Eco-Friendly Synthesis of SnO2-Cu Nanocomposites and Evaluation of Their Peroxidase Mimetic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pod Extract
2.3. Biosynthesis of SnO2-Cu Nanocomposites
2.4. Characterization of SnO2-Cu Nanocomposites
2.5. Evaluation of Peroxidase Mimetic Activity
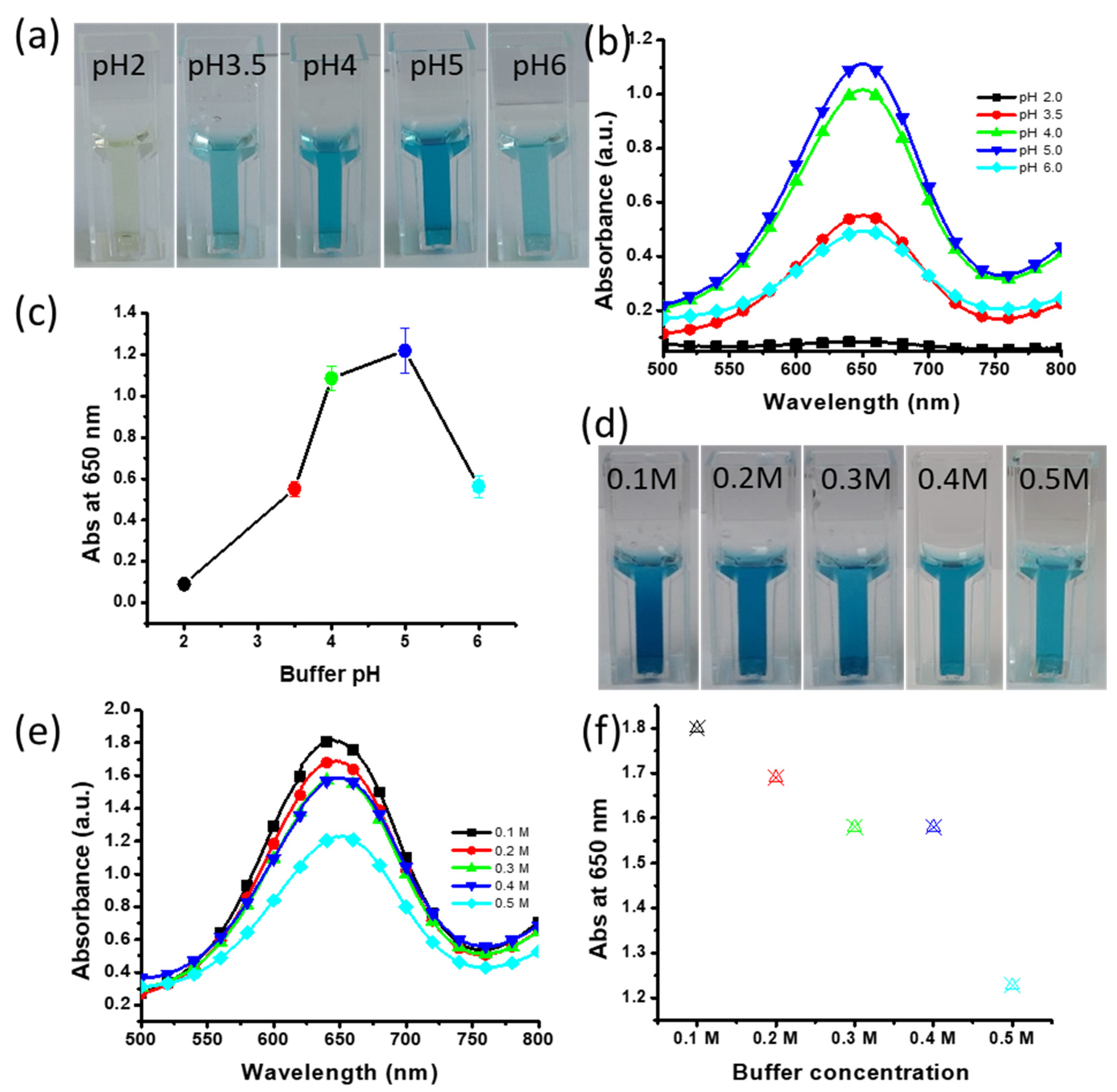
2.6. Effect of Buffer pH
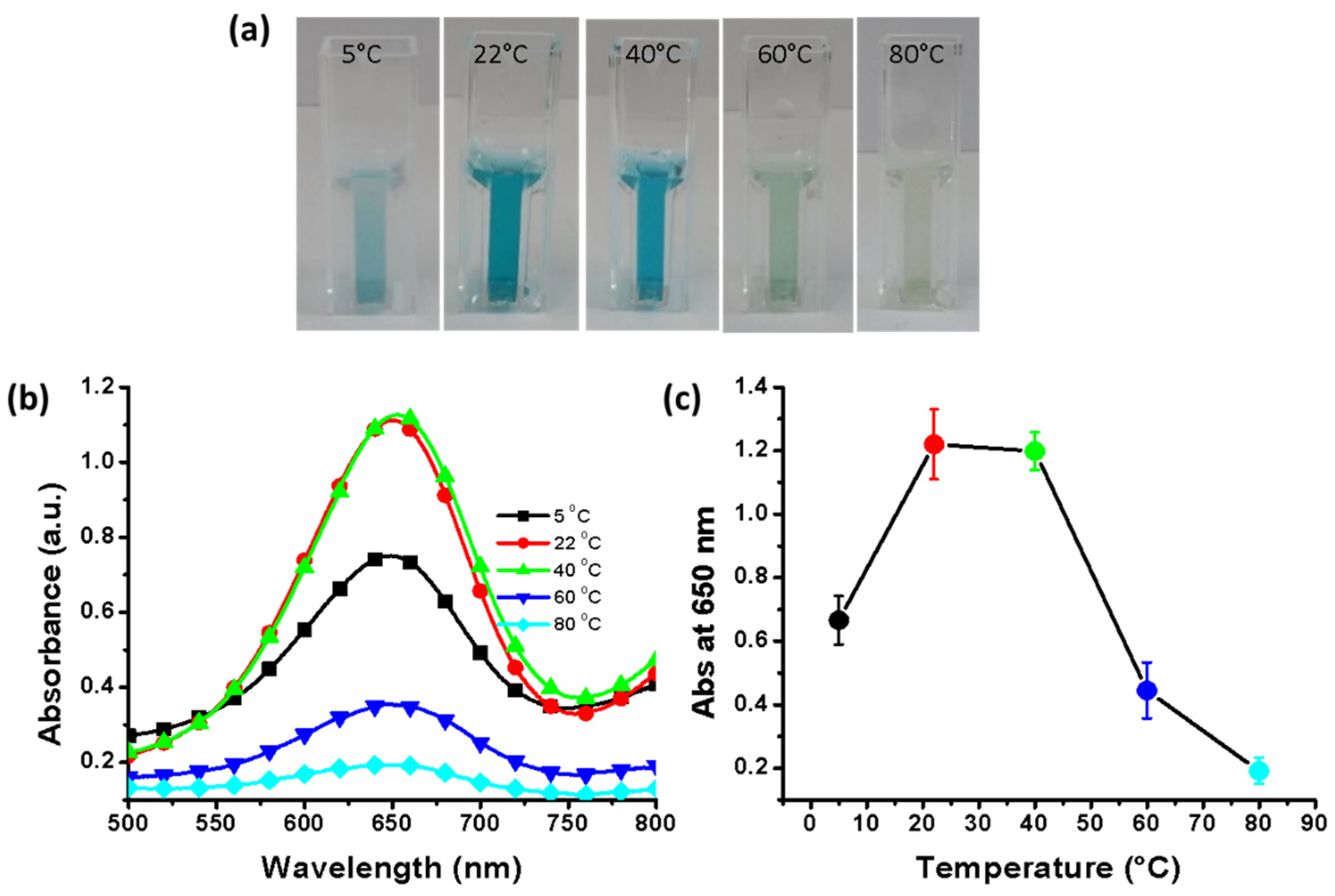
