Trivalent Cations Detection of Magnetic-Sensitive Microcapsules by Controlled-Release Fluorescence Off-On Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Magnetic Nanoparticles (MNPs)—Fe3O4
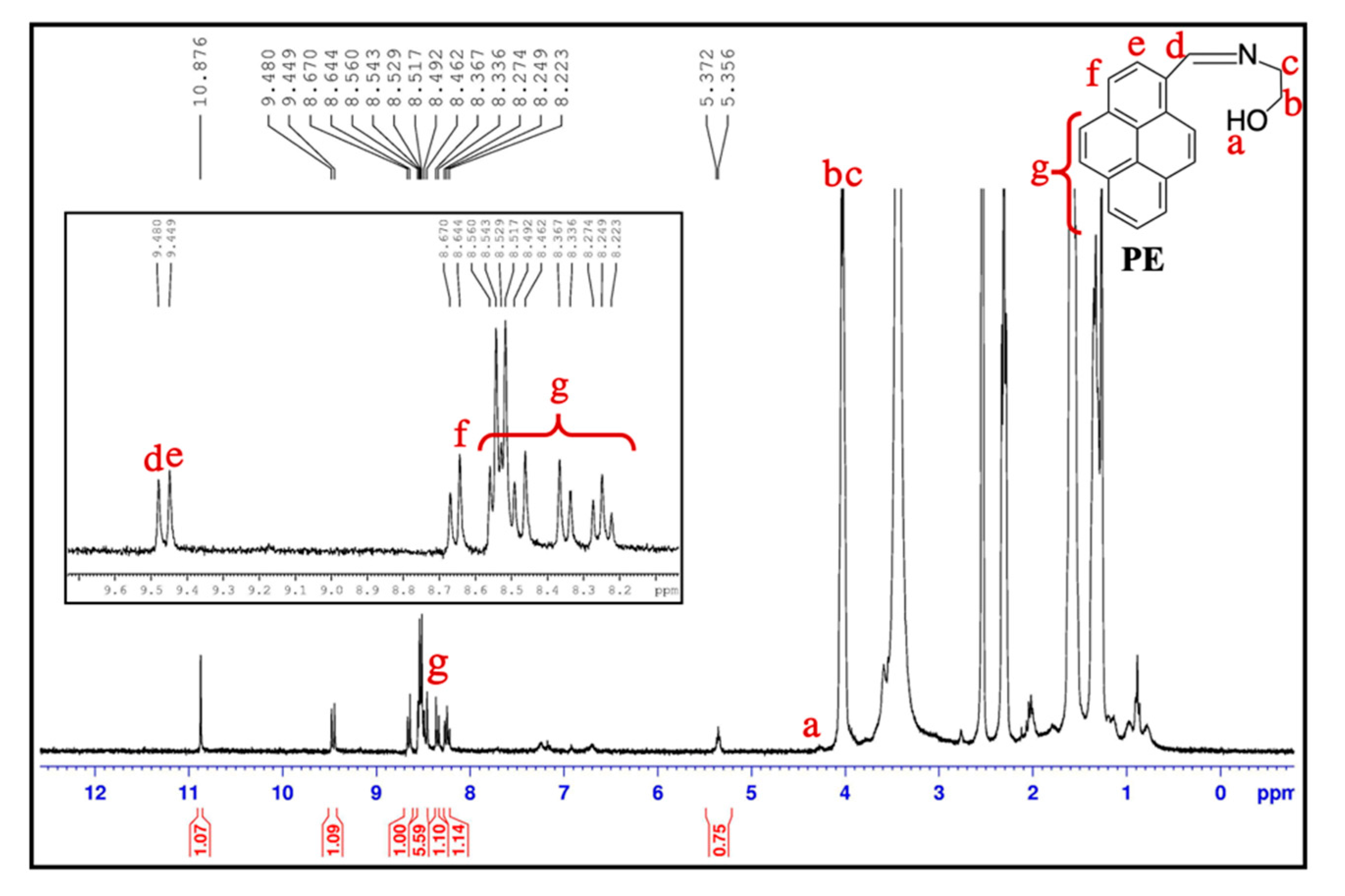
2.3. Preparation of Compound PE (2-((Pyrene-1-Ylmethylene)Amino)Ethanol)
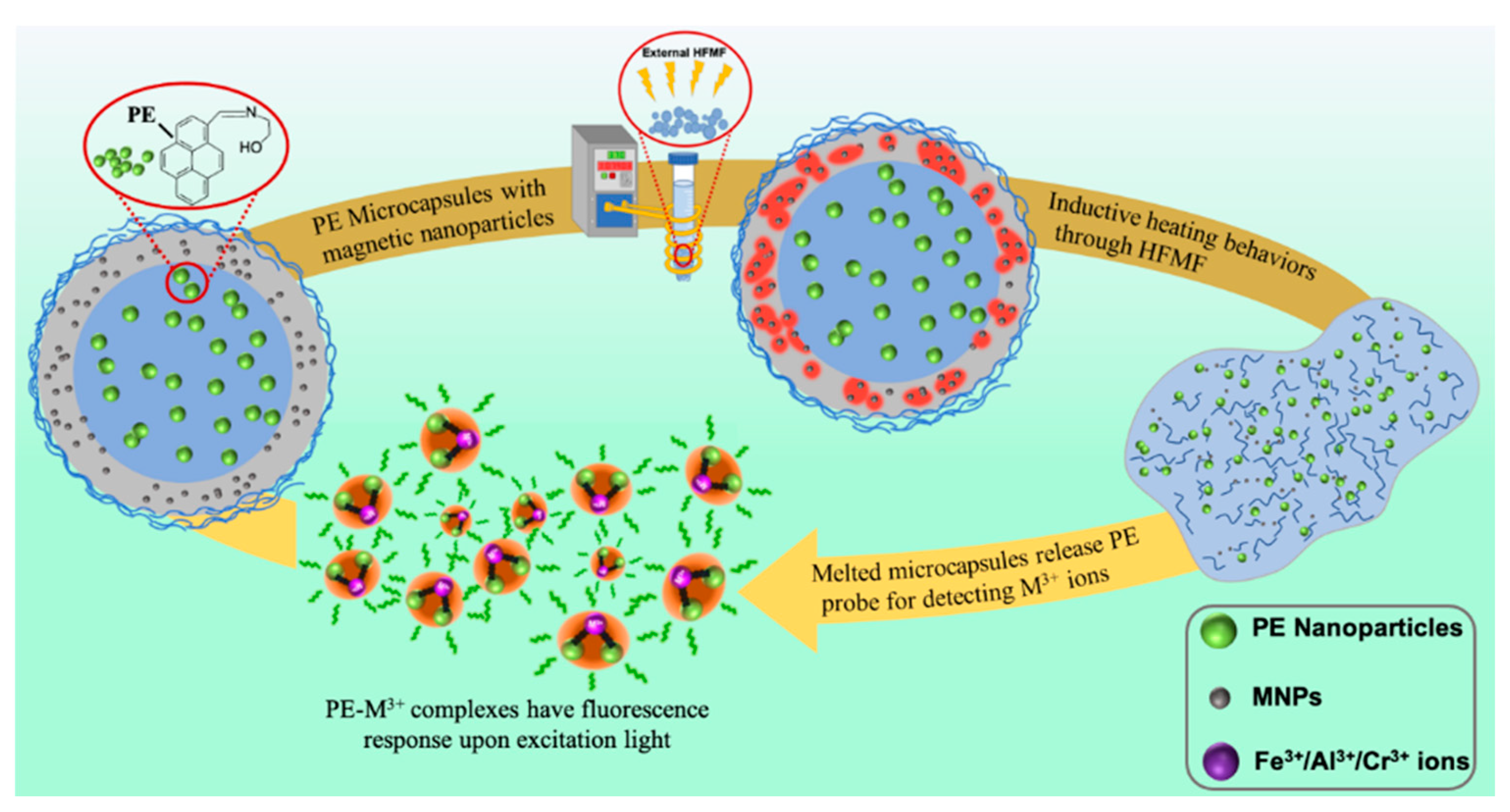
2.4. Preparation of Magnetic-Sensitive PE Microcapsules
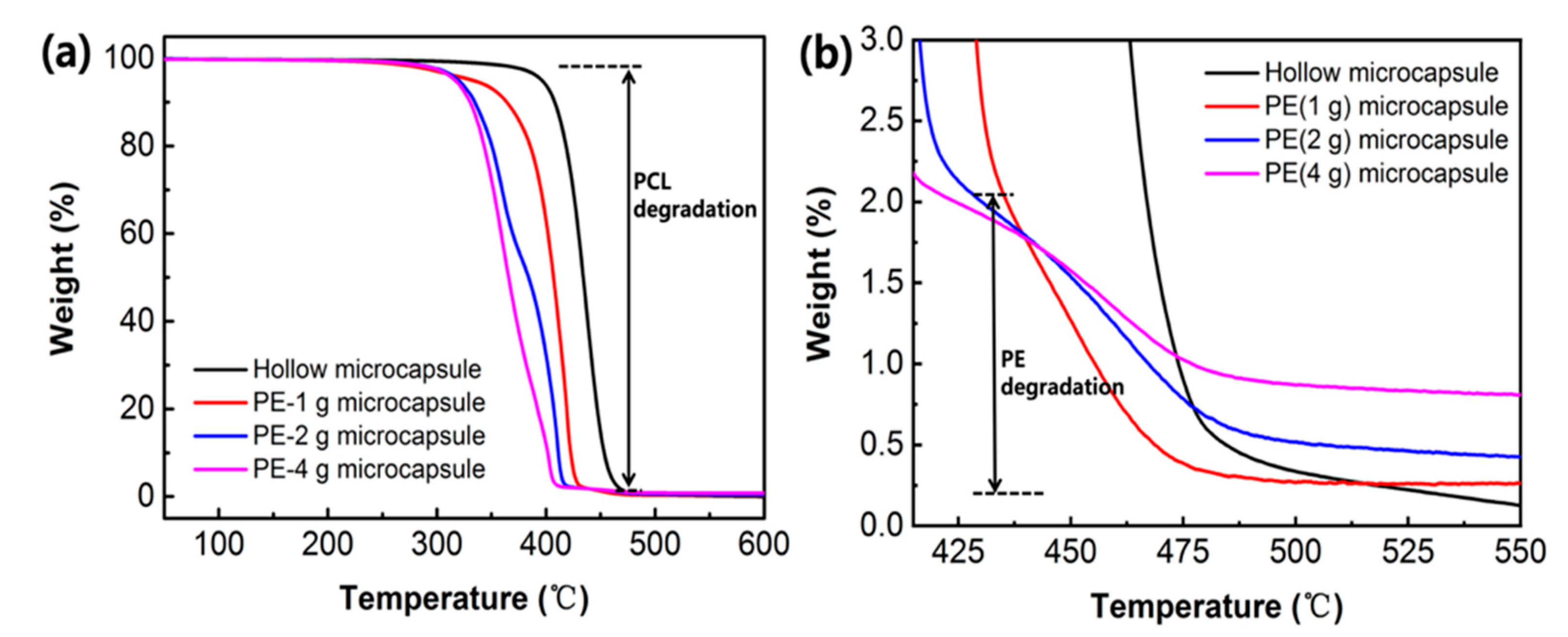
2.5. Characterization of PE/MNPs Microcapsules
2.6. PE Molecule Loading and Release
3. Results and Discussion
3.1. Synthesis and Characterization of PE/MNPs Microcapsules
3.2. Magnetic Properties of the Microcapsules
3.3. PE Release Behaviors with High-Frequency Magnetic Field (HFMF) Treatment
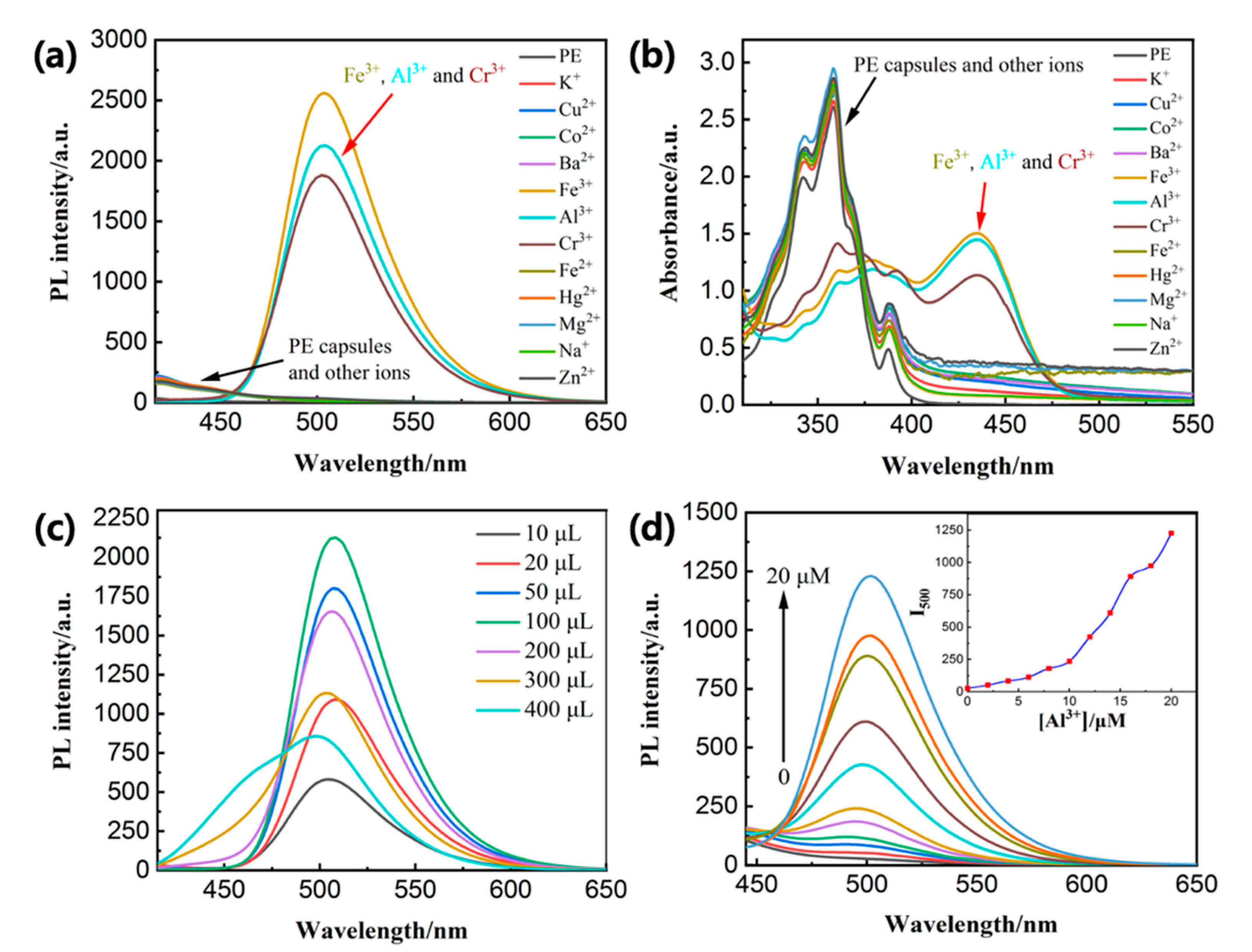
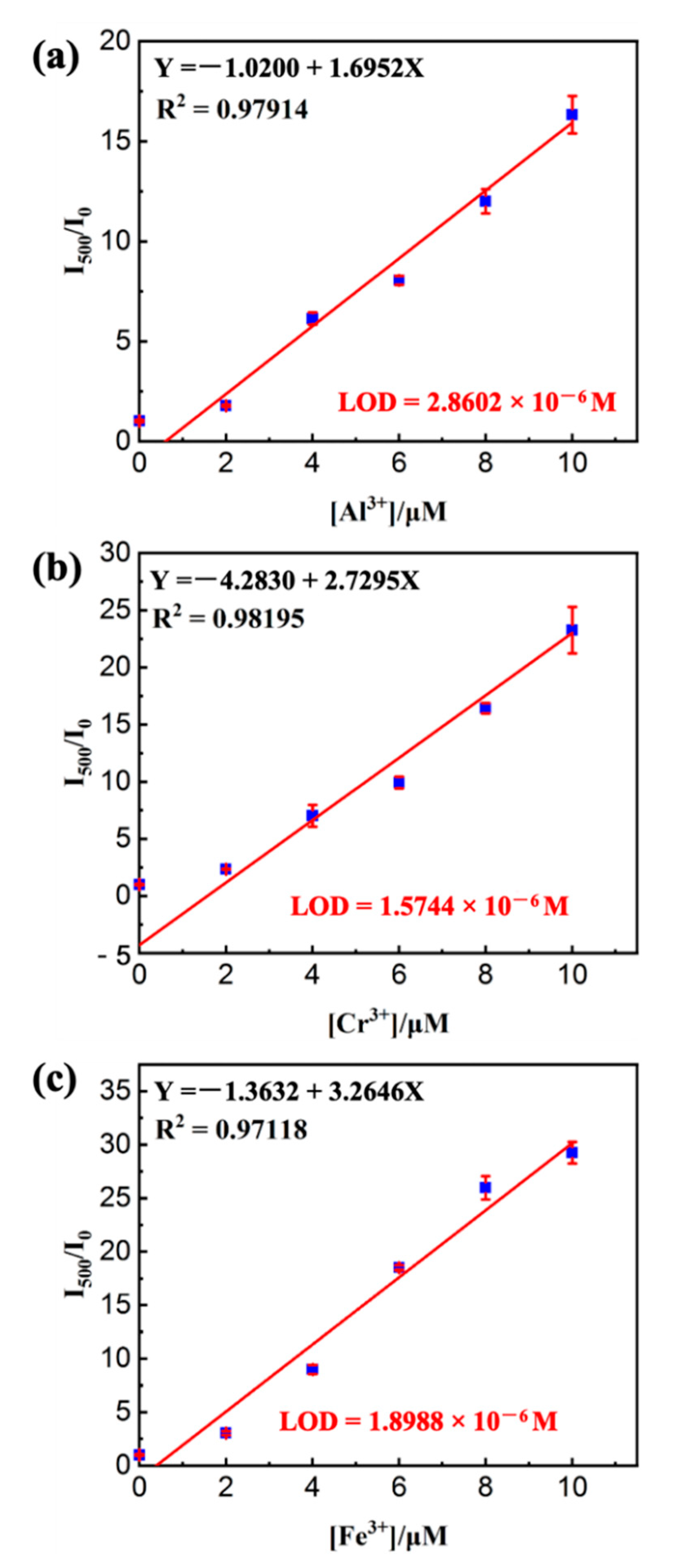
3.4. Sensor Titrations of M3+ (M = Fe/Cr/Al) under HFMF Stimulus
3.5. Comparison of Other Probes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, A.; Xu, W.; Ma, Z.; Jia, X. An ESIPT-Based Fluorescent Switch with AIEE, Solvatochromism, Mechanochromism and Photochromism. Mater. Chem. Front. 2019, 3, 620–625. [Google Scholar] [CrossRef]
- Shyamal, M.; Mazumdar, P.; Maity, S.; Samanta, S.; Sahoo, G.P.; Misra, A. Highly Selective Turn-On Fluorogenic Chemosensor for Robust Quantification of Zn(II) Based on Aggregation Induced Emission Enhancement Feature. ACS Sens. 2016, 1, 739–747. [Google Scholar] [CrossRef]
- Ichiura, H.; Morikawa, M.; Fujiwara, K. Preparation of Microcapsules That Produce Color in Response to Humidity for Use in Intelligent Functional Paper. J. Mater. Sci. 2005, 40, 1987–1991. [Google Scholar] [CrossRef]
- Chang, C.; Wang, F.; Qiang, J.; Zhang, Z.; Chen, Y.; Zhang, W.; Wang, Y.; Chen, X. Benzothiazole-Based Fluorescent Sensor for Hypochlorite Detection and Its Application for Biological Imaging. Sens. Actuators B Chem. 2017, 243, 22–28. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Patel, A.A.; Sukhorukov, G.B.; Lvov, Y.M. Nanoassembly of Biodegradable Microcapsules for DNA Encasing. J. Am. Chem. Soc. 2004, 126, 3374–3375. [Google Scholar] [CrossRef] [PubMed]
- Su, J.F.; Wang, L.X.; Ren, L. Preparation and Characterization of Double-MF Shell MicroPCMs Used in Building Materials. J. Appl. Polym. Sci. 2005, 97, 1755–1762. [Google Scholar] [CrossRef]
