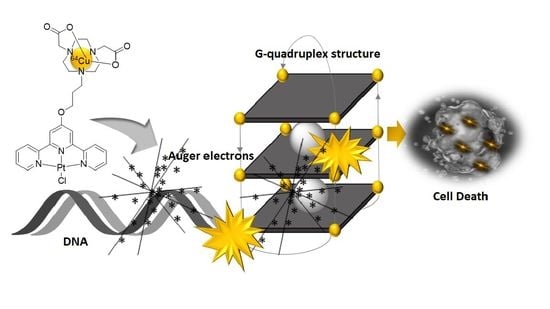
Design, Synthesis, and Cytotoxicity Assessment of [64Cu]Cu-NOTA-Terpyridine Platinum Conjugate: A Novel Chemoradiotherapeutic Agent with Flexible Linker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Stability Studies
2.3. Cell Culture
2.4. Cytotoxicity Assay
2.5. Cellular Uptake, Internalization, and Efflux Assays in HCT116 Cells
2.6. Subcellular Localization of [64Cu]Cu-NOTA-TP in HCT116 and GM05757 Cells
2.7. Data Analysis
3. Results
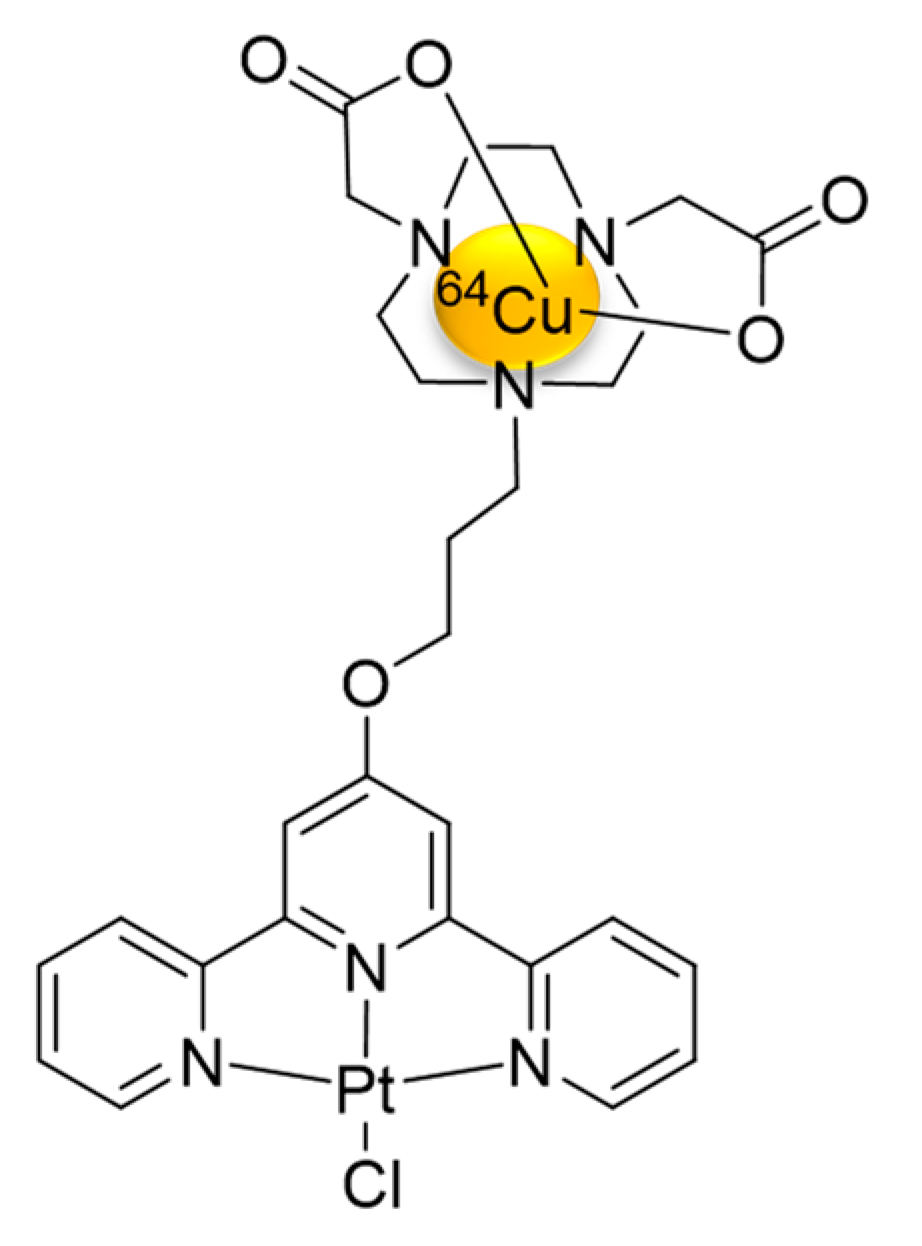
3.1. Synthesis and Characterization of [64Cu]Cu-NOTA-C3-TP
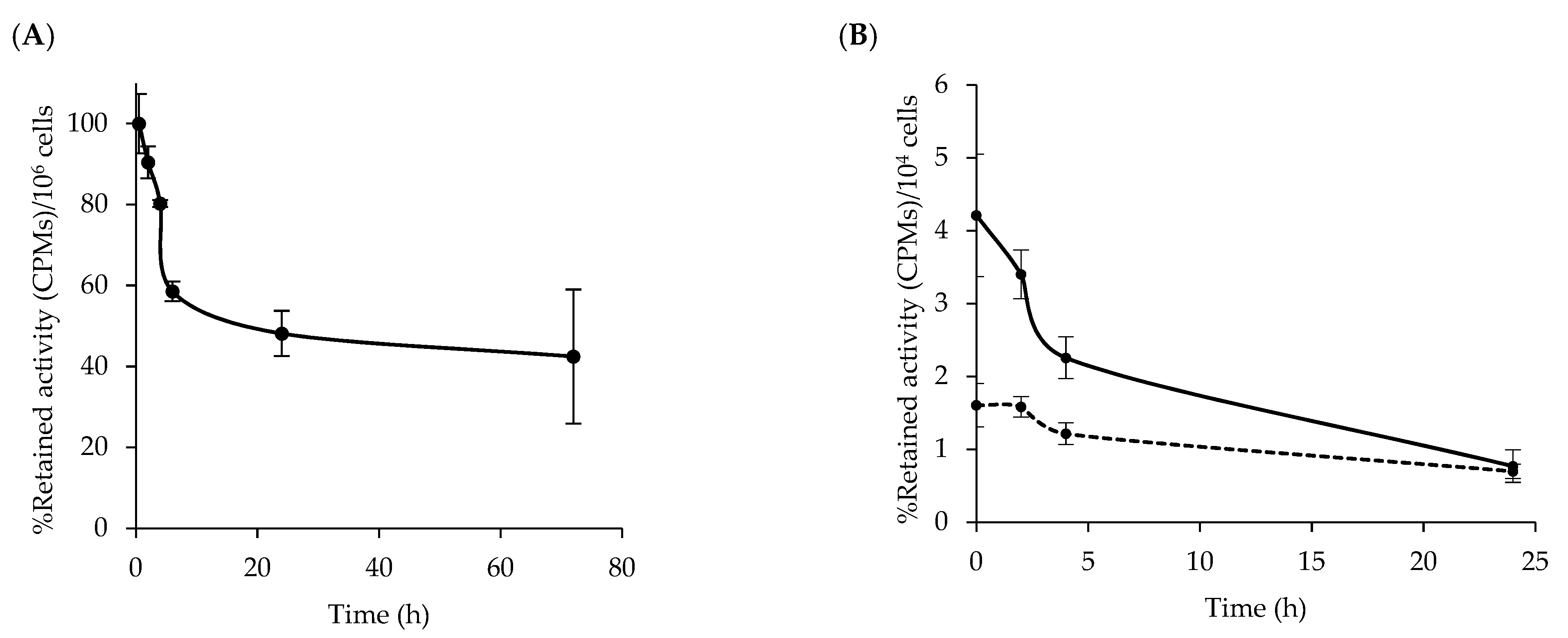
3.2. Stability of [64Cu]Cu-NOTA-C3-TP
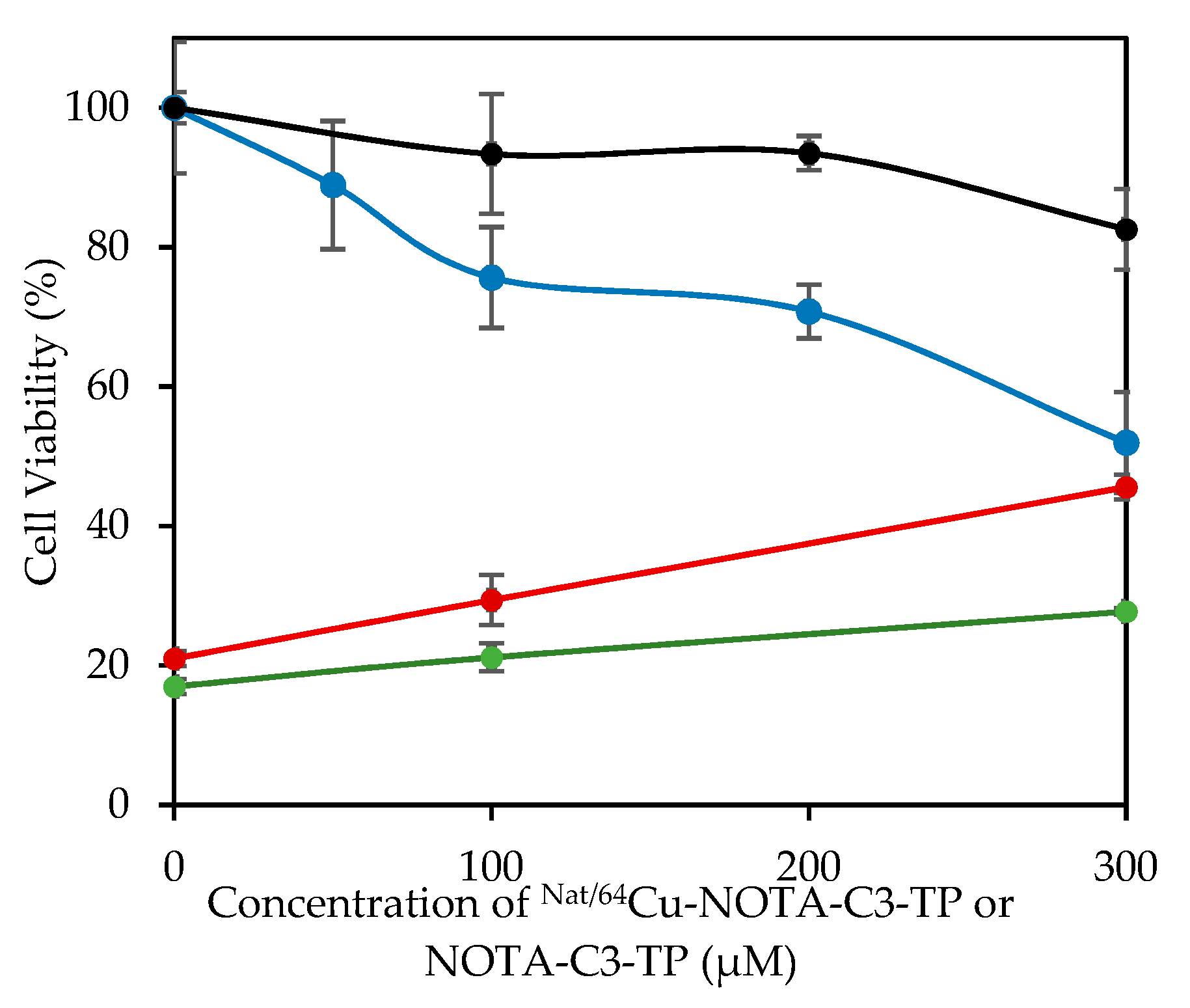
3.3. Cytotoxic Effects of [64Cu]Cu-NOTA-C3-TP
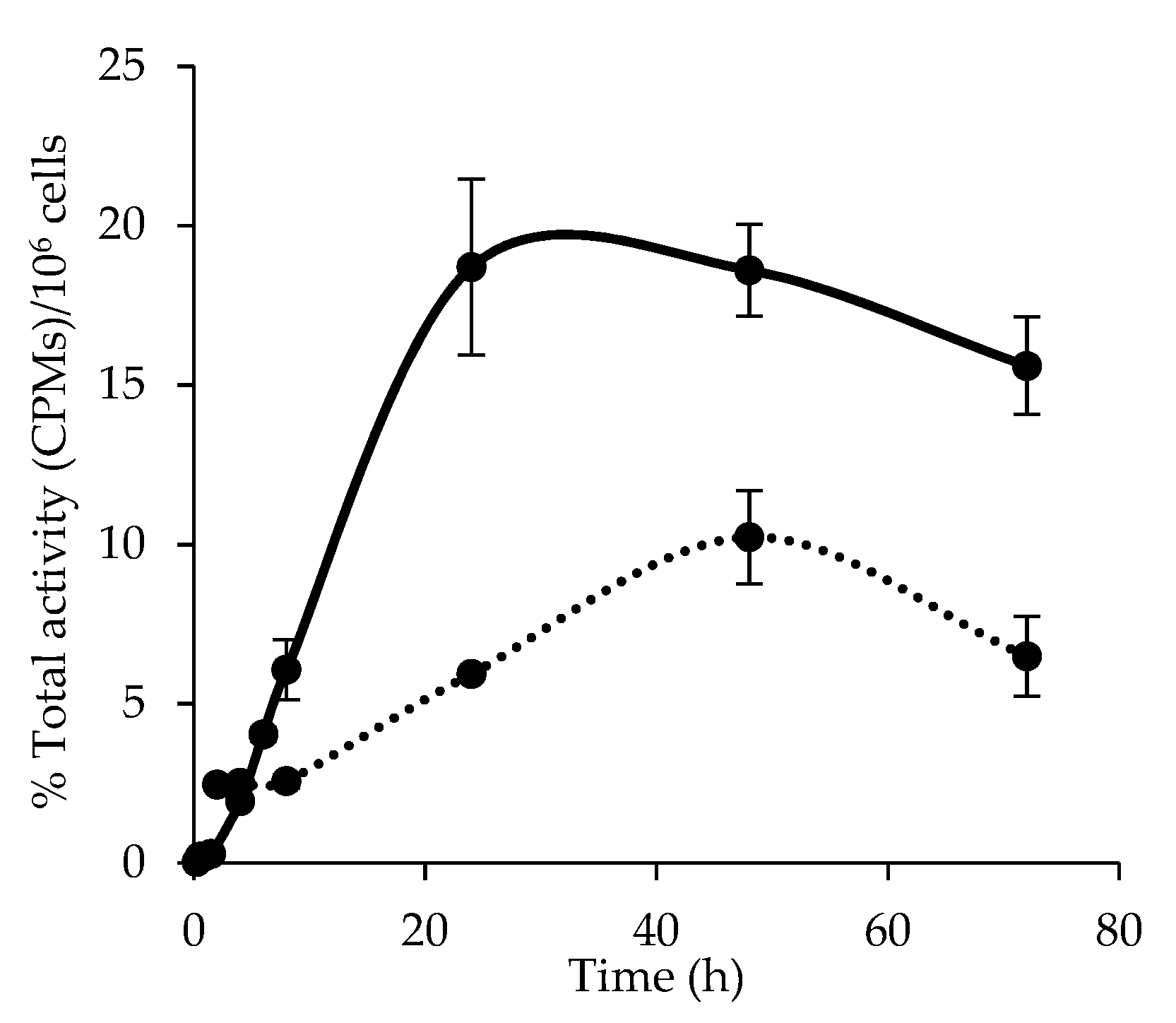
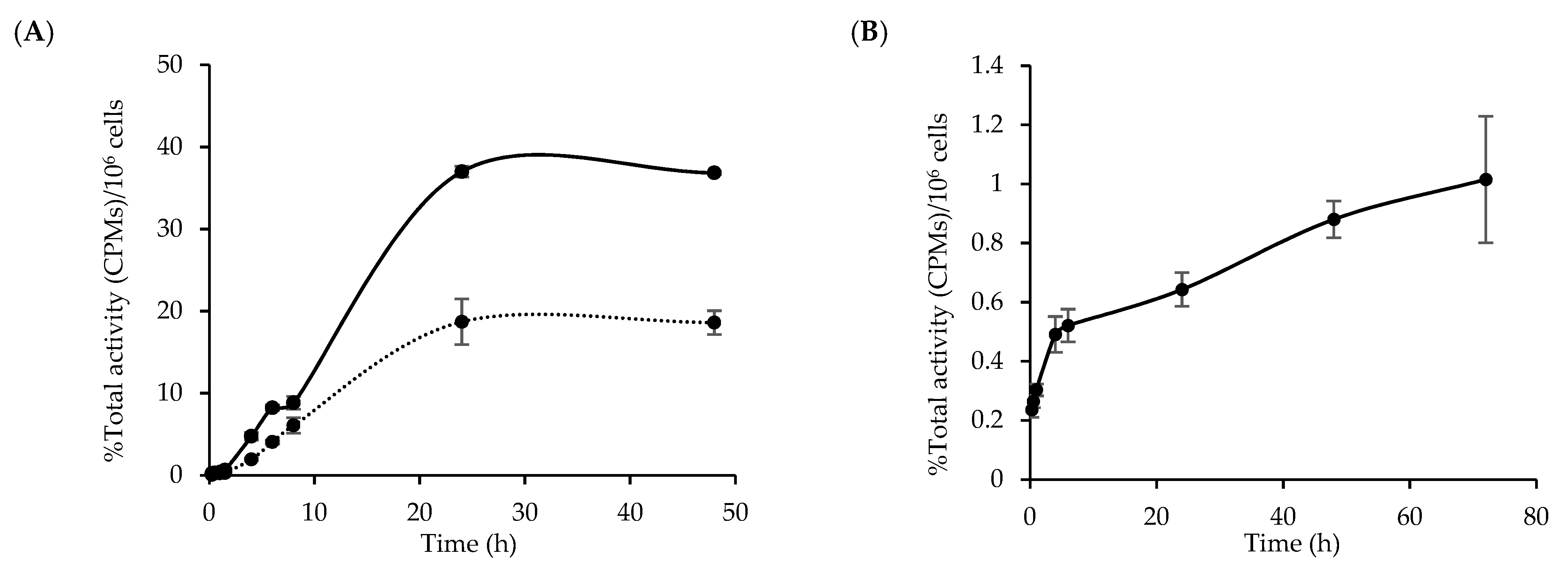
3.4. Cellular Uptake and Internalization of [64Cu]Cu-NOTA-C3-TP
3.5. Kinetic of [64Cu]Cu-NOTA-C3-TP Efflux
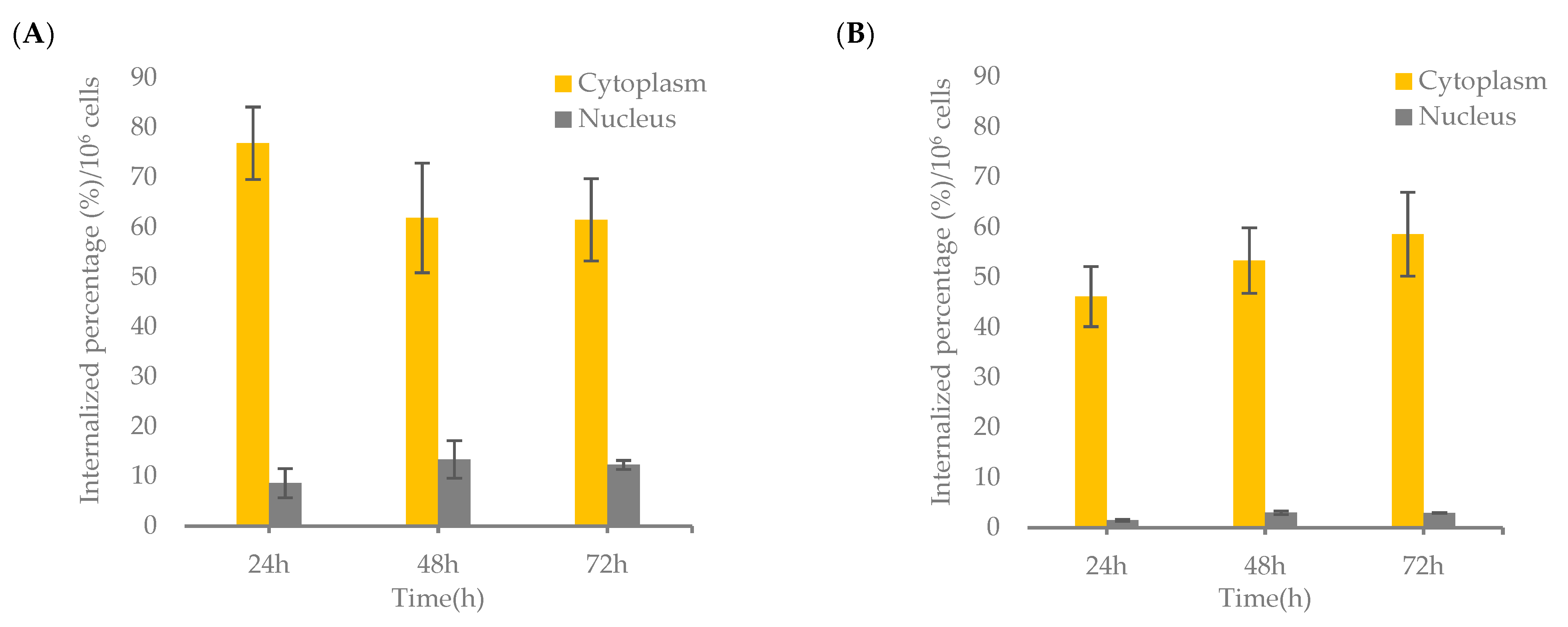
3.6. Nuclear Localization of [64Cu]Cu-NOTA-C3-TP
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [Green Version]
- Barbec, V.; Hrabina, O.; Kasparkova, J. Cytotoxic platinum coordination compounds. DNA binding agents. Coord. Chem. Rev. 2017, 351, 2–31. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. Correction: The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Shen, D.W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef] [Green Version]
