Novel Blue-Wavelength-Blocking Contact Lens with Er3+/TiO2 NPs: Manufacture and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Er3+/TiO2 Preparation
2.2. Er3+/TiO2 Characterization
2.3. Contact Lens Modeling
3. Results
3.1. X-ray Diffraction Pattern
3.2. Surface Morphology
3.3. Energy Gaps
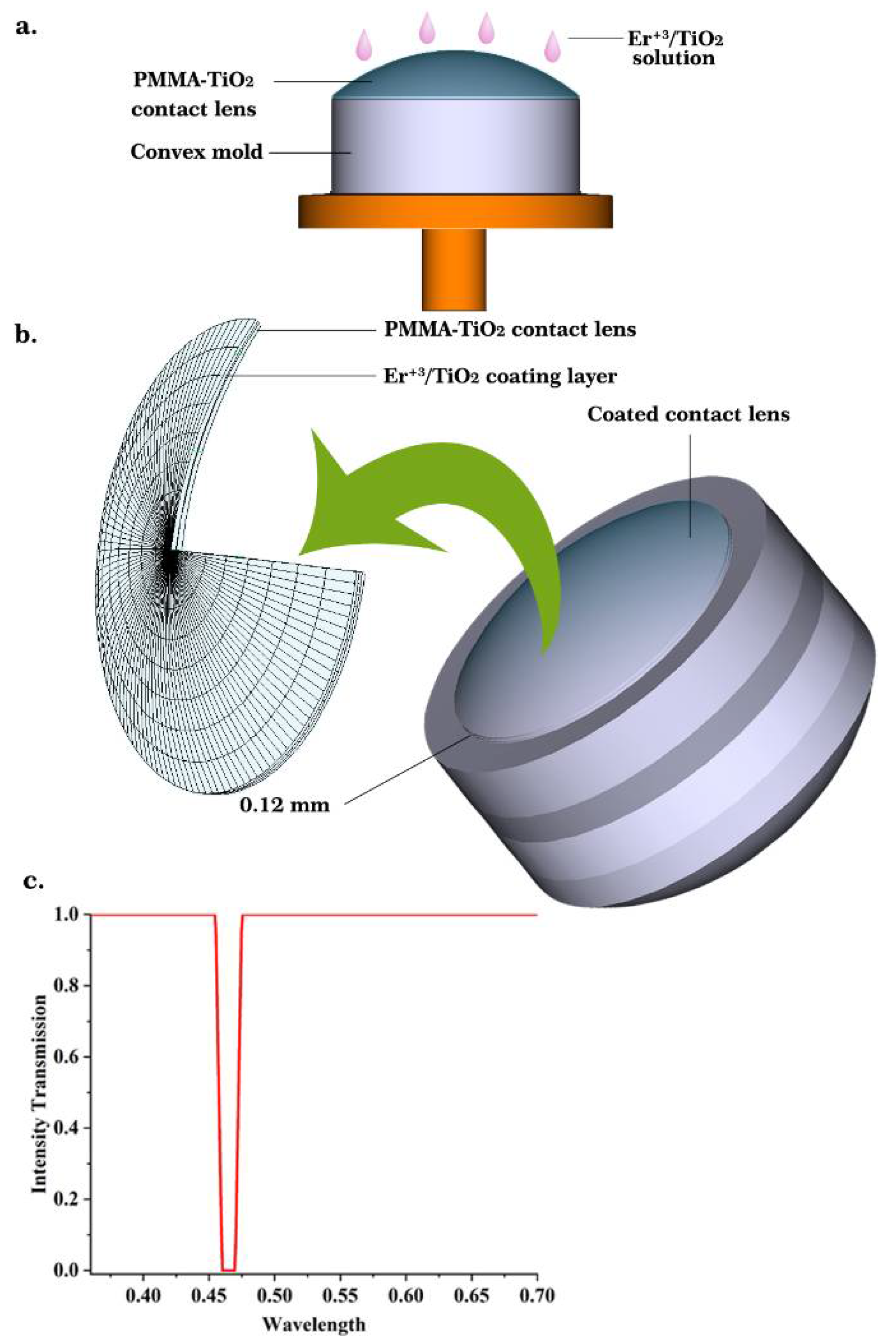
3.4. Application as a Blue-Wavelength-Blocking Contact Lens
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindenthal, L.; Raffael, R.; Harald, S.; Thomas, R.; Janko, P.; Andreas, N.; Stefan, L.; Alexander, K.O.; Peter, B.; Christoph, R. Modifying the Surface Structure of Perovskite-Based Catalysts by Nanoparticle Exsolution. Catalysts 2020, 10, 268. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Khalek, N.A.; Abdel-Khalek, M.A.D.; Selim, K.A.; Abdullah, S.S. Physicochemical study and application for pyrolusite separation from high manganese-iron ore in the presence of microorganisms. Physicochem. Probl. Miner. Process. 2021, 57, 273–283. [Google Scholar]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Hasran, U.A.; Masdar, S.; Daud, W.R.W. Enhancing methanol oxidation with a TiO2-modified semiconductor as a photo-catalyst. Int. J. Hydrog. Energy 2017, 42, 8986–8996. [Google Scholar] [CrossRef]
- Wasmi, B.; Al-Amiery, A.A.; Kadhum, A.A.H.; Takriff, M.S.; Mohamad, A.B. Synthesis of vanadium pentoxide nanoparticles as catalysts for the ozonation of palm oil. Ozone Sci. Eng. 2016, 38, 36–41. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Y.; Feng, P. Bifunctional heterometallic metal-organic frameworks for solvent-free heterogeneous cascade catalysis. Catalysts 2020, 10, 309. [Google Scholar] [CrossRef] [Green Version]
- Sandu, M.P.; Sidelnikov, V.S.; Geraskin, A.A.; Chernyavskii, A.V.; Kurzina, I.A. Influence of the method of preparation of the Pd-Bi/Al2O3 catalyst on catalytic properties in the reaction of liquid-phase oxidation of glucose into gluconic acid. Catalysts 2020, 10, 271. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Song, L.; Hao, Y.; Lu, N.; Quan, X.; Chen, S.; Zhang, Y.; Feng, Y. Fabrication of pilot-scale photocatalytic disinfection device by installing TiO2 coated helical support into UV annular reactor for strengthening sterilization. Chem. Eng. J. 2016, 283, 1506–1513. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Review of the research on anti-protein fouling coatings materials. Prog. Org. Coat. 2020, 147, 105860. [Google Scholar] [CrossRef]
- Melgoza-Ramírez, M.L.; Ramírez-Bon, R. Europium ions as a spectroscopic probe in the study of PMMA-SiO2 hybrid microstructure with variable coupling agent. J. Sol-Gel Sci. Technol. 2021, 99, 1–11. [Google Scholar]
- Shaker, L.M.; Al-Amiery, A.A.; Kadhum, A.A.H.; Takriff, M.S. Manufacture of Contact Lens of Nanoparticle-Doped Polymer Complemented with ZEMAX. Nanomaterials 2020, 10, 2028. [Google Scholar] [CrossRef] [PubMed]