- Guarnido, I.L.; Routh, A.F.; Mantle, M.D.; Serrano, M.F.; Marr, P.C. Ionic Liquid Microcapsules: Formation and Application of Polystyrene Microcapsules with Ionic Liquid Cores. ACS Sustain. Chem. Eng. 2019, 7, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhao, Z.; Liu, Y.; Shao, C.; Bian, F.; Zhao, Y. Biomimetic Enzyme Cascade Reaction System in Microfluidic Electrospray Microcapsules. Sci. Adv. 2018, 4, eaat2816. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huang, L.; Li, Z.; Ma, G.; Zhou, Y.; Han, G. Illuminating Cell Signaling with Near-Infrared Light-Responsive Nanomaterials. ACS Nano. 2016, 10, 3881–3885. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Anderson, D.G.; Chen, X.; Chow, E.K.; Ho, D.; Kabanov, A.V.; Karp, J.M.; Kataoka, K.; Mirkin, C.A.; Petrosko, S.H.; et al. Accelerating the Translation of Nanomaterials in Biomedicine. ACS Nano. 2015, 9, 6644–6654. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Xiong, L.; Wang, S.; Li, S.; Li, Y.; Yang, G. Fluorescent Temperature Sensing Using Triarylboron Compounds and Microcapsules for Detection of a Wide Temperature Range on the Micro- and Macroscale. Adv. Funct. Mater. 2013, 23, 340–345. [Google Scholar] [CrossRef]
- Ravanfar, R.; Celli, G.B.; Abbaspourrad, A. Controlling the Release from Enzyme-Responsive Microcapsules with a Smart Natural Shell. ACS Appl. Mater. Interfaces 2018, 10, 6046–6053. [Google Scholar] [CrossRef]
- de Medeiros, J.A.S.; Blick, A.P.; Galindo, M.V.; Alvim, I.D.; Yamashita, F.; Ueno, C.T.; Shirai, M.A.; Grosso, C.R.F.; Corradini, E.; Sakanaka, L.S. Incorporation of Oregano Essential Oil Microcapsules in Starch-Poly (Butylene Adipate Co-Terephthalate) (PBAT) Films. Macromol. Symp. 2019, 383, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zizi, N.; Ben Abdelkader, M.; Chevalier, Y.; Majdoub, M. New β-Cyclodextrin-Based Microcapsules for Textiles Uses. Fibers. Polym. 2019, 20, 683–689. [Google Scholar]
- Jin, Y.; Wang, J.; Ke, H.; Wang, S.; Dai, Z. Graphene Oxide Modified PLA Microcapsules Containing Gold Nanoparticles for Ultrasonic/CT Bimodal Imaging Guided Photothermal Tumor Therapy. Biomaterials 2013, 34, 4794–4802. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhou, J.; Hu, Y.; Lin, Z.; Ma, Y.; Richardson, J.J.; Caruso, F. Polyphenol-Based Nanoparticles for Intracellular Protein Delivery via Competing Supramolecular Interactions. ACS Nano 2020, 14, 12972–12981. [Google Scholar] [CrossRef]