- Tippayamontri, T.; Kotb, R.; Paqutte, B.; Sanche, L. Synergism in concomitant chemoradiotherapy of cisplatin and oxaliplatin and their liposomal formulation in the human colorectal cancer HCT116 model. Anticancer Res. 2012, 32, 4395–4404. [Google Scholar]
- Shen, D.W.; Goldenberg, S.; Pastan, I.; Gottesmani, M.M. Decreased accumulation of [14c]carboplatin in human cisplatin-resistant cells results from reduced energy-dependent uptake. J. Cell. Physiol. 2000, 183, 108–116. [Google Scholar] [CrossRef]
- Yang, A.D.; Fan, F.; Camp, E.R.; van Buren, G.; Liu, W.; Somcio, R.; Gray, M.J.; Cheng, H.; Hoff, P.M.; Ellis, L.M. Chronic Oxaliplatin Resistance Induces Epithelial-to-Mesenchymal Transition in Colorectal Cancer Cell Lines. Clin. Cancer Res. 2006, 12, 4147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, B.W.J.; Aldrich-Wright, J.R. The synthesis, characterisation and cytotoxicity of bisintercalating (2,2′:6′,2”-terpyridine) platinum(ii) complexes. Dalton Trans. 2015, 44, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, G.; Droz, A.S.; Vilaivan, T.; Weaver, G.W.; Park, J.J.; Pratt, J.M.; Tweedale, L.; Kelland, L.R. Cytotoxicity of 2,2′:6′,2″-Terpyridineplatinum (II) complexes against human ovarian carcinoma. J. Med. Chem. 1999, 42, 3167–3174. [Google Scholar] [CrossRef]