2.7. Effect of Temperature
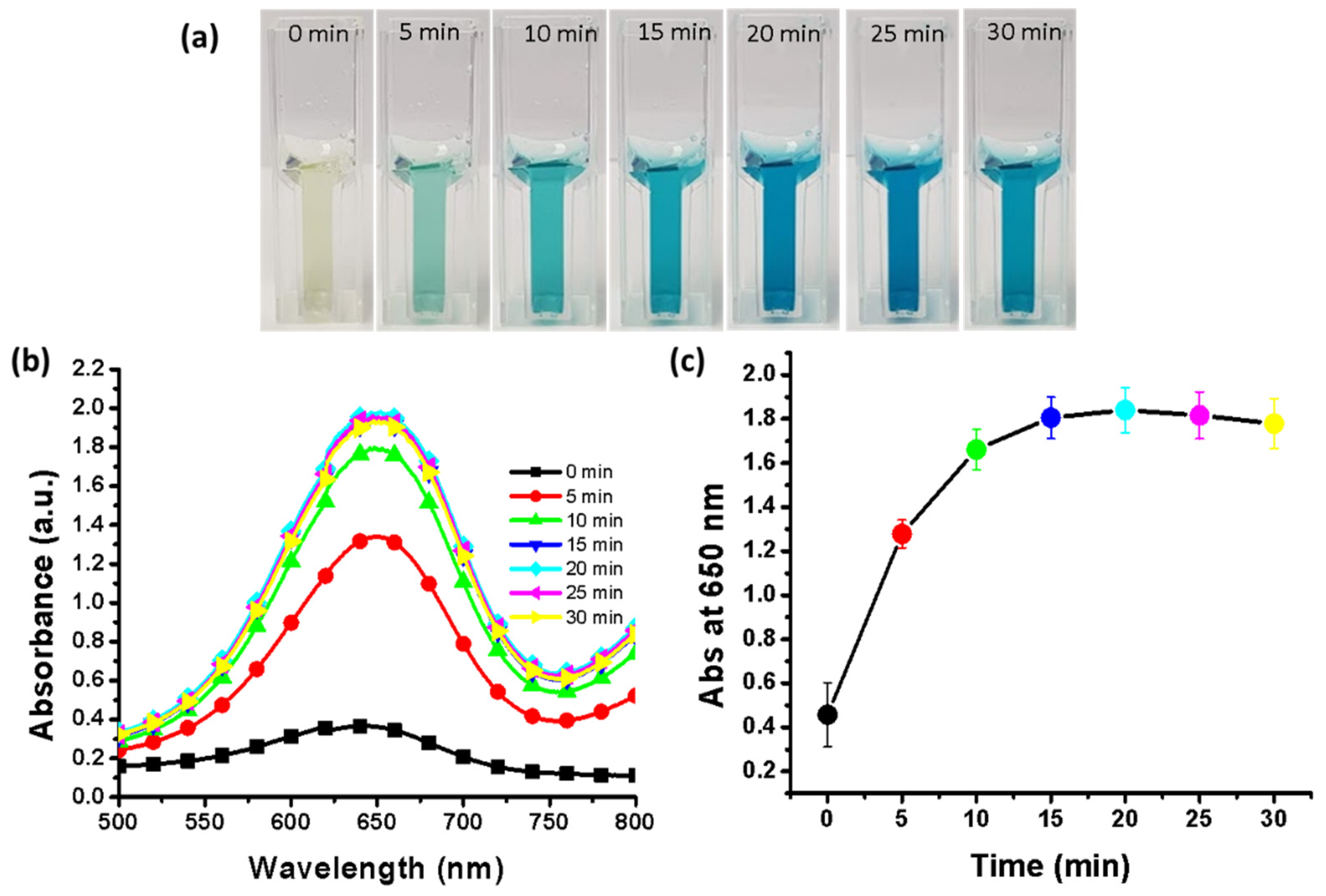
2.8. Effect of Assay Incubation Time
3. Results and Discussion