- Wang, Z.; Möhwald, H.; Gao, C. Nanotubes Protruding from Poly(Allylamine Hydrochloride)-Graft-Pyrene Microcapsules. ACS Nano 2011, 5, 3930–3936. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, G.; Colombo, A.; Dragonetti, C.; Levi, M.; Turri, S.; Griffini, G. Fluorescent Probes Based on Chemically-Stable Core/Shell Microcapsules for Visual Microcrack Detection. Sens. Actuators B Chem. 2017, 248, 35–42. [Google Scholar] [CrossRef]
- Li, W.; Matthews, C.C.; Yang, K.; Odarczenko, M.T.; White, S.R.; Sottos, N.R. Autonomous Indication of Mechanical Damage in Polymeric Coatings. Adv. Mater. 2016, 28, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, M.; Sánchez-González, L.; Ben Messaoud, G.; Desobry, S. Design of Microcapsules Containing Lactococcus Lactis Subsp. Lactis in Alginate Shell and Xanthan Gum with Nutrients Core. LWT-Food Sci. Technol. 2016, 68, 446–453. [Google Scholar] [CrossRef]
- Hong, K.; Park, S. Melamine Resin Microcapsules Containing Fragrant Oil: Synthesis and Characterization. Mater. Chem. Phys. 1999, 58, 128–131. [Google Scholar] [CrossRef]
- Akamatsu, K.; Yamaguchi, T. Novel Preparation Method for Obtaining PH-Responsive Core-Shell Microcapsule Reactors. Ind. Eng. Chem. Res. 2007, 46, 124–130. [Google Scholar] [CrossRef]
- Li, M.; Rouaud, O.; Poncelet, D. Microencapsulation by Solvent Evaporation: State of the Art for Process Engineering Approaches. Int. J. Pharm. 2008, 363, 26–39. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xie, Y.; Li, J.; Peng, Z.H.; Sheinin, Y.; Zhou, J.; Oupický, D. Tumor-Penetrating Nanoparticles for Enhanced Anticancer Activity of Combined Photodynamic and Hypoxia-Activated Therapy. ACS Nano 2017, 11, 2227–2238. [Google Scholar] [CrossRef]
- Pavlov, A.M.; Saez, V.; Cobley, A.; Graves, J.; Sukhorukov, G.B.; Mason, T.J. Controlled Protein Release from Microcapsules with Composite Shells Using High Frequency Ultrasound-Potential for in Vivo Medical Use. Soft Matter 2011, 7, 4341–4347. [Google Scholar] [CrossRef]