- Georgiades, S.N.; Abd Karim, N.H.; Suntharalingam, K.; Vilar, R. Cytotoxicity of 2,2′:6′,2″-Terpyridine Platinum(II) Complexes against Human Ovarian Carcinoma. Angew. Chem. Int. 2010, 49, 4020–4034. [Google Scholar] [CrossRef]
- Stafford, V.S.; Suntharalingam, K.; Shivalingam, A.; White, A.J.; Mann, D.J.; Vilar, R. Syntheses of polypyridyl metal complexes and studies of their interaction with quadruplex DNA. Dalton Trans. 2015, 44, 3686–3700. [Google Scholar] [CrossRef] [Green Version]
- Savić, A.; Marzo, T.; Scaletti, F.; Massai, L.; Bartoli, G.; Hoogenboom, R.; Messori, L.; Van Deun, R.; Van Hecke, K. New platinum (II) and palladium (II) complexes with substituted terpyridine ligands: Synthesis and characterization, cytotoxicity and reactivity towards biomolecules. Biometals 2018, 32, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Choy, H.; MacRae, R.M. Chemoradiation in Cancer Therapy, 1st ed.; Humana Press: Totowa, NJ, USA, 2003. [Google Scholar]
- Mamon, H.J.; Tepper, J.E. Combination chemoradiation therapy: The whole is more than the sum of the parts. J. Clin. Oncol. 2014, 32, 367–369. [Google Scholar] [CrossRef]
- Shirley, L. Radiosensitizers and Radiochemotherapy in the Treatment of Cancer, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–22. [Google Scholar]
- Sanche, L. Interaction of low energy electrons with DNA: Applications to cancer radiation therapy. Radiat. Phys. Chem. 2016, 128, 36–43. [Google Scholar] [CrossRef]
