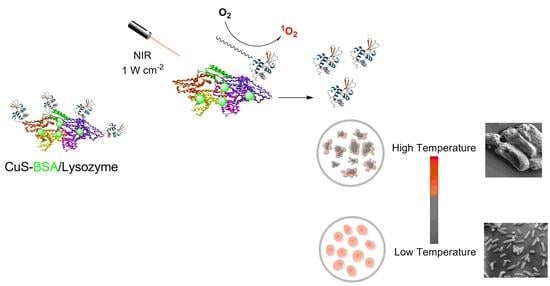
Enhanced Antibacterial Activity of CuS-BSA/Lysozyme under Near Infrared Light Irradiation
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of CuS-BSA Nanoparticles
2.2. Lysozyme Loading onto CuS-BSA Nanoparticles
2.3. NIR Light Induced Photothermal Heating of CuS-BSA Nanoparticles
2.4. In Vitro Photothermal and Passive Release of Lysozyme
2.5. Cell Culture and Cytotoxicity Studies
2.6. Bacterial Culture and Enzyme Activity Assay
2.7. Photodynamic Properties of CuS-BSA Nanoparticles
3. Results and Discussion
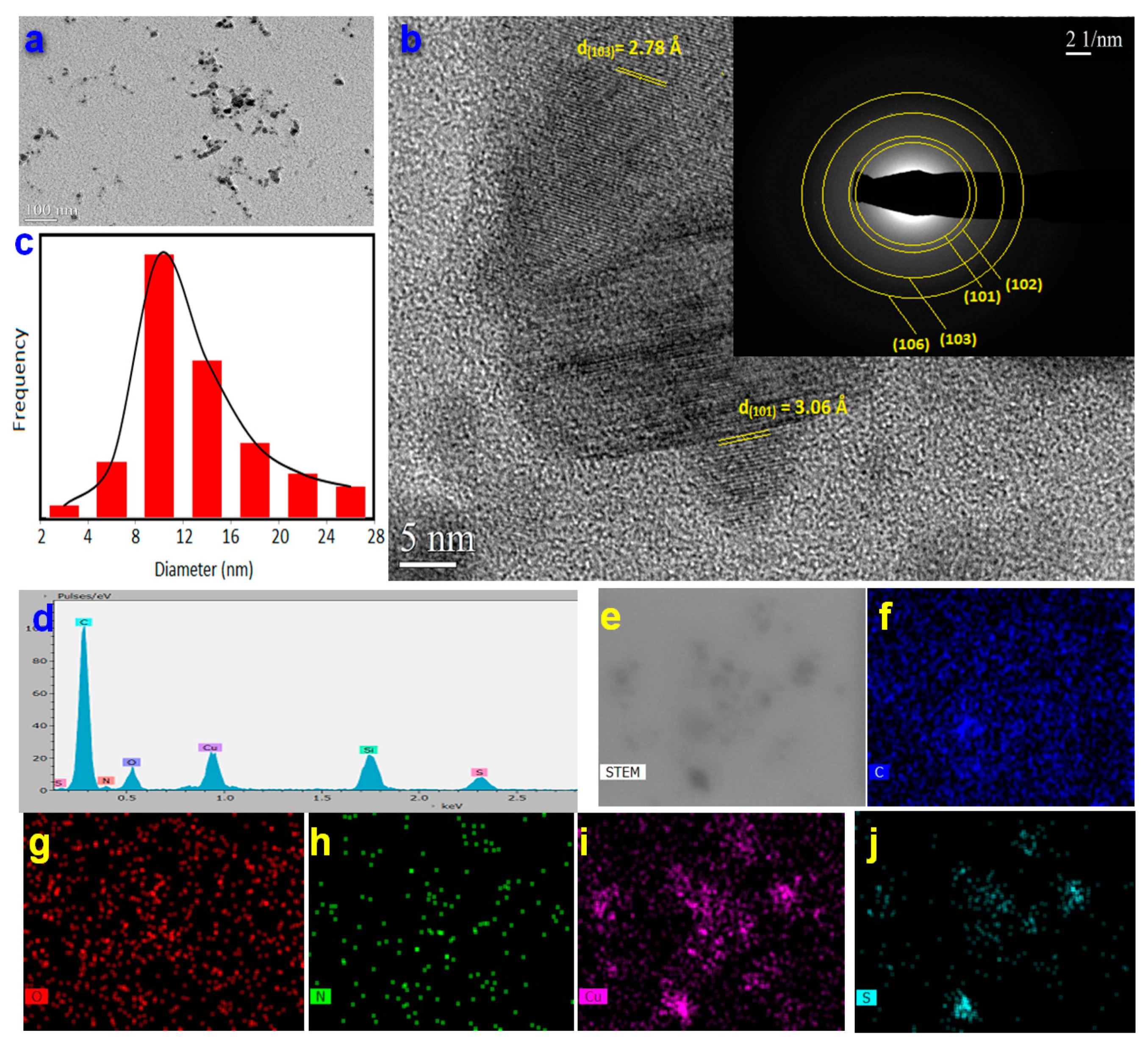
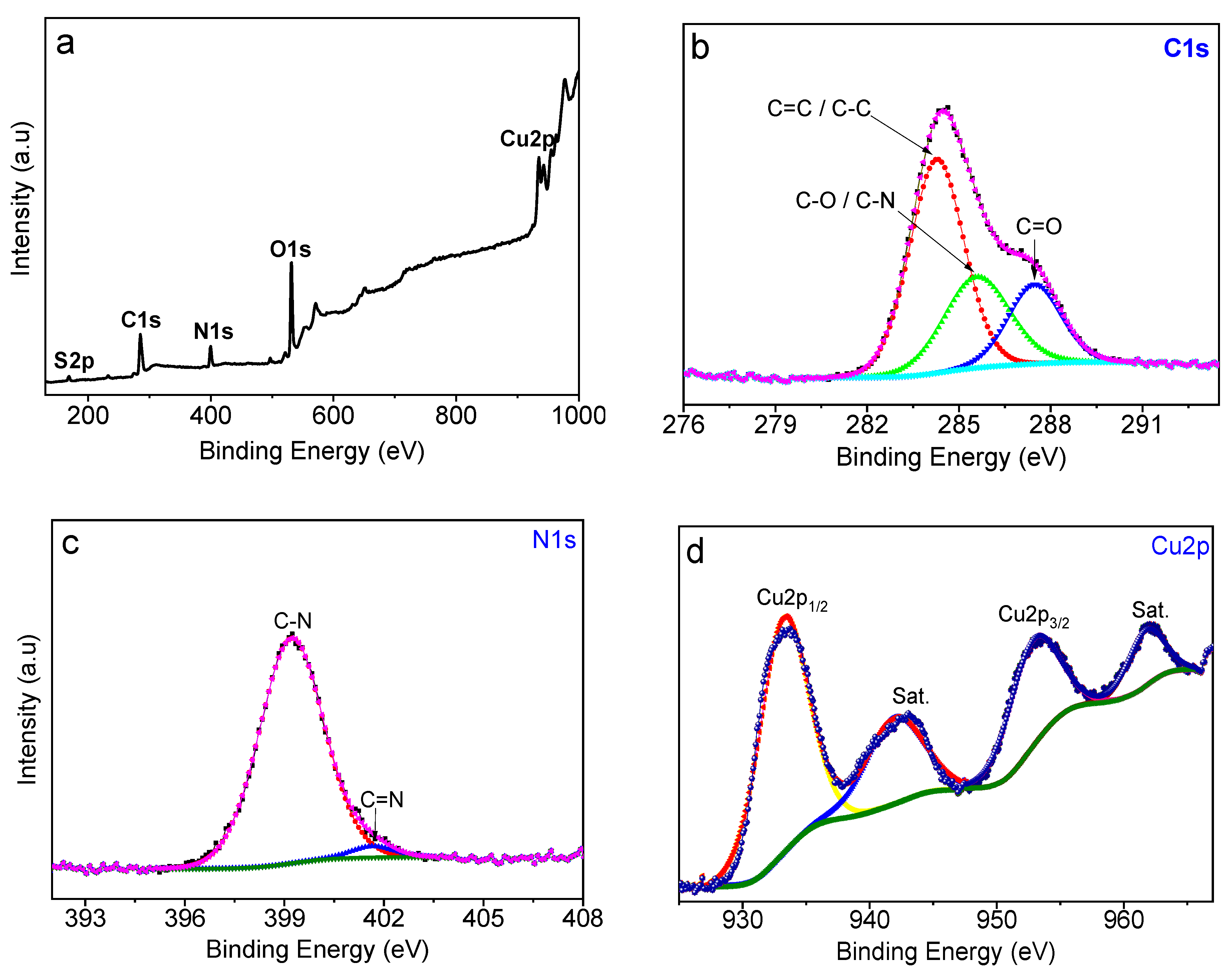
3.1. Preparation and Characterization of CuS-BSA Nanoparticles
3.2. Lysozyme Loading Efficiency
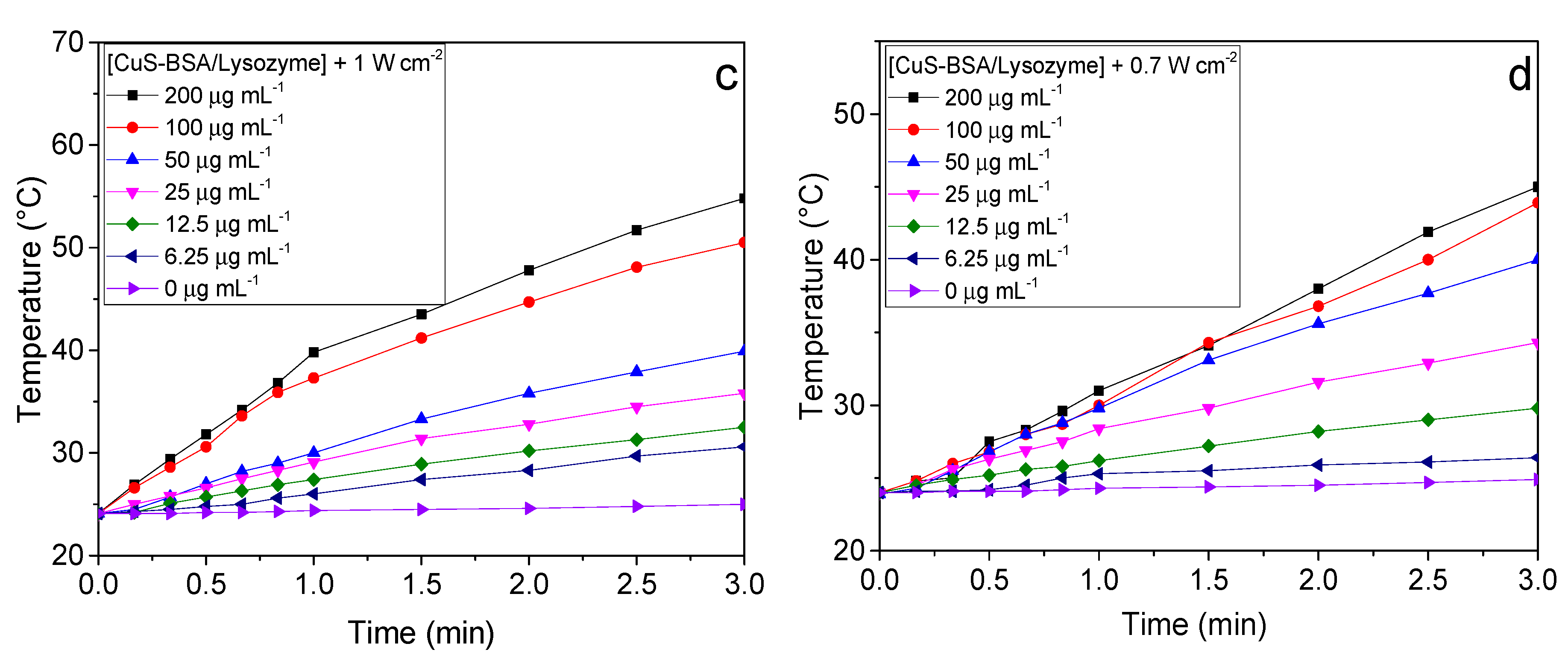
3.3. Photothermal Heating Performance
3.4. In Vitro Photothermal Lysozyme Release
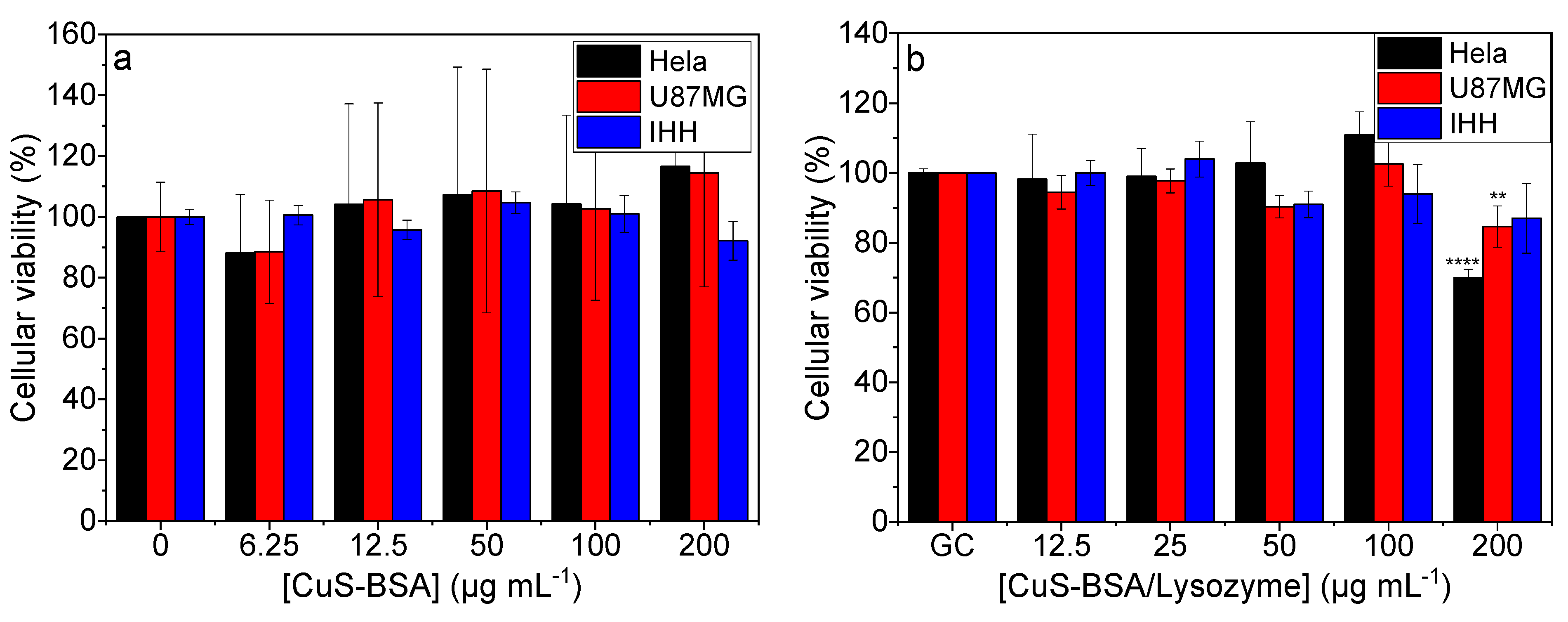
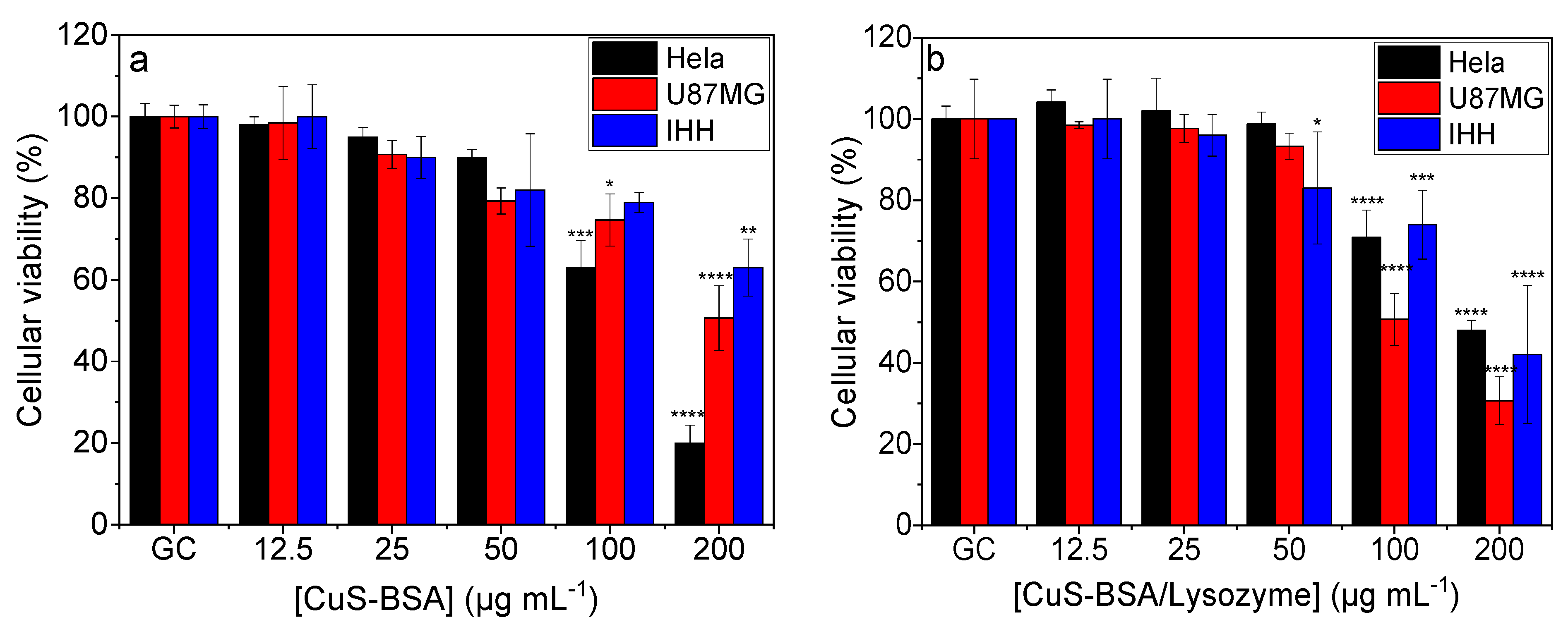
3.5. Cellular Toxicity and Viability Studies
3.6. Singlet Oxygen Generation under NIR Illumination
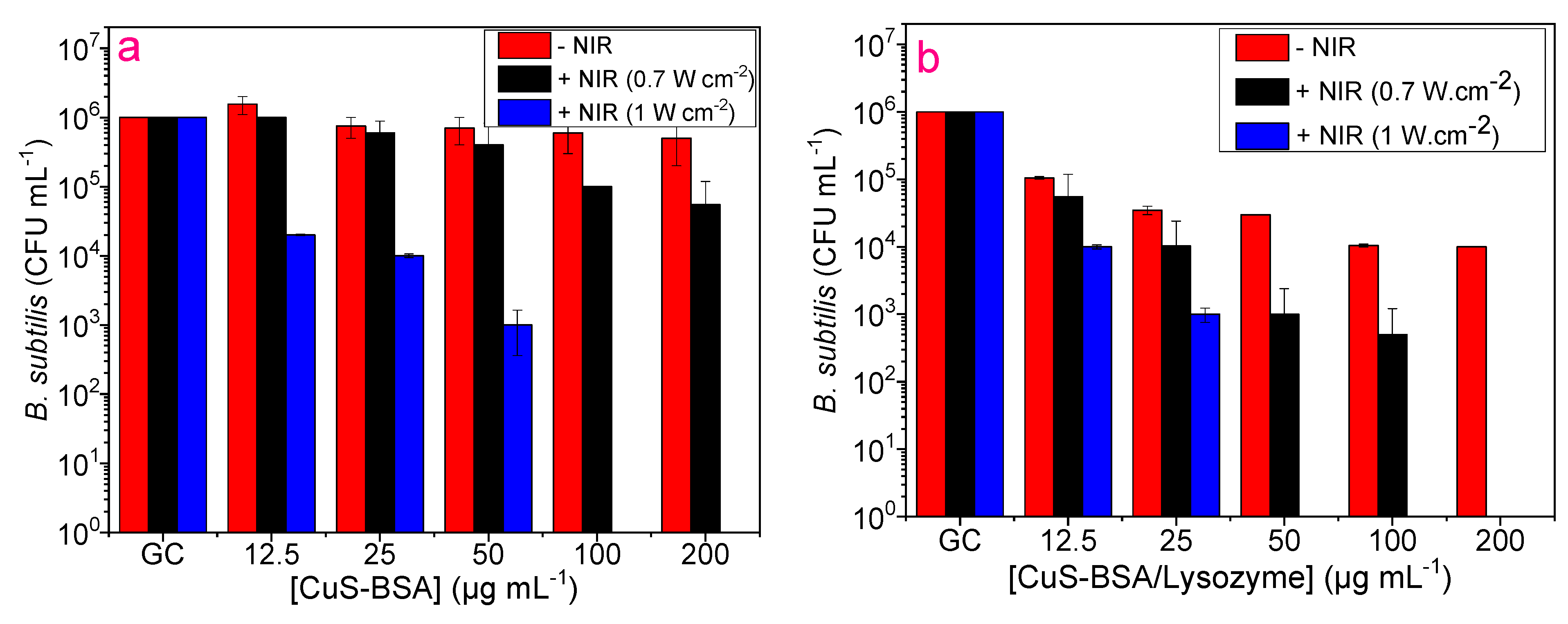
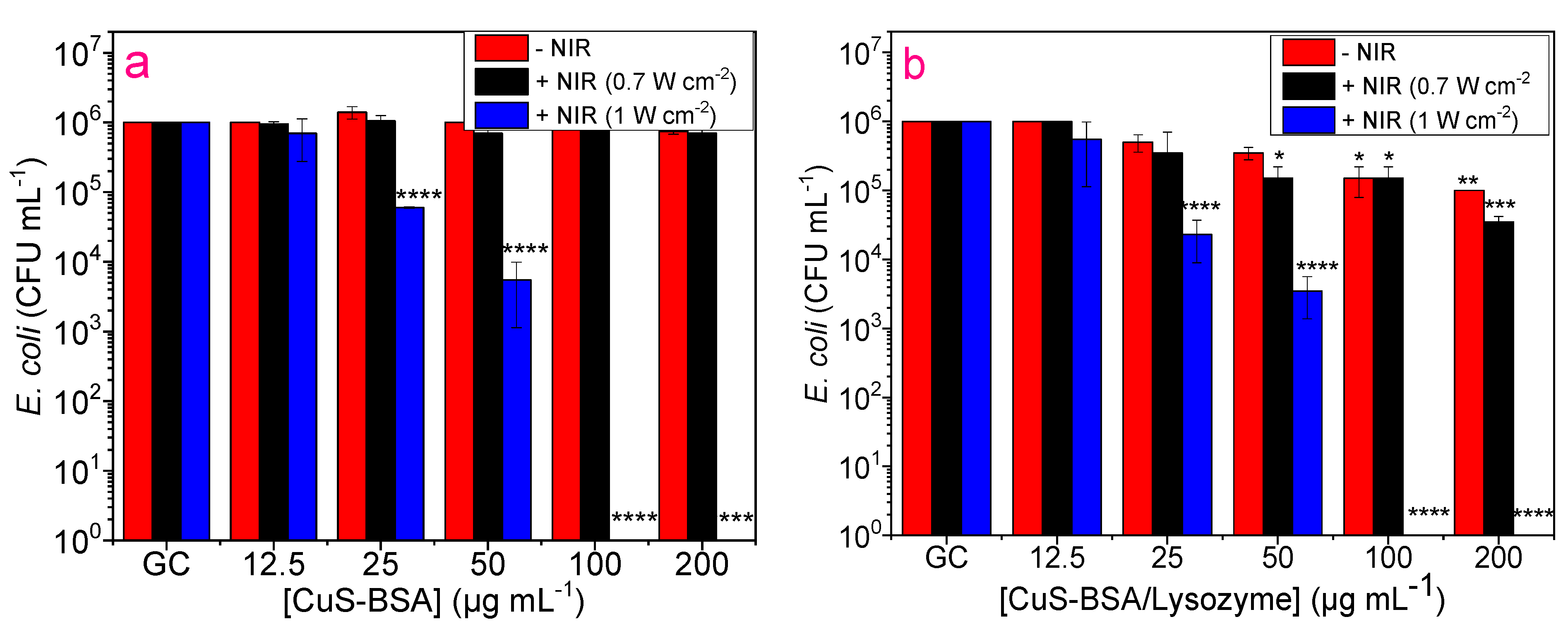
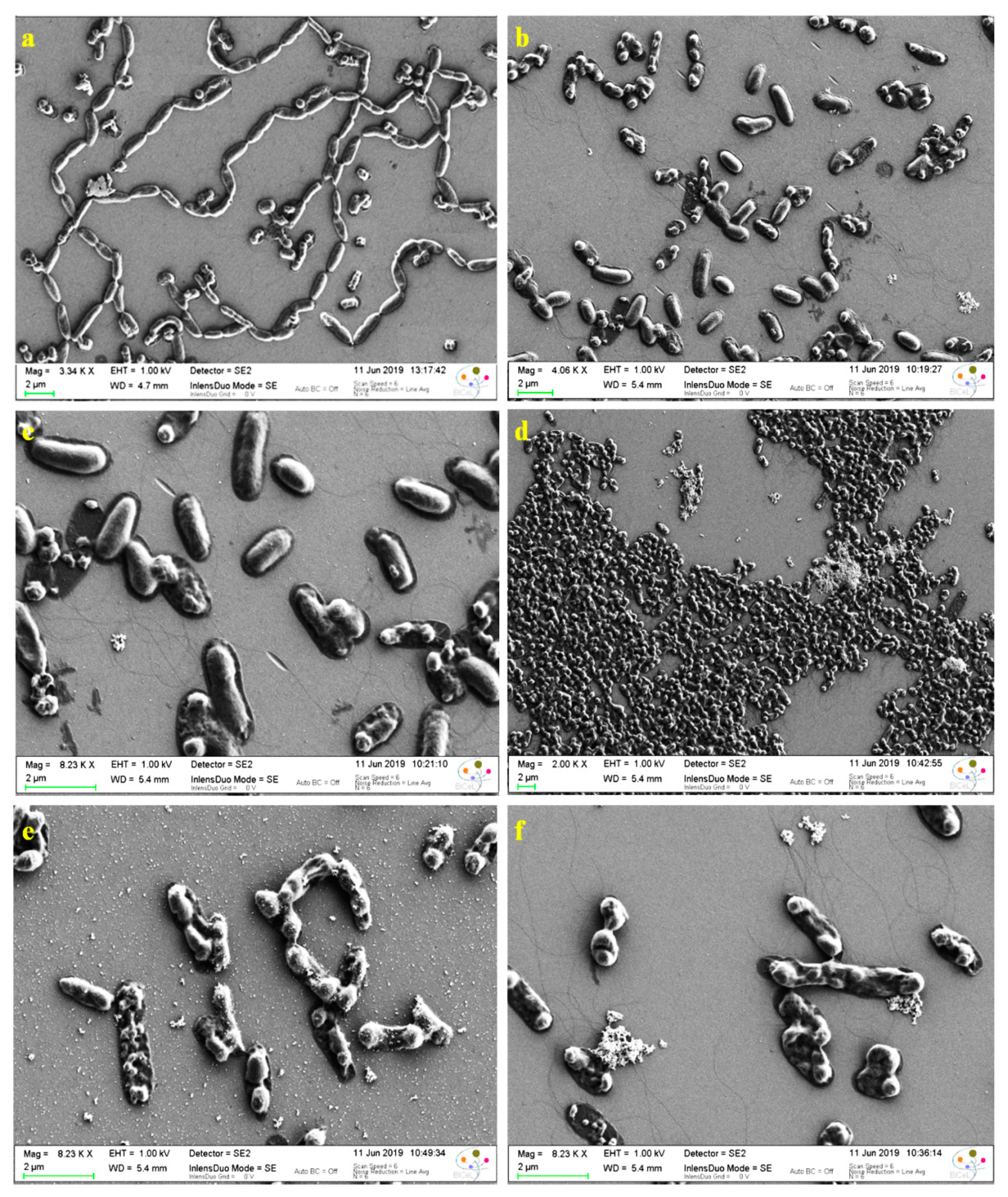
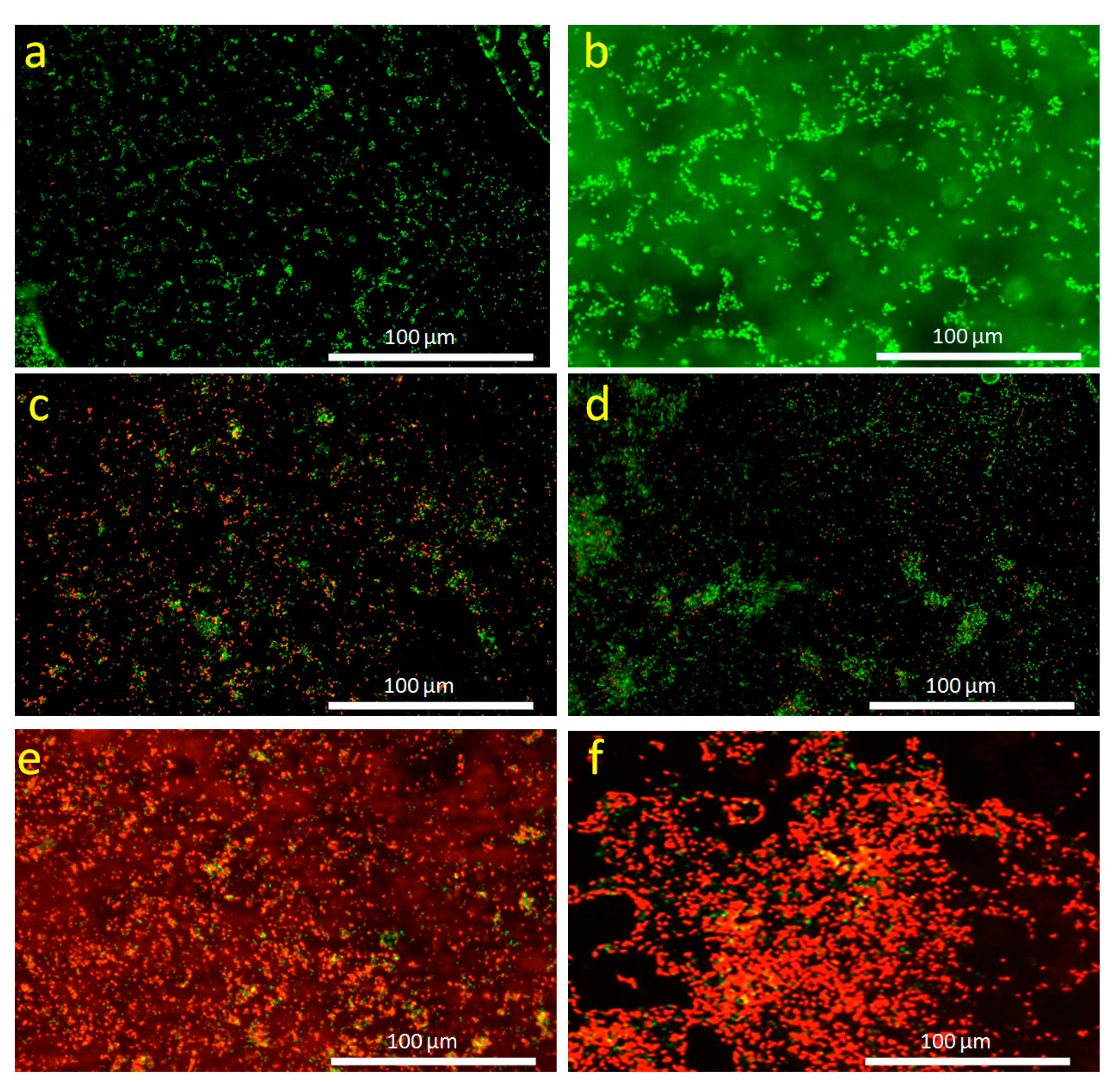
3.7. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, L.-L.; Xu, J.-H.; Qi, G.-B.; Zhao, X.; Yu, F.; Wang, H. Core–shell supramolecular gelatin nanoparticles for adaptive and “on-demand” antibiotic delivery. ACS Nano 2014, 8, 4975–4983. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Cheng, X.; Ren, X.; Huang, T.S. Self-assembled antibacterial coating by N-halamine polyelectrolytes on a cellulose substrate. J. Mater. Chem. B 2015, 3, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, W.; Xiang, Q.; Jin, F.; Peng, X.; Li, Q.; Jiang, M.; Hu, B.; Xing, X. Silver nanoparticle and lysozyme/tannic acid layer-by-layer assembly antimicrobial multilayer on magnetic nanoparticle by an eco-friendly route. Mater. Sci. Eng. C 2017, 76, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. On a remarkable bacteriolytic element found in tissues and secretions. Proc. R. Soc. London. Ser. B Boil. Sci. 1922, 93, 306–317. [Google Scholar] [CrossRef]
