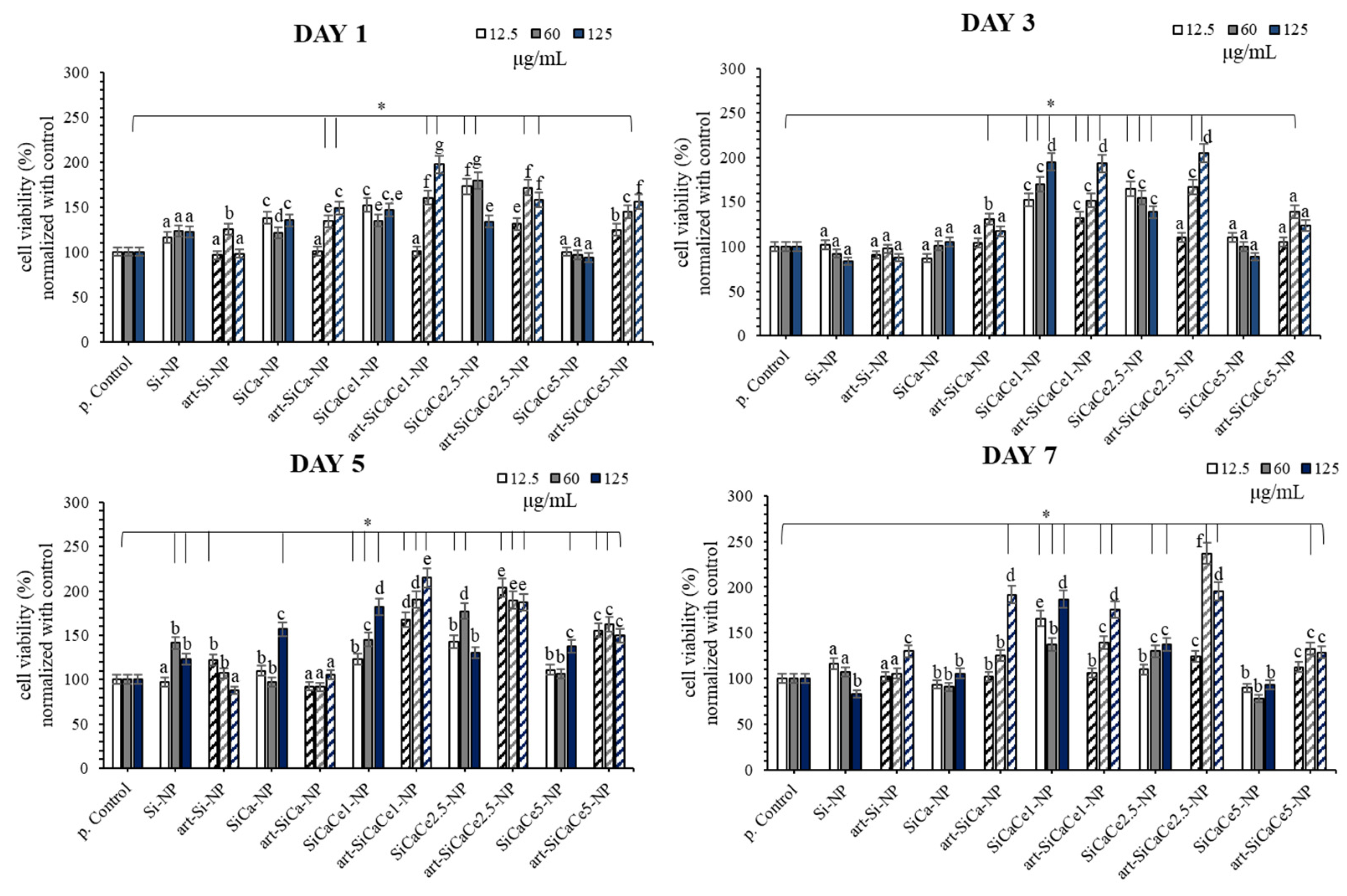
3.3.2. Cytotoxicity Assay
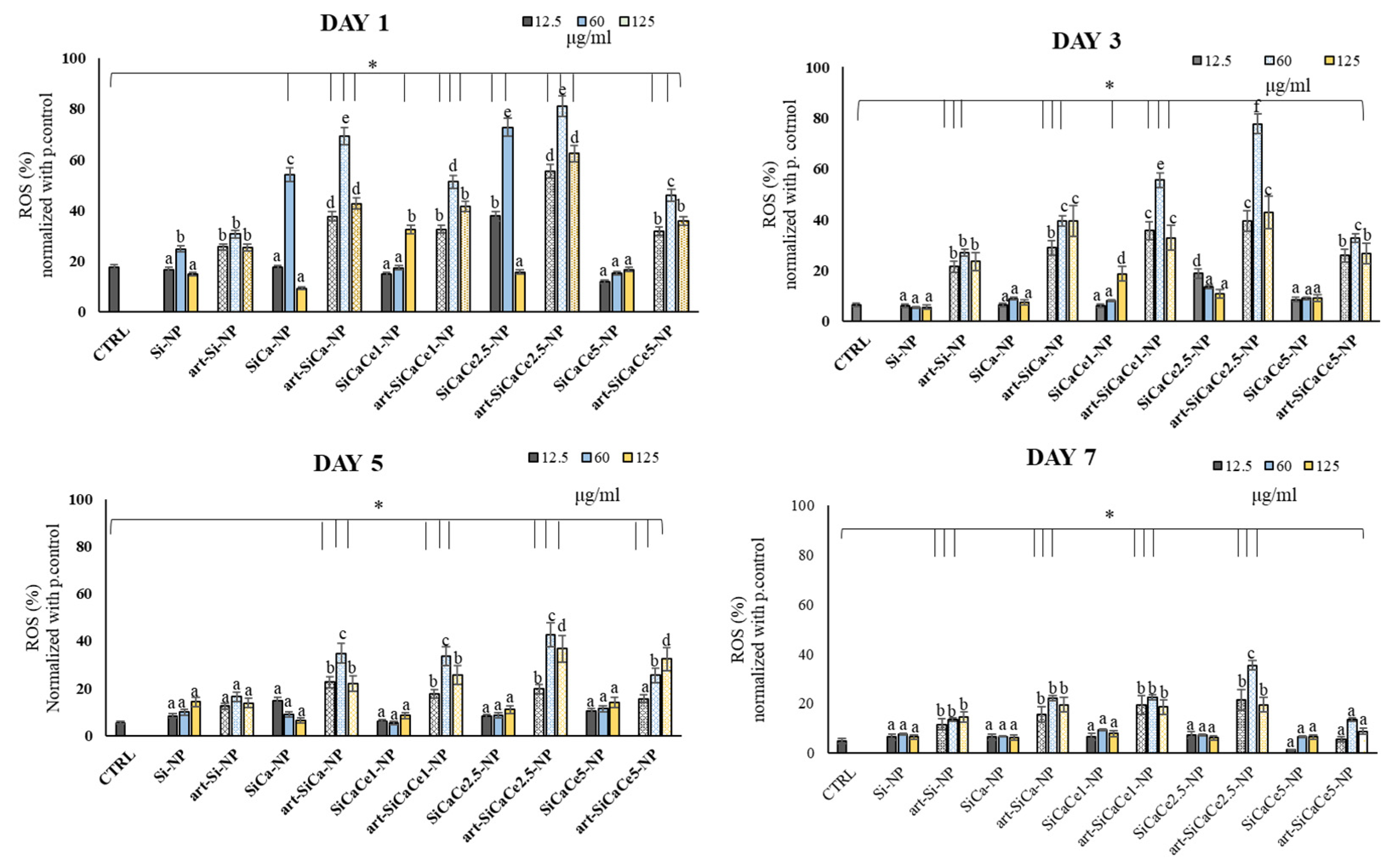
3.3.3. ROS Analysis of hPDLFs after Interaction with NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments

Conflicts of Interest
References
- Kurtuldu, F.; Mutlu, N.; Michálek, M.; Zheng, K.; Masar, M.; Liverani, L.; Chen, S.; Galusek, D.; Boccaccini, A.R. Cerium and gallium containing mesoporous bioactive glass nanoparticles for bone regeneration: Bioactivity, biocompatibility and antibacterial activity. Mater. Sci. Eng. C 2021, 124, 112050. [Google Scholar] [CrossRef]
- Migneco, C.; Fiume, E.; Verné, E.; Baino, F. A guided walk through the world of mesoporous bioactive glasses (MBGs): Fundamentals, processing, and applications. Nanomaterials 2020, 10, 2571. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, K.; Boccaccini, A.R. Multi-functional silica-based mesoporous materials for simultaneous delivery of biologically active ions and therapeutic biomolecules. Acta Biomater. 2021, 129, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liverani, L.; Boccardi, E.; Beltrán, A.M.; Boccaccini, A.R. Incorporation of calcium containing mesoporous (MCM-41-Type) particles in electrospun PCL fibers by using benign solvents. Polymers 2017, 9, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, D.; Zhang, X.; Wang, X.; Cao, L.; Zheng, A.; Du, J.; Li, Y.; Huang, Q.; Jiang, X. Fabrication of large-pore mesoporous Ca-Si-based bioceramics for bone regeneration. Int. J. Nanomed. 2017, 12, 8277–8287. [Google Scholar] [CrossRef] [Green Version]
- Quang, D.V.; Park, J.-K.; Kim, J.-K.; Elineema, G.; Shao, G.; Lee, J.E.; Kim, H.T. Characterization of calcium-doped silica gel prepared in an aqueous solution. Resour. Process. 2012, 59, 33–41. [Google Scholar] [CrossRef]
- Arora, M.; Arora, E. The promise of silicon: Bone regeneration and increased bone density. J. Arthrosc. Jt. Surg. 2017, 4, 103–105. [Google Scholar] [CrossRef]
- Jung, G.Y.; Park, Y.J.; Han, J.S. Effects of HA released calcium ion on osteoblast differentiation. J. Mater. Sci. Mater. Med. 2010, 21, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Rué, E.; Diez-Tercero, L.; Giordano-Kelhoffer, B.; Delgado, L.M.; Bosch, B.M.; Hoyos-Nogués, M.; Mateos-Timoneda, M.A.; Tran, P.A.; Gil, F.J.; Perez, R.A. Biological roles and delivery strategies for ions to promote osteogenic induction. Front. Cell Dev. Biol. 2021, 8, 1809. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J.; Fan, W. Bioactive mesoporous calcium-silicate nanoparticles with excellent mineralization ability, osteostimulation, drug-delivery and antibacterial properties for filling apex roots of teeth. J. Mater. Chem. 2012, 22, 16801–16809. [Google Scholar] [CrossRef]
- Huang, C.Y.; Huang, T.H.; Kao, C.T.; Wu, Y.H.; Chen, W.C.; Shie, M.Y. mesoporous calcium silicate nanoparticles with drug delivery and odontogenesis properties. J. Endod. 2017, 43, 69–76. [Google Scholar] [CrossRef]
- Dai, C.; Guo, H.; Lu, J.; Shi, J.; Wei, J.; Liu, C. Osteogenic evaluation of calcium/magnesium-doped mesoporous silica scaffold with incorporation of rhBMP-2 by synchrotron radiation-based μCT. Biomaterials 2011, 32, 8506–8517. [Google Scholar] [CrossRef]