- Datta, S.S.; Abbaspourrad, A.; Amstad, E.; Fan, J.; Kim, S.H.; Romanowsky, M.; Shum, H.C.; Sun, B.; Utada, A.S.; Windbergs, M.; et al. 25th Anniversary Article: Double Emulsion Templated Solid Microcapsules: Mechanics and Controlled Release. Adv. Mater. 2014, 26, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Fu, C.; Tan, L.; Liu, T.; Mao, J.; Ren, X.; Su, H.; Long, D.; Chai, Q.; Huang, Z.; et al. Imaging-Guided Synergetic Therapy of Orthotopic Transplantation Tumor by Superselectively Arterial Administration of Microwave-Induced Microcapsules. Biomaterials 2017, 133, 144–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.Y.; Liu, T.Y.; Hardiansyah, A.; Lee, C.F.; Wang, M.S.; Chiu, W.Y. Self-Assembly Behaviors of Thermal- and PH- Sensitive Magnetic Nanocarriers for Stimuli-Triggered Release. Nanoscale Res. Lett. 2014, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Hu, S.; Liu, K.; Shaiu, R.; Liu, D.; Chen, S. Instantaneous Drug Delivery of Magnetic/thermally Sensitive Nanospheres by a High-Frequency Magnetic Field Instantaneous Drug Delivery of Magnetic/thermally Sensitive Nanospheres by a High-Frequency Magnetic Field. Drug Deliv. 2008, 13306–13311. [Google Scholar]
- Cui, X.; Guan, X.; Zhong, S.; Chen, J.; Zhu, H.; Li, Z.; Xu, F.; Chen, P.; Wang, H. Multi-Stimuli Responsive Smart Chitosan-Based Microcapsules for Targeted Drug Delivery and Triggered Drug Release. Ultrason. Sonochem. 2017, 38, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Hardiansyah, A.; Yang, M.C.; Liu, T.Y.; Kuo, C.Y.; Huang, L.Y.; Chan, T.Y. Hydrophobic Drug-Loaded PEGylated Magnetic Liposomes for Drug-Controlled Release. Nanoscale Res. Lett. 2017, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Ao, L.; Wu, C.; Liu, K.; Wang, W.; Fang, L.; Huang, L.; Su, W. Polydopamine-Derivated Hierarchical Nanoplatforms for Efficient Dual-Modal Imaging-Guided Combination in Vivo Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 12544–12552. [Google Scholar] [CrossRef]