- Laschtschenko, P. Uber die keimtötende und entwicklungshemmende Wirkung von Hühnereiweiß. Z. Für Hyg. Und Infekt. 1909, 64, 419–427. [Google Scholar] [CrossRef]
- Hughey, V.; Johnson, E. Antimicrobial activity of lysozyme against bacteria involved in food spoilage and food-borne disease. Appl. Environ. Microbiol. 1987, 53, 2165–2170. [Google Scholar] [CrossRef]
- Wiesner, J.; Vilcinskas, A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef]
- Zheng, L.; Wan, Y.; Yu, L.; Zhang, D. Lysozyme as a recognition element for monitoring of bacterial population. Talanta 2016, 146, 299–302. [Google Scholar] [CrossRef]
- Jolles, P. Recent Developments in the Study of Lysozymes. Angew. Chem. Int. Ed. 1964, 3, 28–36. [Google Scholar] [CrossRef]
- Yuan, S.; Yin, J.; Jiang, W.; Liang, B.; Pehkonen, S.; Choong, C. Enhancing antibacterial activity of surface-grafted chitosan with immobilized lysozyme on bioinspired stainless steel substrates. Colloids Surf. B Biointerfaces 2013, 106, 11–21. [Google Scholar] [CrossRef]
- Wang, Y.; Nor, Y.A.; Song, H.; Yang, Y.; Xu, C.; Yu, M.; Yu, C. Small-sized and large-pore dendritic mesoporous silica nanoparticles enhance antimicrobial enzyme delivery. J. Mater. Chem. B 2016, 4, 2646–2653. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, P.; Zhan, Y.; Shi, X.; Lin, J.; Du, Y.; Li, X.; Deng, H. Pectin/lysozyme bilayers layer-by-layer deposited cellulose nanofibrous mats for antibacterial application. Carbohydr. Polym. 2015, 117, 687–693. [Google Scholar] [CrossRef]
- Ben Amara, C.; Eghbal, N.; Degraeve, P.; Gharsallaoui, A. Using complex coacervation for lysozyme encapsulation by spray-drying. J. Food Eng. 2016, 183, 50–57. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, C.; Zeng, G.-M.; Gong, J.-L.; Chang, Y.-N.; Song, B.; Deng, C.-H.; Liu, H.-Y. The disinfection performance and mechanisms of Ag/lysozyme nanoparticles supported with montmorillonite clay. J. Hazard. Mater. 2016, 317, 416–429. [Google Scholar] [CrossRef]