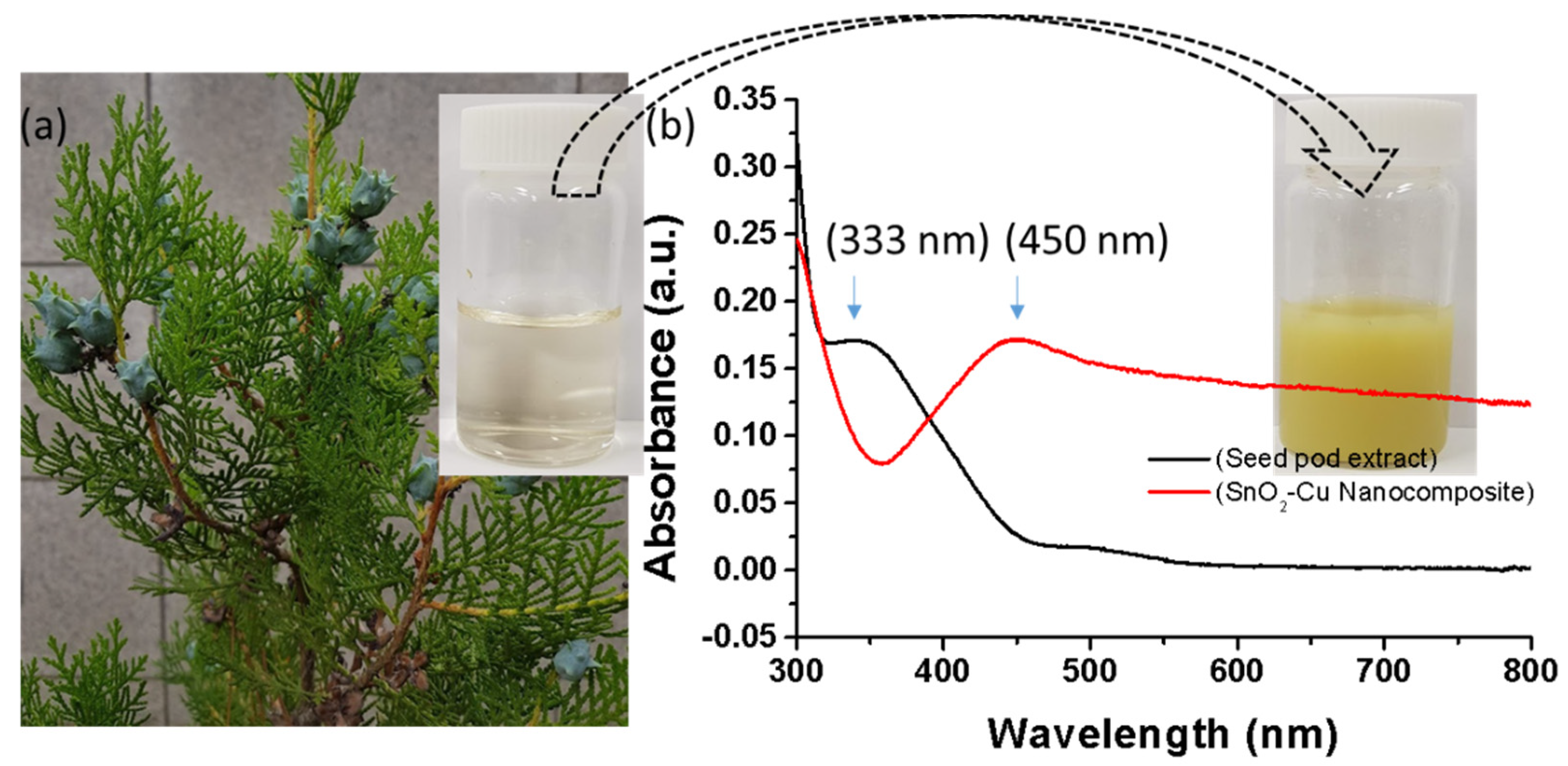
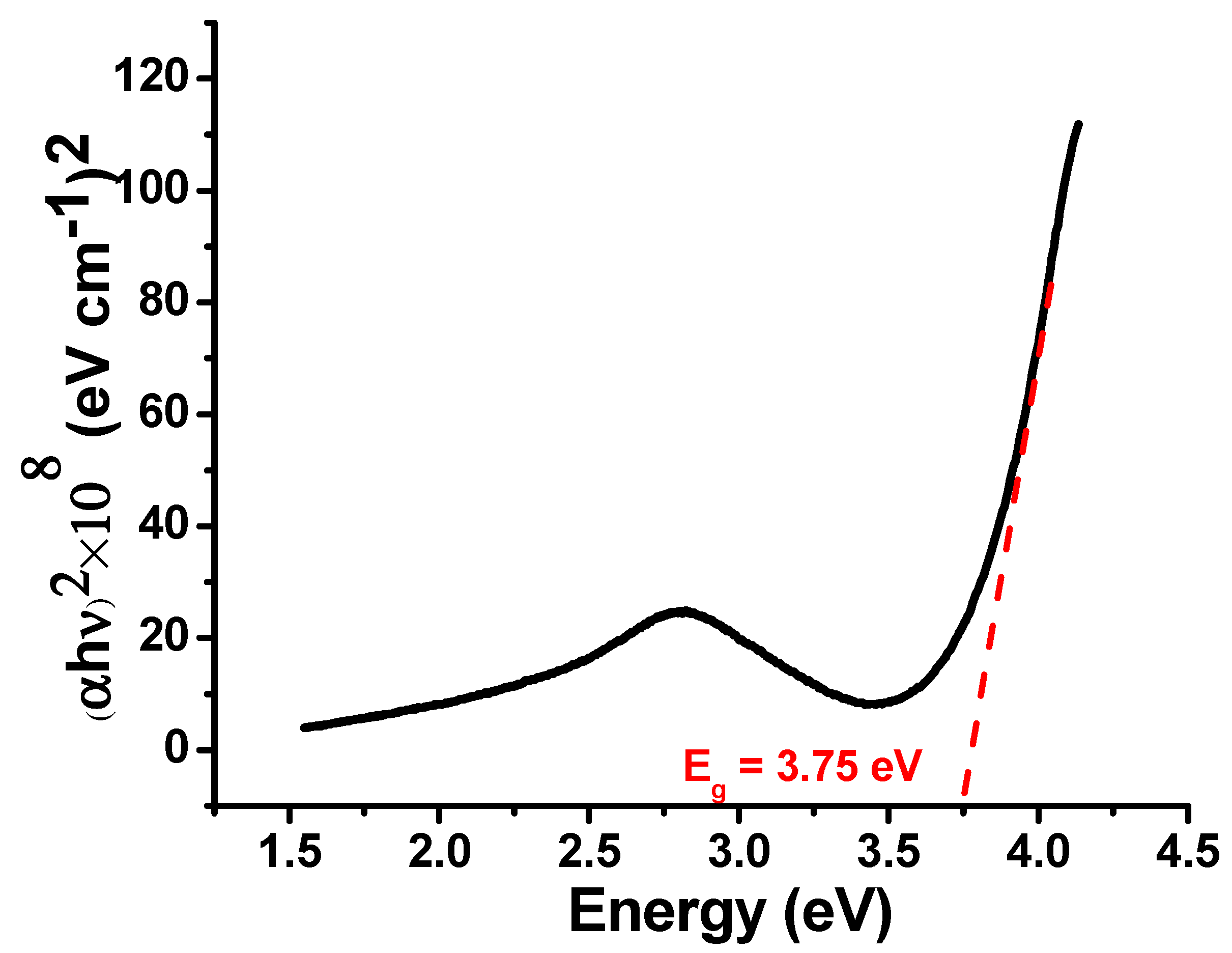
3.1. Ultraviolet–Visible (UV–vis) Spectroscopic Analysis