- Liu, T.Y.; Hu, S.H.; Liu, K.H.; Liu, D.M.; Chen, S.Y. Study on Controlled Drug Permeation of Magnetic-Sensitive Ferrogels: Effect of Fe3O4 and PVA. J. Control. Release 2008, 126, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Butt, H.J.; Landfester, K.; Bannwarth, M.B.; Wooh, S.; Thérien-Aubin, H. Shaping the Assembly of Superparamagnetic Nanoparticles. ACS Nano 2019, 13, 3015–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.Y.; Zhu, J.; Zhang, D.; Lattery, D.M.; Li, M.; Wang, J.P.; Wang, X. Time-Resolved Magneto-Optical Kerr Effect of Magnetic Thin Films for Ultrafast Thermal Characterization. J. Phys. Chem. Lett. 2016, 7, 2328–2332. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Liu, T.Y.; Liu, D.M.; Chen, S.Y. Controlled Pulsatile Drug Release from a Ferrogel by a High-Frequency Magnetic Field. Macromolecules 2007, 40, 6786–6788. [Google Scholar] [CrossRef] [Green Version]
- Niether, C.; Faure, S.; Bordet, A.; Deseure, J.; Chatenet, M.; Carrey, J.; Chaudret, B.; Rouet, A. Improved Water Electrolysis Using Magnetic Heating of FeC-Ni Core-Shell Nanoparticles. Nat. Energy 2018, 3, 476–483. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Joneidi-Yekta, H.; Mohseni, M. Adsorption of Anionic and Cationic Dyes from Aqueous Solution Using Gelatin-Based Magnetic Nanocomposite Beads Comprising Carboxylic Acid Functionalized Carbon Nanotube. Chem. Eng. J. 2017, 308, 1133–1144. [Google Scholar] [CrossRef]
- Salimi, S.; Babra, T.S.; Dines, G.S.; Baskerville, S.W.; Hayes, W.; Greenland, B.W. Composite Polyurethane Adhesives That Debond-on-Demand by Hysteresis Heating in an Oscillating Magnetic Field. Eur. Polym. J. 2019, 121, 109264. [Google Scholar] [CrossRef]
- Verstraeten, S.V.; Aimo, L.; Oteiza, P.I. Aluminium and Lead: Molecular Mechanisms of Brain Toxicity. Arch. Toxicol. 2008, 82, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D. Aluminium, Antiperspirants and Breast Cancer. J. Inorg. Biochem. 2005, 99, 1912–1919. [Google Scholar] [CrossRef]
- Yan, L.; Li, X.; Li, J. A Novel Turn-on Fluorescent Probe Based on Coumarin Schiff’s Base for Multichannel Monitoring of Al3+, Hg2+ and ClO− in Different Solutions and Its Applications. ChemistrySelect 2018, 3, 10157–10163. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Y.; Zhang, L.; Sun, D.; Liu, F.; Meng, Q.; Wang, R.; Sun, D. A Tubular Europium-Organic Framework Exhibiting Selective Sensing of Fe3+ and Al3+ over Mixed Metal Ions. Chem. Commun. 