- Jing, M.; Song, W.; Liu, R. Binding of copper to lysozyme: Spectroscopic, isothermal titration calorimetry and molecular docking studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 164, 103–109. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Xu, Y.; Feng, H.; Fu, S.; Tang, J.; Liu, W.; Sun, D.; Jiang, H.; Xu, S. Preparation and evaluation of lysozyme-loaded nanoparticles coated with poly-γ-glutamic acid and chitosan. Int. J. Biol. Macromol. 2013, 59, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wu, C.; Fu, S.; Wang, L.; Yuan, C.; Chen, S.; Hu, Y. Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity. Carbohydr. Polym. 2017, 155, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Muszanska, A.K.; Busscher, H.J.; Herrmann, A.; van der Mei, H.C.; Norde, W. Pluronic–lysozyme conjugates as anti-adhesive and antibacterial bifunctional polymers for surface coating. Biomaterials 2011, 32, 6333–6341. [Google Scholar] [CrossRef]
- Horn, D.W.; Ao, G.; Maugey, M.; Zakri, C.; Poulin, P.; Davis, V.A. Dispersion State and Fiber Toughness: Antibacterial Lysozyme-Single Walled Carbon Nanotubes. Adv. Funct. Mater. 2013, 23, 6082–6090. [Google Scholar] [CrossRef]
- Chakraborti, S.; Chatterjee, T.; Joshi, P.; Poddar, A.; Bhattacharyya, B.; Singh, S.P.; Gupta, V.; Chakrabarti, P. Structure and Activity of Lysozyme on Binding to ZnO Nanoparticles. Langmuir 2010, 26, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Ping, Y.; Zhang, H.; Zhou, B.; Liu, F.; Yu, Y.; Xie, T.; Li, W.; Zhong, D.; Zhang, Y.; et al. Laser-Activatable CuS Nanodots to Treat Multidrug-Resistant Bacteria and Release Copper Ion to Accelerate Healing of Infected Chronic Nonhealing Wounds. ACS Appl. Mater. Interfaces 2019, 11, 3809–3822. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef]
- Huang, W.-C.; Tsai, P.-J.; Chen, Y.-C. Functional gold nanoparticles as photothermal agents for selective-killing of pathogenic bacteria. Nanomedicine 2007, 2, 777–787. [Google Scholar] [CrossRef]