3.2. Dynamic Light Scattering (DLS) Analysis
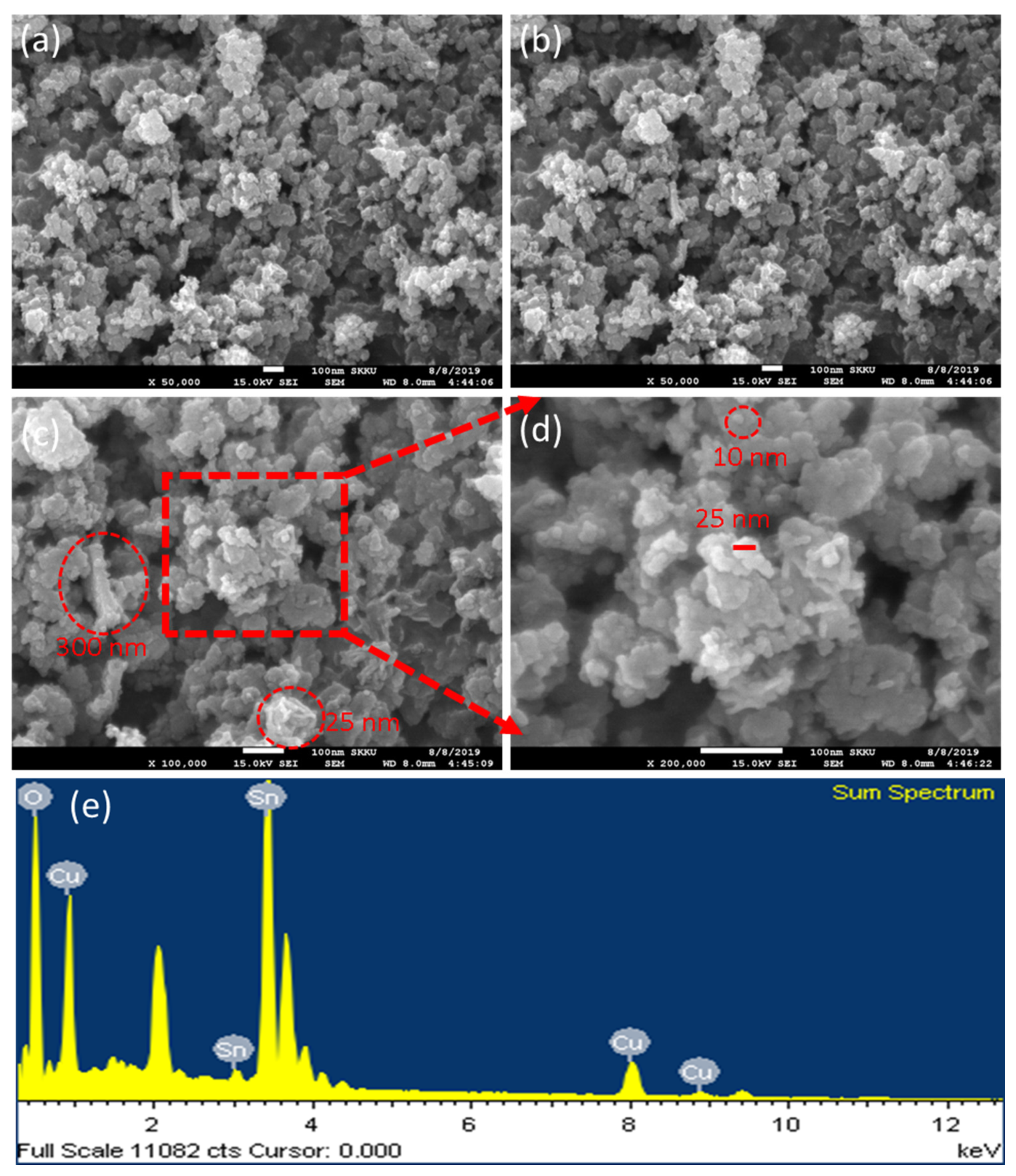
3.3. Field-Emission Scanning