- Rezaee, M.; Hill, R.P.; Jaffray, D.A. The Exploitation of low-energy electrons in cancer treatment. Radiat. Res. 2017, 188, 123–143. [Google Scholar] [CrossRef] [PubMed]
- LaVerne, J.A.; Pimblott, S.M. Electron energy loss distributions in solid and gaseous hydrocarbons. J. Phys. Chem. 1995, 99, 10540–10548. [Google Scholar] [CrossRef]
- Pimblott, S.M.; LaVerne, J.A. Production of low-energy electrons by ionizing radiation. Radiat. Phys. Chem. 2007, 76, 1244–1247. [Google Scholar] [CrossRef]
- Kohanoff, J.; McAllister, M.; Tribello, G.A.; Gu, B. Interactions between low energy electrons and DNA: A perspective from first-principles simulations. J. Phys. Condens. Matter 2017, 29, 383001. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Fortin, D.; Paquette, B.; Sanche, L. Convection-enhancement delivery of liposomal formulation of oxaliplatin shows less toxicity than oxaliplatin yet maintains a similar median survival time in F98 glioma-bearing rat model. Investig. New Drugs 2016, 34, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Tippayamontri, T.; Kotb, R.; Paquette, B.; Sanche, L. Efficacy of Cisplatin and Lipoplatin™ in Combined Treatment with Radiation of a Colorectal Tumor in Nude Mouse. Anticancer Res. 2013, 33, 3005–3014. [Google Scholar] [PubMed]