- Harb, S.V.; Bassous, N.J.; de Souza, T.A.; Trentin, A.; Pulcinelli, S.H.; Santilli, C.V.; Webster, T.J.; Lobo, A.O.; Hammer, P. Hydroxyapatite and β-TCP modified PMMA-TiO2 and PMMA-ZrO2 coatings for bioactive corrosion protection of Ti6Al4V implants. Mater. Sci. Eng. C 2020, 116, 111149. [Google Scholar] [CrossRef]
- Savchyn, V.P.; Popov, A.I.; Aksimentyeva, O.I.; Klym, H.; Horbenko, Y.Y.; Serga, V.; Moskina, A.; Karbovnyk, I. Cathodoluminescence characterization of polystyrene-BaZrO3 hybrid composites. Low Temp. Phys. 2016, 42, 597–600. [Google Scholar] [CrossRef]
- Aksimentyeva, O.I.; Savchyn, V.P.; Dyakonov, V.P.; Piechota, S.; Horbenko, Y.Y.; Opainych, I.Y.; Demchenko, P.Y.; Popov, A.; Szymczak, H. Modification of polymer-magnetic nanoparticles by luminescent and conducting substances. Mol. Cryst. Liq. Cryst. 2014, 590, 35–42. [Google Scholar] [CrossRef]
- Li, F.; Kong, W.; Bhushan, B.; Zhao, X.; Pan, Y. Ultraviolet-driven switchable superliquiphobic/superliquiphilic coating for separation of oil-water mixtures and emulsions and water purification. J. Colloid Interface Sci. 2019, 557, 395–407. [Google Scholar] [CrossRef]
- Umar, A.A.; Saad, S.K.M.; Umar, M.I.A.; Abd Rahman, M.Y.; Oyama, M. Advances in porous and high-energy (001)-faceted anatase TiO2 nanostructures. Opt. Mater. 2018, 75, 390–430. [Google Scholar] [CrossRef]
- Li, X.Z.; Li, F.B. Study of Au/Au3+-TiO2 photocatalysts toward visible photooxidation for water and wastewater treatment. Environ. Sci. Technol. 2001, 35, 2381–2387. [Google Scholar] [CrossRef]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Evaluation of UV and visible light activity of TiO2 catalysts for water remediation. Chem. Eng. J. 2013, 225, 378–386. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of Photoluminescence Performance of Nano-Sized Semiconductor Materials and Its Relationships with Photocatalytic Activity. Sol. Energ. Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Linsebigler, A.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Li, H.; Bian, Z.; Zhu, J.; Huo, Y.; Li, H.; Lu, Y. Mesoporous Au/TiO2 Nanocomposites with Enhanced Photocatalytic Activity. J. Am. Chem. Soc. 2007, 129, 4538–4539. [Google Scholar] [CrossRef] [PubMed]
- Zaleska, A. Doped-TiO2: A Review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Di Paola, A.; MarcI, G.; Palmisano, L.; Schiavello, M.; Uosaki, K.; Ikeda, S.; Ohtani, B. Preparation of Polycrystalline TiO2 Photocatalysts Impregnated with Various Transition Metal Ions: Characterization and Photocatalytic Activity for the Degradation of 4-Nitrophenol. J. Phys. Chem. B 2002, 106, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.W.; Murugesan, V. Enhancement of Photocatalytic Activity by Metal Deposition: Characterization and Photonic Efficiency of Pt, Au and Pd Deposited on TiO2 Catalyst. Water Res. 2004, 38, 3001–3008. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Ohtani, B.; Iwai, K.; Nishimoto, S.; Sato, S. Role of Platinum Deposits on Titanium(IV) Oxide Particles: Structural and Kinetic Analyses of Photocatalytic Reaction in Aqueous Alcohol and Amino Acid Solutions. J. Phys. Chem. B 1997, 101, 3349–3359. [Google Scholar] [CrossRef] [Green Version]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Estrellan, C.R.; Salim, C.; Hinode, H. Photocatalytic activity of sol–gel derived TiO2 Co-doped with iron and niobium. React. Kinet. Catal. Lett. 2009, 98, 187–192. [Google Scholar] [CrossRef]
- Yu, C.L.; Yang, K.; Yu, J.; Peng, P.; Cao, F.; Li, X.; Zhou, X.C. Effects of rare earth Ce doping on the structure and photocatalytic performance of ZnO. Acta Phys. Chim. Sin. 2011, 27, 505–512. [Google Scholar]
- Minero, C.; Pelizzatti, E.; Sega, M.; Friberg, S.E.; Sjoblom, J. The role of humic substances in photocatalytic degradation of water contaminants. J. Dispers. Sci. Technol. 1999, 20, 643–661. [Google Scholar] [CrossRef]
- Mohammad, G.; Mohammed, W.; Marzoog, T.R.; Al-Amiery, A.; Kadhumc, A.; Mohamad, A. Green synthesis, antimicrobial and cytotoxic effects of silver nanoparticles using Eucalyptus chapmaniana leaves extract. Asian Pac. J. Trop. Biomed. 2013, 3, 58–63. [Google Scholar]
- Gaaz, T.S.; Sulong, A.B.; Kadhum, A.A.H.K.; Nassir, M.H.; Al-Amiery, A.A. Impact of sulfuric acid treatment of halloysite on physico-chemic property modification. Materials 2016, 9, 620. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [Green Version]
- Obayes, H.; Al-Gebori, A.; Khazaal, S.; Jarad, A.; Alwan, G.; Al-Amiery, A. Hypothetical design of carbon nanotube materials based on [8] circulene. J. Nanoelectron. Optoelectron. 2015, 10, 711–716. [Google Scholar] [CrossRef]
- Wasmi, W.; Al-Amiery, A.; Kadhum, A.; Mohamad, A. Novel approach: Tungsten oxide nanoparticle as a catalyst for malonic acid ester synthesis via ozonolysis. J. Nanomater. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of Poly(Titanium Oxide) on the Viscoelastic and Thermophysical Properties of Interpenetrating Polymer Networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Pankratova, V.; Popov, A.I.; Pankratov, V. Study of phase composition, photocatalytic activity, and photoluminescence of TiO2 with Eu additive produced by the extraction-pyrolytic method. J. Mater. Res. Technol. 2021, 13, 2350–2360. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite titanium dioxide nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Rajendran, V. Synthesis and characterization of nano-TiO2 via different methods. Arch. Appl. Sci. Res. 2012, 4, 1183–1190. [Google Scholar]
- Rohatgi, S.; Hanna, T.N.; Sliker, C.W.; Abbott, R.M.; Nicola, R. After-hours radiology: Challenges and strategies for the radiologist. Am. J. Roentgenol. 2015, 205, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Waite, S.; Kolla, S.; Jeudy, J.; Legasto, A.; Macknik, S.L.; Martinez-Conde, S.; Krupinski, E.A.; Reede, D.L. Tired in the reading room: The influence of fatigue in radiology. J. Am. Coll Radiol. 2017, 14, 191–197. [Google Scholar] [CrossRef]