- Shoaib, M.; Bahadur, A.; Iqbal, S.; AL-Anazy, M.M.; Laref, A.; Tahir, M.A.; Channar, P.A.; Noreen, S.; Yasir, M.; Iqbal, A.; et al. Magnesium doped mesoporous bioactive glass nanoparticles: A promising material for apatite formation and mitomycin c delivery to the MG-63 cancer cells. J. Alloy. Compd. 2021, 866, 159013. [Google Scholar] [CrossRef]
- Kargozar, S.; Fiume, E.; Fiume, E.; Baino, F. Multiple and promising applications of strontium (Sr)-containing bioactive glasses in bone tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khamsehashari, N.; Hassanzadeh-Tabrizi, S.A.; Bigham, A. Effects of strontium adding on the drug delivery behavior of silica nanoparticles synthesized by P123-assisted sol-gel method. Mater. Chem. Phys. 2018, 205, 283–291. [Google Scholar] [CrossRef]
- Rubio, L.; Annangi, B.; Vila, L.; Hernández, A.; Marcos, R. Antioxidant and anti-genotoxic properties of cerium oxide nanoparticles in a pulmonary-like cell system. Arch. Toxicol. 2016, 90, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Gojova, A.; Lee, J.T.; Jung, H.S.; Guo, B.; Barakat, A.I.; Kennedy, I.M. Effect of cerium oxide nanoparticles on inflammation in vascular endothelial cells. Inhal. Toxicol. 2009, 21, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farias, I.A.P.; Dos Santos, C.C.L.; Sampaio, F.C. Antimicrobial activity of cerium oxide nanoparticles on opportunistic microorganisms: A systematic review. Biomed. Res. Int. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, G.; Harja, M. Cerium-doped hydroxyapatite/collagen coatings on titanium for bone implants. Ceram. Int. 2018, 45, 2852–2857. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Hashemian, S.J.; Brouki Milan, P.; Hamzehlou, S.; Soleimani, M.; Joghataei, M.T.; Gholipourmalekabadi, M.; Korourian, A.; Mousavizadeh, K.; et al. Osteogenic potential of stem cells-seeded bioactive nanocomposite scaffolds: A comparative study between human mesenchymal stem cells derived from bone, umbilical cord Wharton’s jelly, and adipose tissue. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Torre, E.; Bari, A.; Taccardi, N.; Cassinelli, C.; Morra, M.; Fiorilli, S.; Vitale-Brovarone, C.; Iviglia, G.; Boccaccini, A.R. Antioxidant mesoporous Ce-doped bioactive glass nanoparticles with anti-inflammatory and pro-osteogenic activities. Mater. Today Bio. 2020, 5, 100041. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.M.; Al-Rashidy, Z.M.; Ahmed, M.M. In Vitro drug release behavior of Ce-doped nano-bioactive glass carriers under oxidative stress. J. Mater. Sci. Mater. Med. 2019, 30, 18. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Cresswell, M.; Boccaccini, A.R. Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater. Sci. Eng. C 2018, 83, 99–107. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control. Release 2014, 193, 282–295. [Google Scholar] [CrossRef]