- Charest, G.; Sanche, L.; Fortin, D.; Mathieu, D.; Paquette, B. Optimization of the route of platinum drugs administration to optimize the concomitant treatment with radiotherapy for glioblastoma implanted in the Fischer rat brain. J. Neurooncol. 2013, 115, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charest, G.; Sanche, L.; Fortin, D.; Mathieu, D.; Paquette, B. Glioblastoma treatment: Bypassing the toxicity of platinum compounds by using liposomal formulation and increasing treatment efficiency with concomitant radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Rezaee, M.; Alizadeh, E.; Cloutier, P.; Hunting, D.J.; Sanche, L. A single subexcitation-energy electron can induce a double-strand break in DNA modified by platinum chemotherapeutic drugs. ChemMedChem 2014, 9, 1145–1149. [Google Scholar] [CrossRef] [Green Version]
- Khosravifarsani, M.; Ait-Mohand, S.; Paquette, B.; Sanche, L.; Guérin, B. High cytotoxic effect by combining copper-64 with a NOTA-terpyridine platinum conjugate. J. Med. Chem. 2021, 64, 6765–6776. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.A.; Jain, S.; Lears, K.A.; Chong, H.S.; Kang, C.S.; Sun, X.; Rogers, B.E. Copper-64 radiolabeling and biological evaluation of bifunctional chelators for radiopharmaceutical development. Nucl. Med. Biol. 2012, 39, 1099–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ait-Mohand, S.; Denis, C.; Tremblay, G.; Paquette, M.; Guérin, B. Development of bifunctional chelates bearing hydroxamate arms for highly efficient 64Cu radiolabeling. Org. Lett. 2014, 16, 4512–4515. [Google Scholar] [CrossRef]