2013, 49, 11557–11559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apollaro, C.; Fuoco, I.; Brozzo, G.; De Rosa, R. Release and Fate of Cr(VI) in the Ophiolitic Aquifers of Italy: The Role of Fe(III) as a Potential Oxidant of Cr(III) Supported by Reaction Path Modelling. Sci. Total Environ. 2019, 660, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Ye, S.L.; Han, Y.H.; Shi, X.X.; Chen, D.L.; Li, M. Biosorption and Bioaccumulation of Chromate from Aqueous Solution by a Newly Isolated: Bacillus Mycoides Strain 200AsB1. RSC Adv. 2016, 6, 101153–101161. [Google Scholar] [CrossRef]
- Simon, T.; Shellaiah, M.; Srinivasadesikan, V.; Lin, C.C.; Ko, F.H.; Sun, K.W.; Lin, M.C. A Simple Pyrene Based AIEE Active Schiff Base Probe for Selective Naked Eye and Fluoresence Off-On Detection of Trivalent Cations with Live Cell Application. Sens. Actuators B Chem. 2016, 231, 18–29. [Google Scholar] [CrossRef]
- Shin, M.J.; Shin, Y.J.; Hwang, S.W.; Shin, J.S. Microencapsulation of Imidazole Curing Agent by Solvent Evaporation Method Using W/O/W Emulsion. J. Appl. Polym. Sci. 2013, 129, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xu, C.; Liu, J.; Fang, X.; Zhang, Z. Optical Absorption Property and Photo-Thermal Conversion Performance of Graphene Oxide/Water Nanofluids with Excellent Dispersion Stability. Sol. Energy 2017, 148, 17–24. [Google Scholar] [CrossRef]
- Guo, X.M.; Guo, B.; Zhang, Q.; Sun, X. Absorption of 10-Hydroxycamptothecin on Fe3O4 Magnetite Nanoparticles with Layer-by-Layer Self-Assembly and Drug Release Response. Dalt. Trans. 2011, 40, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.; Song, F.; Wei, G.; Cheng, Y.; Zhu, C. A Highly Selective and Sensitive Polymer-Based Off-On Fluorescent Sensor for Hg2+ Detection Incorporating Salen and Perylenyl Moieties. J. Mater. Chem. 2012, 22, 478–482. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, Y.; Zhou, Y.; Liu, M.; Xu, W.; Bian, B.; Tao, Z.; Xiao, X. A Highly Selective Fluorescent Chemosensor Probe for Detection of Fe3+ and Ag+ Based on Supramolecular Assembly of Cucurbit Uril with a Pyrene Derivative. Dye. Pigment. 2020, 176, 108235. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, X.; Cao, Y.; Xin, Y.; Ding, L. Fluorescent Binary Ensemble Based on Pyrene Derivative and Sodium Dodecyl Sulfate Assemblies as a Chemical Tongue for Discriminating Metal Ions and Brand Water. ACS Sens. 2017, 2, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.; Roy, M.; Bergamini, G.; Ceroni, P.; Gingras, M. Highly Emissive Water-Soluble Polysulfurated Pyrene-Based Chromophores as Dual Mode Sensors of Metal Ions. Chempluschem 2020, 85, 1481–1486. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, H.; Liu, H.B.; Liang, L.; Tao, J. Pyrene–Imidazole Conjugate as a Fluorescent Sensor for the Sequential Detection of Iron(III) and Histidine in Aqueous Solution. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 228, 117725. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wen, X.; Fan, Z. An AIE Active Pyrene Based Fluorescent Probe for Selective Sensing Hg2+ and Imaging in Live Cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117315. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Chakraborty, A.; Das, S. A Pyrene Based Fluorescent Turn on Chemosensor for Detection of Cu2+ Ions with Antioxidant Nature. J. Lumin. 2018, 199, 302–309. [Google Scholar] [CrossRef]
- Ruan, Z.; Shan, Y.; Gong, Y.; Wang, C.; Ye, F.; Qiu, Y.; Liang, Z.; Li, Z. Novel AIE-Active Ratiometric Fluorescent Probes for Mercury(II) Based on the Hg2+-Promoted Deprotection of Thioketal, and Good Mechanochromic Properties. J. Mater. Chem. C 2018, 6, 773–780. [Google Scholar] [CrossRef]
- Phapale, D.; Gaikwad, A.; Das, D. Selective Recognition of Cu (II) and Fe (III) Using a Pyrene Based Chemosensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 178, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Padghan, S.D.; Puyad, A.L.; Bhosale, R.S.; Bhosale, S.V.; Bhosale, S.V. A Pyrene Based Fluorescent Turn-on Chemosensor: Aggregation-Induced Emission Enhancement and Application towards Fe3+ and Fe2+ Recognition. Photochem. Photobiol. Sci. 2017, 16, 1591–1595. [Google Scholar] [CrossRef] [PubMed]




| Probe | Metal Ions | Source of Sample | LOD (μM) | Ref. |
|---|---|---|---|---|
| Aminopropyl−1-pyrenebutanamide cucurbit [10] uril | Fe3+ | Deionized water | 550 | [54] |
| Bis(2-picolyl)amine-modified pyrene derivative with sodium dodecyl sulfate | Fe3+, Al3+, Mg2+, Pb2+, Ca2+ and Ba2+ | Mineral Water Samples | 50 | [55] |
| 3,3′,3′′,3′′′-(pyrene-1,3,6,8-tetrayltetrakis(sulfanediyl))tetrabenzoic acid | Pb2+ | - | 0.2 | [56] |