- Liu, H.J.; Xu, P. Smart mesoporous silica nanoparticles for protein delivery. Nanomaterials 2019, 9, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.; Li, X.; Qian, J.; Lv, G.; Yan, Y.; Su, J.; Wei, J. Mesoporous calcium-silicon xerogels with mesopore size and pore volume influence hMSC behaviors by load and sustained release of rhBMP-2. Int. J. Nanomed. 2015, 10, 1715–1726. [Google Scholar]
- Ambrogi, V.; Pietrella, D.; Marmottini, F.; Riva, F.; Tiralti, M.C.; Ricci, M. Chlorhexidine-loaded functionalized mesoporous MCM-41 poly(methylmethacrylate) based composites with Candida antibiofilm activity. RSC Adv. 2015, 5, 84827–84835. [Google Scholar] [CrossRef]
- Yan, H.; Wang, S.; Han, L.; Peng, W.; Yi, L.; Guo, R.; Liu, S.; Yang, H.; Huang, C. Chlorhexidine-encapsulated mesoporous silica-modified dentin adhesive. J. Dent. 2018, 78, 83–90. [Google Scholar] [CrossRef]
- Lu, M.M.; Ge, Y.; Qiu, J.; Shao, D.; Zhang, Y.; Bai, J.; Zheng, X.; Chang, Z.M.; Wang, Z.; Dong, W.F.; et al. Redox/pH dual-controlled release of chlorhexidine and silver ions from biodegradable mesoporous silica nanoparticles against oral biofilms. Int. J. Nanomed. 2018, 13, 7697–7709. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Yang, J.; Feng, Q.; Shu, Y.; Liu, C.; Zeng, S.; Guan, H.; Ge, L.; Pathak, J.L.; Zeng, S. Mesoporous bioactive glass nanoparticles promote odontogenesis and neutralize pathophysiological acidic pH. Front. Mater. 2020, 7, 241. [Google Scholar] [CrossRef]
- Pouroutzidou, G.K.; Liverani, L.; Theocharidou, A.; Tsamesidis, I.; Lazaridou, M.; Christodoulou, E.; Beketova, A.; Pappa, C.; Triantafyllidis, K.S.; Anastasiou, A.D.; et al. Synthesis and characterization of mesoporous mg-and sr-doped nanoparticles for moxifloxacin drug delivery in promising tissue engineering applications. Int. J. Mol. Sci. 2021, 22, 577. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Izquierdo-Barba, I.; Colilla, M. Structure and functionalization of mesoporous bioceramics for bone tissue regeneration and local drug delivery. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 1400–1421. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Arcos, D.; Sakamoto, Y.; Terasaki, O.; López-Noriega, A.; Vallet-Regí, M. High-performance mesoporous bioceramics mimicking bone mineralization. Chem. Mater. 2008, 20, 3191–3198. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, D.; Li, N.; Jiang, X.; Liu, C.; Li, Y. Large-pore mesoporous Ca-Si-based bioceramics with high: In vitro bioactivity and protein adsorption capability for bone tissue regeneration. J. Mater. Chem. B 2016, 4, 3916–3924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. The osteoprotective effects of artemisinin compounds and the possible mechanisms associated with intracellular iron: A review of In Vivo and In Vitro studies. Environ. Toxicol. Pharmacol. 2020, 76, 103358. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Kuang, Z.; Gong, Z.; Xue, D.; Zheng, Q. Dihydroartemisinin promotes the osteogenesis of human mesenchymal stem cells via the ERK and Wnt/β-catenin signaling pathways. Biomed. Res. Int. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.M.; Mao, M.H.; Hu, Y.H.; Zhou, X.C.; Li, S.; Chen, C.F.; Li, C.N.; Yuan, Q.L.; Li, W. Artemisinin protects DPSC from hypoxia and TNF-α mediated osteogenesis impairments through CA9 and Wnt signaling pathway. Life Sci. 2021, 277, 119471. [Google Scholar] [CrossRef]