- Anderson, C.J.; Ferdani, R. Copper-64 radiopharmaceuticals for PET imaging of cancer: Advances in preclinical and clinical research. Cancer Biother. Radiopharm. 2009, 24, 379–393. [Google Scholar] [CrossRef]
- Eiblmaier, M.; Meyer, L.A.; Anderson, C.J. The role of p53 in the trafficking of copper-64 to tumor cell nuclei. Cancer Biol. Ther. 2008, 7, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournier, P.; Dumulon-Perreault, V.; Ait-Mohand, S.; Langlois, R.; Bénard, F.; Lecomte, R.; Guérin, B. Comparative study of 64Cu/NOTA-[D-Tyr6,βAla11,Thi13,Nle14]BBN(6-14) monomer and dimers for prostate cancer PET imaging. EJNMMI Res. 2012, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Suntharalingam, K.; Mendoza, O.; Duarte, A.A.; Mann, D.J.; Vilar, R. A platinum complex that binds non-covalently to DNA and induces cell death via a different mechanism than cisplatin. Metallomics 2013, 5, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Tippayamontri, T.; Kotb, R.; Paquette, B.; Sanche, L. Cellular uptake and cytoplasm/DNA distribution of cisplatin and oxaliplatin and their liposomal formulation in human colorectal cancer cell HCT116. Investig. New Drugs 2011, 29, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.A.; Neefjes, J.J.; Mathari, A.E.; Flens, M.J.; Scheffer, G.L.; Scheper, R.J. Overexpression of the ABC transporter TAP in multidrug-resistant human cancer cell lines. Br. J. Cancer 1996, 74, 1961–1967. [Google Scholar] [CrossRef] [Green Version]