| 1-(3,5-dihydropyren-1-yl)-2-((1-methyl-1,6-dihydropyrimidin-2-yl)thio)ethan-1-one | Fe3+ | Deionized water | 3.06 | [57] |
| (E)-1-(pyren-1-yl)-N-tritylmethanimine | Hg2+ | Deionized water | 0.4 | [58] |
| 1,5-dimethyl-2-phenyl-4-((pyren-1-ylmethylene)amino)-1H-pyrazol-3(2H)-one | Cu2+ | - | 2.5 | [59] |
| 1,2-bis(2-(4-(1,2,2-triphenylvinyl)phenyl)-1,3-dithiolan-2-yl)benzene | Hg2+ | Distilled water | 10 | [60] |
| 1-(pyren-1-yl)-N,N-bis-(pyridine-2-ylmethyl)methanamine | Cu2+, Fe3+ | Distilled water | 4.9 | [61] |
| 6-methoxy-N-(pyren-1-ylmethylene)benzo[d]thiazol-2-amine | Fe3+, Fe2+ | Distilled water | 2.61 | [62] |
| 2-((pyren-1-ylmethylene)amino)ethanol | Fe3+, Al3+ and Cr3+ | Distilled water | 0.106–0.117 | [49] |
| 2-((pyren-1-ylmethylene)amino)ethanol magnetic-sensitive microcapsules | Fe3+, Al3+ and Cr3+ | Tap water | 1.574–2.860 | This work |
| Sample | Added Fe3+/Cr3+/Al3+ Concentration (μM) | Detected Concentration a (μM) | Recovery b (%) | ||||
|---|---|---|---|---|---|---|---|
| Fe3+ | Cr3+ | Al3+ | Fe3+ | Cr3+ | Al3+ | ||
| 1 | 5 | 4.874 ± 0.136 | 4.933 ± 0.232 | 4.894 ± 0.184 | 97.4 | 98.7 | 97.9 |
| 2 | 20 | 19.739 ± 0.337 | 19.349 ± 0.748 | 18.972 ± 0.612 | 98.7 | 96.7 | 94.7 |
| 3 | 50 | 48.241 ± 0.619 | 49.721 ± 1.281 | 49.434 ± 0.941 | 96.5 | 99.4 | 98.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, B.-W.; Lin, C.-C.; Ko, F.-H. Trivalent Cations Detection of Magnetic-Sensitive Microcapsules by Controlled-Release Fluorescence Off-On Sensor. Nanomaterials 2021, 11, 1801. https://doi.org/10.3390/nano11071801
Du B-W, Lin C-C, Ko F-H. Trivalent Cations Detection of Magnetic-Sensitive Microcapsules by Controlled-Release Fluorescence Off-On Sensor. Nanomaterials. 2021; 11(7):1801. https://doi.org/10.3390/nano11071801
Chicago/Turabian StyleDu, Bo-Wei, Ching-Chang Lin, and Fu-Hsiang Ko. 2021. "Trivalent Cations Detection of Magnetic-Sensitive Microcapsules by Controlled-Release Fluorescence Off-On Sensor" Nanomaterials 11, no. 7: 1801. https://doi.org/10.3390/nano11071801
APA StyleDu, B.-W., Lin, C.-C., & Ko, F.-H. (2021). Trivalent Cations Detection of Magnetic-Sensitive Microcapsules by Controlled-Release Fluorescence Off-On Sensor. Nanomaterials, 11(7), 1801. https://doi.org/10.3390/nano11071801