- Fang, J.; Zhao, X.; Li, S.; Xing, X.; Wang, H.; Lazarovici, P.; Zheng, W. Protective mechanism of artemisinin on rat bone marrow-derived mesenchymal stem cells against apoptosis induced by hydrogen peroxide via activation of c-Raf-Erk1/2-p90rsk-CREB pathway. Stem Cell Res. Ther. 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamum, T. Surface-structure changes in bioactive. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Rahman, I.A.; Vejayakumaran, P.; Sipaut, C.S.; Ismail, J.; Chee, C.K. Effect of the drying techniques on the morphology of silica nanoparticles synthesized via sol-gel process. Ceram. Int. 2008, 34, 2059–2066. [Google Scholar] [CrossRef]
- Vazquez, N.I.; Gonzalez, Z.; Ferrari, B.; Castro, Y. Synthesis of mesoporous silica nanoparticles by sol-gel as nanocontainer for future drug delivery applications. Boletín Soc. Española Cerámica Vidr. 2017, 56, 139–145. [Google Scholar] [CrossRef]
- Ismail, A.R.; Vejayakumaran, P. Synthesis of silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—A review. J. Nanomater. 2012, 2012, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.H.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Suzuki, K.; Ikari, K.; Imai, H. Synthesis of silica nanoparticles having a well-ordered mesostructure using a double surfactant system. J. Am. Chem. Soc. 2004, 126, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Ikari, K.; Suzuki, K.; Imai, H. Structural control of mesoporous silica nanoparticles in a binary surfactant system. Langmuir 2006, 22, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Haynes, C.L. Synthesis and characterization of biocompatible and size-tunable multifunctional porous silica nanoparticles. Chem. Mater. 2009, 21, 3979–3986. [Google Scholar] [CrossRef]
- Yokoi, T.; Sakamoto, Y.; Terasaki, O.; Kubota, Y.; Okubo, T.; Tatsumi, T. Periodic arrangement of silica nanospheres assisted by amino acids. J. Am. Chem. Soc. 2006, 128, 13664–13665. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Xu, T.; Gu, J.; Liu, Q.; Zhou, S.; Shi, G.; Zhu, Z. Preparation of bioactive glass nanoparticles with highly and evenly doped calcium ions by reactive flash nanoprecipitation. J. Mater. Sci. Mater. Med. 2021, 32, 48. [Google Scholar] [CrossRef] [PubMed]
- Neščáková, Z.; Zheng, K.; Liverani, L.; Nawaz, Q.; Galusková, D.; Kaňková, H.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Multifunctional zinc ion doped sol-gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019, 4, 312–321. [Google Scholar] [CrossRef]
- Yasutaka, K.; Takato, Y.; Takashi, K.; Kohsuke, M.; Hiromi, Y. Enhancement in adsorption and catalytic activity of enzymes immobilized on phosphorus-and calcium-modified MCM-41. J. Phys. Chem. B 2011, 115, 10335–10345. [Google Scholar] [CrossRef]