- Howell, R.W.; Kassis, A.I.; Adelstein, S.J.; Rao, D.V.; Wright, H.A.; Hamm, R.N.; Turner, J.E.; Sastry, K.S.R. Radiotoxicity of platinum-195m-labeled trans-platinum (II) in mammalian cells. Radiat. Res. 1994, 140, 55–62. [Google Scholar] [CrossRef]
- Muscella, A.; Vetrugno, C.; Fanizzi, F.P.; Manca, C.; De Pascali, S.A.; Marsigliante, S. A new platinum(II) compound anticancer drug candidate with selective cytotoxicity for breast cancer cells. Cell Death Dis. 2013, 4, e796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Xu, F.; Zhao, Y.; Zheng, W.; Zeng, W.; Luo, Q.; Wang, Z.; Kui, W.; Jun, D.; Fuyi, W. Platinum(II) terpyridine anticancer complexes possessing multiple mode of DNA interaction and EGFR inhibiting activity. Front. Chem. 2020, 8, 210. [Google Scholar] [CrossRef] [PubMed]

| Entry | Compound | 24 h | 48 h | 72 h | |||
|---|---|---|---|---|---|---|---|
| GM05757 | HCT116 | GM05757 | HCT116 | GM05757 | HCT116 | ||
| 1 | NOTA-C3-TP 4 | 504 ± 4 | >700 a | 202 ± 5 | 63 ± 2 | 51 ± 3 | 24 ± 1 a |
| 2 | [NatCu]Cu-NOTA-C3-TP | >1000 | 298 ± 2 | 839 ± 2 | 481 ± 25 | 747 ± 26 | 330 ± 51 |
| 3 | [64Cu]Cu-NOTA-C3-TP b | >200 | 59 ± 3 | N/A | 9 ± 2 | 12 ± 2 | <5 |
| 4 | [64Cu]Cu-NOTA-C3-TP c | >0.066 | 0.017 ± 0.004 | 0.025 ± 0.005 | 0.012 ± 0.006 | 0.019 ± 0.004 | 0.006 ± 0.002 |