- Michael, R.; Wegener, A. Estimation of safe exposure time from an ophthalmic operating microscope with regard to ultraviolet radiation and blue-light hazards to the eye. J. Opt. Soc. Am. A 2004, 21, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.S.; Schwiegerling, J.; Fest, E.; Hamilton, R. Visual Optics, in Molded Optics: Design and Manufacture; CRC Press: Boca Raton, FL, USA, 2016; pp. 2–58. [Google Scholar]
- Brennan, N.A.; Liou, H.-L. Anatomically accurate, finite model eye for optical modeling. Opt. Soc. Am. 1997, 14, 1684–1695. [Google Scholar]








| 2θ | Intensity | ||||
|---|---|---|---|---|---|
| TiO2 | 0.6 wt % Er3+/TiO2 | 1.2 wt % Er3+/TiO2 | 1.8 wt % Er3+/TiO2 | 2.4 wt % Er3+/TiO2 | |
| 25.2712 | 2158 | 1984 | 1911 | 1879 | 1855 |
| 27.4136 | 515 | 498 | 493 | 481 | 474 |
| 37.3038 | 127 | 105 | 100 | 90 | 90 |
| 48.0908 | 555 | 536 | 481 | 478 | 473 |
| 53.8206 | 311 | 310 | 288 | 262 | 230 |
| 53.97 | 379 | 368 | 351 | 346 | 336 |
| 55.116 | 367 | 340 | 313 | 291 | 283 |
| 62.7142 | 292 | 285 | 272 | 249 | 241 |
| 68.8675 | 165 | 149 | 143 | 131 | 120 |
| 70.2128 | 145 | 114 | 109 | 108 | 104 |
| 75.0956 | 185 | 164 | 163 | 151 | 147 |
| Er3+/TiO2 (wt %) | Crystallite (nm) |
|---|---|
| TiO2 | 28.72 |
| 0.6 | 28.84 |
| 1.2 | 31.53 |
| 1.8 | 33.93 |
| 2.4 | 35.93 |
| Er3+/TiO2 (wt %) | Calculated Metal (wt %) | Actual Metal (wt %) by EDX |
|---|---|---|
| 0.6 | 0.6 | 0.72 |
| 1.2 | 1.2 | 1.31 |
| 1.8 | 1.8 | 2.06 |
| 2.4 | 2.4 | 2.45 |
| Er3+/TiO2 (wt %) | Energy Gap (eV) |
|---|---|
| TiO2 | 3.13 ± 0.02 |
| 0.6 | 3.20 ± 0.02 |
| 1.2 | 3.00 ± 0.02 |
| 1.8 | 2.63 ± 0.02 |
| 2.4 | 2.70 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaker, L.M.; Alamiery, A.; Takriff, M.; Wan Isahak, W.N.R. Novel Blue-Wavelength-Blocking Contact Lens with Er3+/TiO2 NPs: Manufacture and Characterization. Nanomaterials 2021, 11, 2190. https://doi.org/10.3390/nano11092190
Shaker LM, Alamiery A, Takriff M, Wan Isahak WNR. Novel Blue-Wavelength-Blocking Contact Lens with Er3+/TiO2 NPs: Manufacture and Characterization. Nanomaterials. 2021; 11(9):2190. https://doi.org/10.3390/nano11092190
Chicago/Turabian StyleShaker, Lina Mohammed, Ahmed Alamiery, Mohd Takriff, and Wan Nor Roslam Wan Isahak. 2021. "Novel Blue-Wavelength-Blocking Contact Lens with Er3+/TiO2 NPs: Manufacture and Characterization" Nanomaterials 11, no. 9: 2190. https://doi.org/10.3390/nano11092190
APA StyleShaker, L. M., Alamiery, A., Takriff, M., & Wan Isahak, W. N. R. (2021). Novel Blue-Wavelength-Blocking Contact Lens with Er3+/TiO2 NPs: Manufacture and Characterization. Nanomaterials, 11(9), 2190. https://doi.org/10.3390/nano11092190