- Serra, J.; González, P.; Liste, S.; Chiussi, S.; León, B.; Pérez-Amor, M.; Ylänen, H.O.; Hupa, M. Influence of the non-bridging oxygen groups on the bioactivity of silicate glasses. J. Mater. Sci. Mater. Med. 2002, 13, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Kalampounias, A.G. IR and Raman spectroscopic studies of sol-gel derived alkaline-earth silicate glasses. Bull. Mater. Sci. 2011, 34, 299–303. [Google Scholar] [CrossRef]
- Riti, P.I.; Vulpoi, A.; Ponta, O.; Simon, V. The effect of synthesis route and magnesium addition on structure and bioactivity of sol-gel derived calcium-silicate glasses. Ceram. Int. 2014, 40, 14741–14748. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Tsuru, K.; Nagai, H.; Fujisawa, K.; Kudoh, T.; Ohe, G.; Ishikawa, K.; Miyamoto, Y. Fabrication and evaluation of carbonate apatite-coated calcium carbonate bone substitutes for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, 2077–2087. [Google Scholar] [CrossRef]
- Papa, F.; Cortese, A.; Sagliocco, R.; Farella, M.; Banzi, C.; Maltarello, M.C.; Pellegrini, C.; D’Agostino, E.; Aimola, P.; Claudio, P.P. Outcome of 47 consecutive sinus lift operations using aragonitic calcium carbonate associated with autologous platelet-rich plasma: Clinical, histologic, and histomorphometrical evaluations. J. Craniofac. Surg. 2009, 20, 2067–2074. [Google Scholar] [CrossRef]
- Smith, B. Infrared Spectral Interpretation: A System Approach; CRC Press: Boca Raton, FL, USA, 1998; ISBN 0849324637. [Google Scholar]
- Ogino, M.; Ohuchi, F.; Hench, L.L. Compositional dependence of the formation of calcium phosphate films on bioglass. J. Biomed. Mater. Res. 1980, 14, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, M.R.T.; La Torre, G.; Hench, L.L. Solution effects on the surface reactions of three bioactive glass compositions. J. Biomed. Mater. Res. 1993, 27, 1485–1493. [Google Scholar] [CrossRef]
- Pouroutzidou, G.K.; Theodorou, G.S.; Kontonasaki, E.; Tsamesidis, I.; Pantaleo, A.; Patsiaoura, D.; Papadopoulou, L.; Rhoades, J.; Likotrafiti, E.; Lioutas, C.B.; et al. Effect of ethanol/TEOS ratios and amount of ammonia on the properties of copper-doped calcium silicate nanoceramics. J. Mater. Sci. Mater. Med. 2019, 30, 1–13. [Google Scholar] [CrossRef]
- Pouroutzidou, G.K.; Theodorou, G.S.; Kontonasaki, E.; Papadopoulou, L.; Kantiranis, N.; Patsiaoura, D.; Chrissafis, K.; Lioutas, C.B.; Paraskevopoulos, K.M. Synthesis of a bioactive nanomaterial in the ternary system SiO2-CaO-MgO doped with CuO: The effect of Ball milling on the particle size, morphology and bioactive behavior. AIP Conf. Proc. 2019, 2075, 200005. [Google Scholar]
- Chen, X.; Hu, Q. Bioactive glasses. Front. Nanobiomedical Res. 2017, 9, 147–182. [Google Scholar]
- Tsamesidis, I.; Pouroutzidou, G.K.; Lymperaki, E.; Kazeli, K.; Lioutas, C.B.; Christodoulou, E.; Perio, P.; Reybier, K.; Pantaleo, A.; Kontonasaki, E. Effect of ion doping in silica-based nanoparticles on the hemolytic and oxidative activity in contact with human erythrocytes. Chem. Biol. Interact. 2020, 318, 108974. [Google Scholar] [CrossRef]