| 5 | Cisplatin | 88 ± 4 | 31 ± 2 | 84 ± 2 | 42 ± 8 | 77 ± 1 | 23 ± 3 |
| 6 | Oxaliplatin | >200 | >200 | 165 ± 9 | 64 ± 1 | 65 ± 3 | 16 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khosravifarsani, M.; Ait-Mohand, S.; Paquette, B.; Sanche, L.; Guérin, B. Design, Synthesis, and Cytotoxicity Assessment of [64Cu]Cu-NOTA-Terpyridine Platinum Conjugate: A Novel Chemoradiotherapeutic Agent with Flexible Linker. Nanomaterials 2021, 11, 2154. https://doi.org/10.3390/nano11092154
Khosravifarsani M, Ait-Mohand S, Paquette B, Sanche L, Guérin B. Design, Synthesis, and Cytotoxicity Assessment of [64Cu]Cu-NOTA-Terpyridine Platinum Conjugate: A Novel Chemoradiotherapeutic Agent with Flexible Linker. Nanomaterials. 2021; 11(9):2154. https://doi.org/10.3390/nano11092154
Chicago/Turabian StyleKhosravifarsani, Meysam, Samia Ait-Mohand, Benoit Paquette, Léon Sanche, and Brigitte Guérin. 2021. "Design, Synthesis, and Cytotoxicity Assessment of [64Cu]Cu-NOTA-Terpyridine Platinum Conjugate: A Novel Chemoradiotherapeutic Agent with Flexible Linker" Nanomaterials 11, no. 9: 2154. https://doi.org/10.3390/nano11092154
APA StyleKhosravifarsani, M., Ait-Mohand, S., Paquette, B., Sanche, L., & Guérin, B. (2021). Design, Synthesis, and Cytotoxicity Assessment of [64Cu]Cu-NOTA-Terpyridine Platinum Conjugate: A Novel Chemoradiotherapeutic Agent with Flexible Linker. Nanomaterials, 11(9), 2154. https://doi.org/10.3390/nano11092154