- Prodi, A.; Filon, F.L. Nano-Scaled Particles and Fibres Occupational Exposure Assessment: An Integrated Approach from Air Sampling to Skin and Surface Contamination. Nano Biomed. Eng. 2016, 8, 91–104. [Google Scholar] [CrossRef][Green Version]
- Huang, J.; Zhou, J.; Zhuang, J.; Gao, H.; Huang, D.; Wang, L.; Wu, W.; Li, Q.; Junyang, Z.; Han, M.-Y. Strong Near-Infrared Absorbing and Biocompatible CuS Nanoparticles for Rapid and Efficient Photothermal Ablation of Gram-Positive and -Negative Bacteria. ACS Appl. Mater. Interfaces 2017, 9, 36606–36614. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, Q.; Qi, W.; Jia, Y.; Xiong, T.; Fan, Z.; Liu, S.; Yang, J.; Li, N.; Chang, B. BSA-CuS Nanoparticles for Photothermal Therapy of Diabetic Wound Infection In Vivo. ChemistrySelect 2018, 3, 9510–9516. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Anbazhagan, V. Synthesis of copper sulfide nanoparticles and evaluation of in vitro antibacterial activity and in vivo therapeutic effect in bacteria-infected zebrafish. RSC Adv. 2017, 7, 36644–36652. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Chen, L.; Zhou, Y.; Dang, W.; Chang, J.; Wu, C. Ultrathin Cu-TCPP MOF nanosheets: A new theragnostic nanoplatform with magnetic resonance/near-infrared thermal imaging for synergistic phototherapy of cancers. Theranostics 2018, 8, 4086–4096. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Chen, F.; Cai, W. Synthesis and Biomedical Applications of Copper Sulfide Nanoparticles: From Sensors to Theranostics. Small 2014, 10, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, W.; Huang, Q.; Li, C.; Chen, W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine 2010, 5, 1161–1171. [Google Scholar] [CrossRef]
- Ramadan, S.; Guo, L.; Li, Y.; Yan, B.; Lu, W. Hollow Copper Sulfide Nanoparticle-Mediated Transdermal Drug Delivery. Small 2012, 8, 3143–3150. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Y.; Ke, H.; Wang, Q.; Lv, X.; Wu, H.; Tang, Y.; Yang, X.; Chen, C.; Zhao, Y.; et al. Protein-Nanoreactor-Assisted Synthesis of Semiconductor Nanocrystals for Efficient Cancer Theranostics. Adv. Mater. 2016, 28, 5923–5930. [Google Scholar] [CrossRef]