- Cui, H.; Chen, X.; Bai, M.; Han, D.; Lin, L.; Dong, M. Multipathway antibacterial mechanism of a nanoparticle-supported artemisinin promoted by nitrogen plasma treatment. ACS Appl. Mater. Interfaces 2019, 11, 47299–47310. [Google Scholar] [CrossRef]
- Ismail, M.; Ling, L.; Du, Y.; Yao, C.; Li, X. Liposomes of dimeric artesunate phospholipid: A combination of dimerization and self-assembly to combat malaria. Biomaterials 2018, 163, 76–87. [Google Scholar] [CrossRef]
- Jenkins, W.; Perone, P.; Walker, K.; Bhagavathula, N.; Aslam, M.N.; DaSilva, M.; Dame, M.K.; Varani, J. Fibroblast response to lanthanoid metal ion stimulation: Potential contribution to fibrotic tissue injury. Biol. Trace Elem. Res. 2011, 144, 621–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudryk, Y.; Gschneidner, K.A.; Pecharsky, V.K. Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2014; Volume 44, pp. 283–449. [Google Scholar]
- Zhang, J.; Liu, C.; Li, Y.; Sun, J.; Wang, P.; Di, K.; Zhao, Y. Effect of cerium ion on the proliferation, differentiation and mineralization function of primary mouse osteoblasts In Vitro. J. Rare Earths 2010, 28, 138–142. [Google Scholar] [CrossRef]
- Chou, A.M.; Sae-Lim, V.; Lim, T.M.; Schantz, J.T.; Teoh, S.H.; Chew, C.L.; Hutmacher, D.W. Culturing and characterization of human periodontal ligament fibroblasts—A preliminary study. Mater. Sci. Eng. C 2002, 20, 77–83. [Google Scholar] [CrossRef]
- Inan, B.; Elcin, A.E.; Elcin, Y.M. Osteogenic induction of human periodontal ligament fibroblasts under two-and three-dimensional culture conditions. Tissue Eng. 2006, 12, 257–266. [Google Scholar] [CrossRef]
- Zhang, W. Nanoparticle aggregation: Principles and modeling. Adv. Exp. Med. Biol. 2014, 811, 20–43. [Google Scholar]
- Wang, Y.; Pi, C.; Feng, X.; Hou, Y.; Zhao, L.; Wei, Y. The influence of nanoparticle properties on oral bioavailability of drugs. Int. J. Nanomed. 2020, 15, 6295–6310. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bélteky, P.; Rónavári, A.; Zakupszky, D.; Boka, E.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Vágvölgyi, C.; Kiricsi, M.; Kónya, Z. Are smaller nanoparticles always better? Understanding the biological effect of size-dependent silver nanoparticle aggregation under biorelevant conditions. Int. J. Nanomed. 2021, 16, 3021–3040. [Google Scholar] [CrossRef]
- Cui, W.; Li, J.; Zhang, Y.; Rong, H.; Lu, W.; Jiang, L. Effects of aggregation and the surface properties of gold nanoparticles on cytotoxicity and cell growth. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 46–53. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [Green Version]
- Mahl, D.; Greulich, C.; Meyer-Zaika, W.; Köller, M.; Epple, M. Gold nanoparticles: Dispersibility in biological media and cell-biological effect. J. Mater. Chem. 2010, 20, 6176–6181. [Google Scholar] [CrossRef]