- Swaidan, A.; Borthakur, P.; Boruah, P.K.; Das, M.R.; Barras, A.; Hamieh, S.; Toufaily, J.; Hamieh, T.; Szunerits, S.; Boukherroub, R. A facile preparation of CuS-BSA nanocomposite as enzyme mimics: Application for selective and sensitive sensing of Cr (VI) ions. Sens. Actuators B Chem. 2019, 294, 253–262. [Google Scholar] [CrossRef]
- Al Atya, A.K.; Drider-Hadiouche, K.; Vachee, A.; Drider, D. Potentialization of β-lactams with colistin: In case of extended spectrum β-lactamase producing Escherichia coli strains isolated from children with urinary infections. Res. Microbiol. 2016, 167, 215–221. [Google Scholar] [CrossRef]
- Gollavelli, G.; Ling, Y.-C. Magnetic and fluorescent graphene for dual modal imaging and single light induced photothermal and photodynamic therapy of cancer cells. Biomaterials 2014, 35, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, S.; Wang, Q.; Geng, B. A facile solution chemical route to self-assembly of CuS ball-flowers and their application as an efficient photocatalyst. CrystEngComm 2010, 12, 144–149. [Google Scholar] [CrossRef]
- Riyaz, S.; Parveen, A.; Azam, A. Microstructural and optical properties of CuS nanoparticles prepared by sol–gel route. Perspect. Sci. 2016, 8, 632–635. [Google Scholar] [CrossRef]
- Li, Z.; Mi, L.; Chen, W.; Hou, H.; Liu, C.; Wang, H.; Zheng, Z.; Shen, C. Three-dimensional CuS hierarchical architectures as recyclable catalysts for dye decolorization. CrystEngComm 2012, 14, 3965–3971. [Google Scholar] [CrossRef]
- Tanveer, M.; Cao, C.; Ali, Z.; Aslam, I.; Idrees, F.; Khan, W.S.; But, F.K.; Tahir, M.; Mahmood, N. Template free synthesis of CuS nanosheet-based hierarchical microspheres: An efficient natural light driven photocatalyst. CrystEngComm 2014, 16, 5290–5300. [Google Scholar] [CrossRef]
- Pal, M.; Mathews, N.R.; Sanchez-Mora, E.; Pal, U.B.; Paraguay-Delgado, F.; Mathew, X. Synthesis of CuS nanoparticles by a wet chemical route and their photocatalytic activity. J. Nanoparticle Res. 2015, 17, 301. [Google Scholar] [CrossRef]
- Yang, H.; Liang, H.; Xie, Y.; Chen, Q. A cancer cell turn-on protein-CuSMn nanoparticle as the sensor of breast cancer cell and CH3O-PEG-phosphatide. Chin. Chem. Lett. 2018, 29, 1528–1532. [Google Scholar] [CrossRef]
- Safrani, T.; Jopp, J.; Golan, Y. A comparative study of the structure and optical properties of copper sulfide thin films chemically deposited on various substrates. RSC Adv. 2013, 3, 23066–23074. [Google Scholar] [CrossRef]
- Milekhin, A.; Yeryukov, N.; Sveshnikova, L.; Duda, T.; Rodyakina, E.; Gridchin, V.; Sheremet, E.; Zahn, D. Combination of surface- and interference-enhanced Raman scattering by CuS nanocrystals on nanopatterned Au structures. Beilstein J. Nanotechnol. 2015, 6, 749–754. [Google Scholar] [CrossRef]
- Kuhar, N.; Sil, S.; Verma, T.; Umapathy, S. Challenges in application of Raman spectroscopy to biology and materials. RSC Adv. 2018, 8, 25888–25908. [Google Scholar] [CrossRef]
- Ratnayake, D.; Martin, M.D.; Gowrishetty, U.R.; Porter, A.; Berfield, T.; McNamara, S.P.; Walsh, K.M. Engineering stress in thin films for the field of bistable MEMS. J. Micromech. Microeng. 2015, 25, 125025. [Google Scholar] [CrossRef]
- Koshani, R.; Aminlari, M.; Niakosari, M.; Farahnaky, A.; Mesbahi, G. Production and properties of tragacanthin-conjugated lysozyme as a new multifunctional biopolymer. Food Hydrocoll. 2015, 47, 69–78. [Google Scholar] [CrossRef]
- Huang, P.; Li, Z.; Hu, H.; Cui, D. Synthesis and Characterization of Bovine Serum Albumin-Conjugated Copper Sulfide Nanocomposites. J. Nanomater. 2010, 2010, 641545. [Google Scholar] [CrossRef]
- Prosapio, V.; Reverchon, E.; De Marco, I. Production of lysozyme microparticles to be used in functional foods, using an expanded liquid antisolvent process. J. Supercrit. Fluids 2015, 107, 106–113. [Google Scholar] [CrossRef]
- Zhou, Y.; Bai, Y.; Liu, H.; Jiang, X.; Tong, T.; Fang, L.; Wang, D.; Ke, Q.; Liang, J.; Xiao, S. Tellurium/Bovine Serum Albumin Nanocomposites Inducing the Formation of Stress Granules in a Protein Kinase R-Dependent Manner. ACS Appl. Mater. Interfaces 2018, 10, 25241–25251. [Google Scholar] [CrossRef]