- Graf, C.; Gao, Q.; Schütz, I.; Noufele, C.N.; Ruan, W.; Posselt, U.; Korotianskiy, E.; Nordmeyer, D.; Rancan, F.; Hadam, S.; et al. Surface functionalization of silica nanoparticles supports colloidal stability in physiological media and facilitates internalization in cells. Langmuir 2012, 28, 7598–7613. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Z.; Jin, X.; George, S.; Xia, T.; Meng, H.; Wang, X.; Suarez, E.; Zhang, H.; Hoek, E.M.V.; Godwin, H.; et al. Dispersion and stability optimization of TiO2 nanoparticles in cell culture media. Environ. Sci. Technol. 2010, 44, 7309–7314. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.P.; Zhang, Y.; Zhang, S.; Xia, J.G.; Liu, J.W.; Xu, K.; Gu, N. Preparation and characterization of water-soluble monodisperse magnetic iron oxide nanoparticles via surface double-exchange with DMSA. Colloids Surf. A Physicochem. Eng. Asp. 2008, 316, 210–216. [Google Scholar] [CrossRef]
- Ren, S.; Zhou, Y.; Zheng, K.; Xu, X.; Yang, J.; Wang, X.; Miao, L.; Wei, H.; Xu, Y. Cerium oxide nanoparticles loaded nanofibrous membranes promote bone regeneration for periodontal tissue engineering. Bioact. Mater. 2021, 7, 242–253. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nibali, L.; Donos, N. Periodontitis and redox status: A review. Curr. Pharm. Des. 2013, 19, 2687–2697. [Google Scholar] [CrossRef]
- Liu, C.; Mo, L.; Niu, Y.; Li, X.; Zhou, X.; Xu, X. The role of reactive oxygen species and autophagy in periodontitis and their potential linkage. Front. Physiol. 2017, 8, 439. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol. 2000 2007, 43, 160–232. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, Z.; Huang, W.; Liu, L.; Li, J.; Li, J.; Wu, Q. Redox enzyme-mimicking activities of CeO2 nanostructures: Intrinsic influence of exposed facets. Sci. Rep. 2016, 6, 35344. [Google Scholar] [CrossRef]
- Pulido-Reyes, G.; Rodea-Palomares, I.; Das, S.; Sakthivel, T.S.; Leganes, F.; Rosal, R.; Seal, S.; Fernández-Pinãs, F. Untangling the biological effects of cerium oxide nanoparticles: The role of surface valence states. Sci. Rep. 2015, 5, 15613. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Wang, K.; Kolattukudy, P.E. Cerium oxide nanoparticles inhibits oxidative stress and nuclear Factor-κB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J. Pharmacol. Exp. Ther. 2011, 338, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef] [Green Version]
- Lord, M.S.; Jung, M.S.; Teoh, W.Y.; Gunawan, C.; Vassie, J.A.; Amal, R.; Whitelock, J.M. Cellular uptake and reactive oxygen species modulation of cerium oxide nanoparticles in human monocyte cell line U937. Biomaterials 2012, 33, 7915–7924. [Google Scholar] [CrossRef]
- Lee, S.S.; Song, W.; Cho, M.; Puppala, H.L.; Nguyen, P.; Zhu, H.; Segatori, L.; Colvin, V.L. Antioxidant properties of cerium oxide nanocrystals as a function of nanocrystal diameter and surface coating. ACS Nano 2013, 7, 9693–9703. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Tsoi, B.; Gunawan, C.; Teoh, W.Y.; Amal, R.; Whitelock, J.M. Anti-angiogenic activity of heparin functionalised cerium oxide nanoparticles. Biomaterials 2013, 34, 8808–8818. [Google Scholar] [CrossRef] [PubMed]
- Ece, A.; Chenxi, X.; Hilal, Y.; Amit, R.; Thomas, W. pH and time dependent antioxidant activity of dextran coated cerium oxide nanoparticles. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef]


| Sample | Molar Ratio of Reactants |
|---|---|