- Ieva, E.; Trapani, A.; Cioffi, N.; Ditaranto, N.; Monopoli, A.; Sabbatini, L. Analytical characterization of chitosan nanoparticles for peptide drug delivery applications. Anal. Bioanal. Chem. 2009, 393, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Lim, S.; Tian, Z.; Shang, J.; Lai, L.; Macdonald, B.J.; Fu, C.; Shen, Z.; Yu, T.; Lin, J. Pyridinic N doped graphene: Synthesis, electronic structure, and electrocatalytic property. J. Mater. Chem. 2011, 21, 8038–8044. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal treatment of grass: A low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu (II) ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, J.; Zhang, Y.; Li, Q.; Gong, J.R. Visible Light Photocatalytic H2-Production Activity of CuS/ZnS Porous Nanosheets Based on Photoinduced Interfacial Charge Transfer. Nano Lett. 2011, 11, 4774–4779. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Zhou, P.; Yin, J.; Liu, H.; Chen, F.; Liu, H.; Du, Y.; Xi, P. Phase Transformation Fabrication of a Cu2S Nanoplate as an Efficient Catalyst for Water Oxidation with Glycine. Inorg. Chem. 2015, 54, 3281–3289. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Bajpai, P. Synthesis of copper sulfide nanoparticles: pH dependent phase stabilization. Nano-Struct. Nano-Objects 2017, 10, 151–158. [Google Scholar] [CrossRef]
- Gorle, G.; Bathinapatla, A.; Chen, Y.-Z.; Ling, Y.-C. Near infrared light activatable PEI-wrapped bismuth selenide nanocomposites for photothermal/photodynamic therapy induced bacterial inactivation and dye degradation. RSC Adv. 2018, 8, 19827–19834. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Yao, Y.; Zhang, S.; Gu, Z. Reverse micelle-based water-soluble nanoparticles for simultaneous bioimaging and drug delivery. Org. Biomol. Chem. 2017, 15, 3232–3238. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, H.; Liu, C.; Guan, Y.; Xu, D.; Zhang, J.; Su, Y.; Zhao, L.; Luo, J. Biodegradable multi-blocked polyurethane micelles for intracellular drug delivery: The effect of disulfide location on the drug release profile. RSC Adv. 2016, 6, 9082–9089. [Google Scholar] [CrossRef]
- Barras, A.; Boussekey, L.; Courtade, E.; Boukherroub, R. Hypericin-loaded lipid nanocapsules for photodynamic cancer therapy in vitro. Nanoscale 2013, 5, 10562–10572. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, X.; Xiong, J.; Peng, S.; Huang, W.; Joshi, R.; Cai, Y.; Liu, Y.; Li, R.; Yuan, K.; et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Carloni, P.; Damiani, E.; Greci, L.; Stipa, P.; Tanfani, F.; Tartaglini, E.; Woźniak, M. On the use of 1,3-diphenylisobenzofuran (DPBF). Reactions with carbon and oxygen centered radicals in model and natural systems. Res. Chem. Intermed. 1993, 19, 395–405. [Google Scholar] [CrossRef]
- Jijie, R.; Barras, A.; Bouckaert, J.; Dumitrascu, N.; Szunerits, S.; Boukherroub, R. Enhanced antibacterial activity of carbon dots functionalized with ampicillin combined with visible light triggered photodynamic effects. Colloids Surf. B Biointerfaces 2018, 170, 347–354. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swaidan, A.; Ghayyem, S.; Barras, A.; Addad, A.; Szunerits, S.; Boukherroub, R. Enhanced Antibacterial Activity of CuS-BSA/Lysozyme under Near Infrared Light Irradiation. Nanomaterials 2021, 11, 2156. https://doi.org/10.3390/nano11092156
Swaidan A, Ghayyem S, Barras A, Addad A, Szunerits S, Boukherroub R. Enhanced Antibacterial Activity of CuS-BSA/Lysozyme under Near Infrared Light Irradiation. Nanomaterials. 2021; 11(9):2156. https://doi.org/10.3390/nano11092156
Chicago/Turabian StyleSwaidan, Abir, Sena Ghayyem, Alexandre Barras, Ahmed Addad, Sabine Szunerits, and Rabah Boukherroub. 2021. "Enhanced Antibacterial Activity of CuS-BSA/Lysozyme under Near Infrared Light Irradiation" Nanomaterials 11, no. 9: 2156. https://doi.org/10.3390/nano11092156
APA StyleSwaidan, A., Ghayyem, S., Barras, A., Addad, A., Szunerits, S., & Boukherroub, R. (2021). Enhanced Antibacterial Activity of CuS-BSA/Lysozyme under Near Infrared Light Irradiation. Nanomaterials, 11(9), 2156. https://doi.org/10.3390/nano11092156