| Si-NP | 1 TEOS/0.13 CTAB/0.4 NaOH/1240 H2O |
| SiCa-NP | 0.6 TEOS/0.13 CTAB/0.4 NaOH/1240 H2O/0.4 Ca |
| SiCaCe1-NP | 0.6 TEOS/0.13 CTAB/0.4 NaOH/1240 H2O/0.39 Ca/0.01 Ce |
| SiCaCe2.5-NP | 0.6 TEOS/0.13 CTAB/0.4 NaOH/1240 H2O/0.375 Ca/0.025 Ce |
| SiCaCe5-NP | 0.6 TEOS/0.13 CTAB/0.4 NaOH/1240 H2O/0.35 Ca/0.05 Ce |
| Order | Reagent | Amount |
|---|---|---|
| 1 | NaCl | 16.072 g |
| 2 | NaHCO3 | 0.704 g |
| 3 | KCl | 0.450 g |
| 4 | K2HPO4·3H2O | 0.460 g |
| 5 | MgCl2·6H2O | 0.622 g |
| 6 | 1.0 M-HCl | 6 mL |
| 7 | CaCl2 | 0.586 g |
| 8 | Na2SO4 | 0.144 g |
| 9 | (CH2OH)3CNH2(TRIS) a | 12.126 g |
| Sample | SiO2 | CaO | CeO2 | Total N | Total XRF | |||
|---|---|---|---|---|---|---|---|---|
| N | XRF | N | XRF | N | XRF | |||
| Si-NP | 100 | 100 | - | - | - | - | 100 | 100 |
| SiCa-NP | 61.64 | 61.48 | 38.36 | 38.52 | - | - | 100 | 100 |
| SiCaCe1-NP | 60.44 | 70.74 | 36.67 | 27.92 | 2.89 | 1.34 | 100 | 100 |
| SiCaCe2.5-NP | 58.73 | 72.13 | 34.26 | 23.19 | 7.01 | 4.68 | 100 | 100 |
| SiCaCe5-NP | 56.08 | 71.84 | 30.53 | 19.64 | 13.39 | 8.52 | 100 | 100 |
| Sample | XRD | N2 Porosimetry | |||
|---|---|---|---|---|---|
| d-Spacing | α0 | SBET | dp | Vp | |
| (nm) | (nm) | (m2/g) | (nm) | (cc/g) | |
| Si-NP | 3.71 | 12.85 | 1312 | 2.5 | 2.82 |
| SiCa-NP | 4.96 | 17.18 | 650 | 4.1 | 5.24 |
| SiCaCe1-NP | 5.07 | 17.57 | 495 | 3.9 | 3.07 |
| SiCaCe2.5-NP | 5.32 | 18.42 | 495 | 3.9 | 3.68 |
| SiCaCe5-NP | 5.52 | 19.11 | 495 | 3.9 | 2.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsamesidis, I.; Gkiliopoulos, D.; Pouroutzidou, G.K.; Lymperaki, E.; Papoulia, C.; Reybier, K.; Perio, P.; Paraskevopoulos, K.M.; Kontonasaki, E.; Theocharidou, A. Effect of Artemisinin-Loaded Mesoporous Cerium-Doped Calcium Silicate Nanopowder on Cell Proliferation of Human Periodontal Ligament Fibroblasts. Nanomaterials 2021, 11, 2189. https://doi.org/10.3390/nano11092189
Tsamesidis I, Gkiliopoulos D, Pouroutzidou GK, Lymperaki E, Papoulia C, Reybier K, Perio P, Paraskevopoulos KM, Kontonasaki E, Theocharidou A. Effect of Artemisinin-Loaded Mesoporous Cerium-Doped Calcium Silicate Nanopowder on Cell Proliferation of Human Periodontal Ligament Fibroblasts. Nanomaterials. 2021; 11(9):2189. https://doi.org/10.3390/nano11092189
Chicago/Turabian StyleTsamesidis, Ioannis, Dimitrios Gkiliopoulos, Georgia K. Pouroutzidou, Evgenia Lymperaki, Chrysanthi Papoulia, Karine Reybier, Pierre Perio, Konstantinos M. Paraskevopoulos, Eleana Kontonasaki, and Anna Theocharidou. 2021. "Effect of Artemisinin-Loaded Mesoporous Cerium-Doped Calcium Silicate Nanopowder on Cell Proliferation of Human Periodontal Ligament Fibroblasts" Nanomaterials 11, no. 9: 2189. https://doi.org/10.3390/nano11092189
APA StyleTsamesidis, I., Gkiliopoulos, D., Pouroutzidou, G. K., Lymperaki, E., Papoulia, C., Reybier, K., Perio, P., Paraskevopoulos, K. M., Kontonasaki, E., & Theocharidou, A. (2021). Effect of Artemisinin-Loaded Mesoporous Cerium-Doped Calcium Silicate Nanopowder on Cell Proliferation of Human Periodontal Ligament Fibroblasts. Nanomaterials, 11(9), 2189. https://doi.org/10.3390/nano11092189