Electron Microscopy (FESEM) Analysis
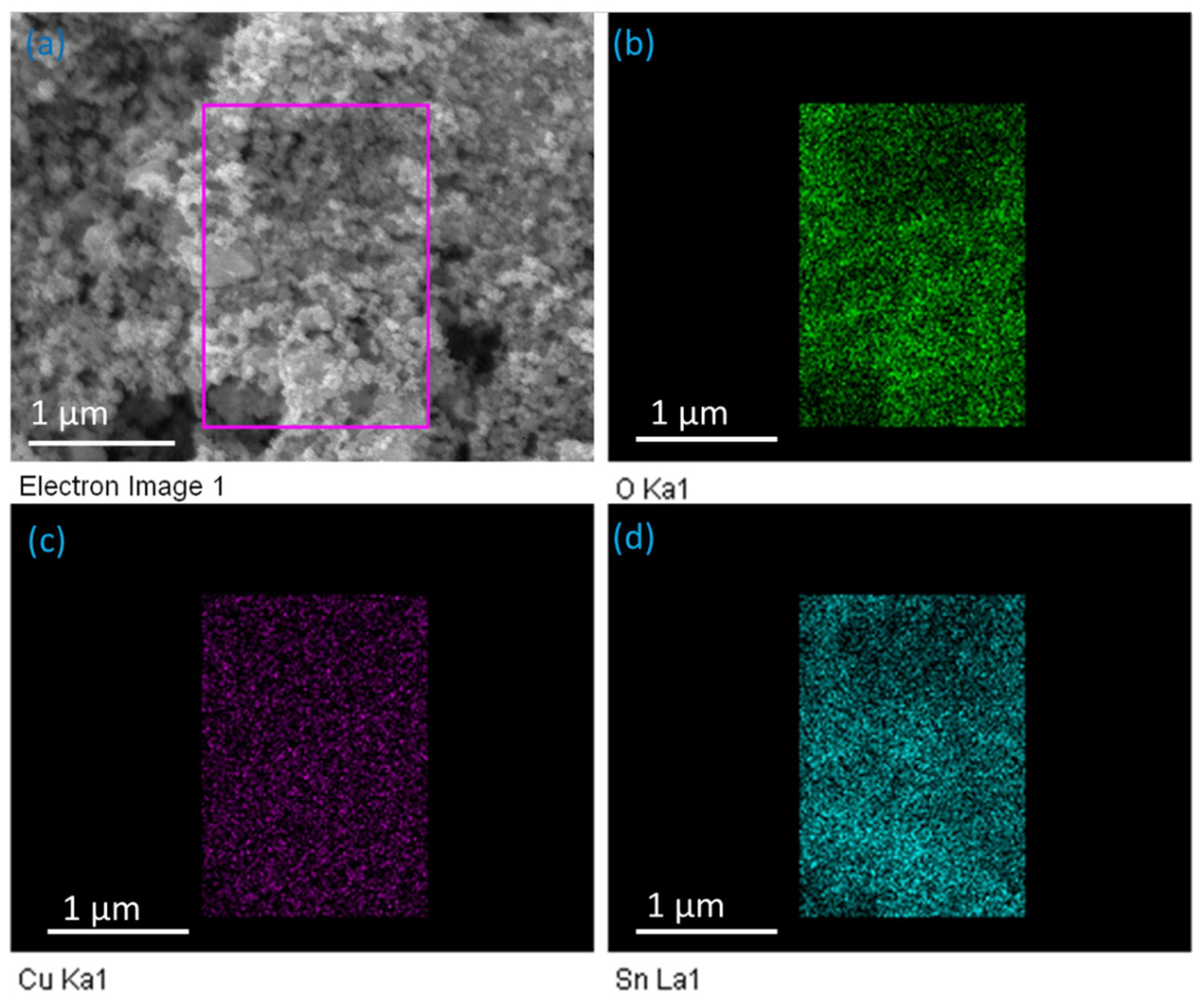
3.4. Energy-Dispersive X-ray Spectroscopy (EDX) Analysis
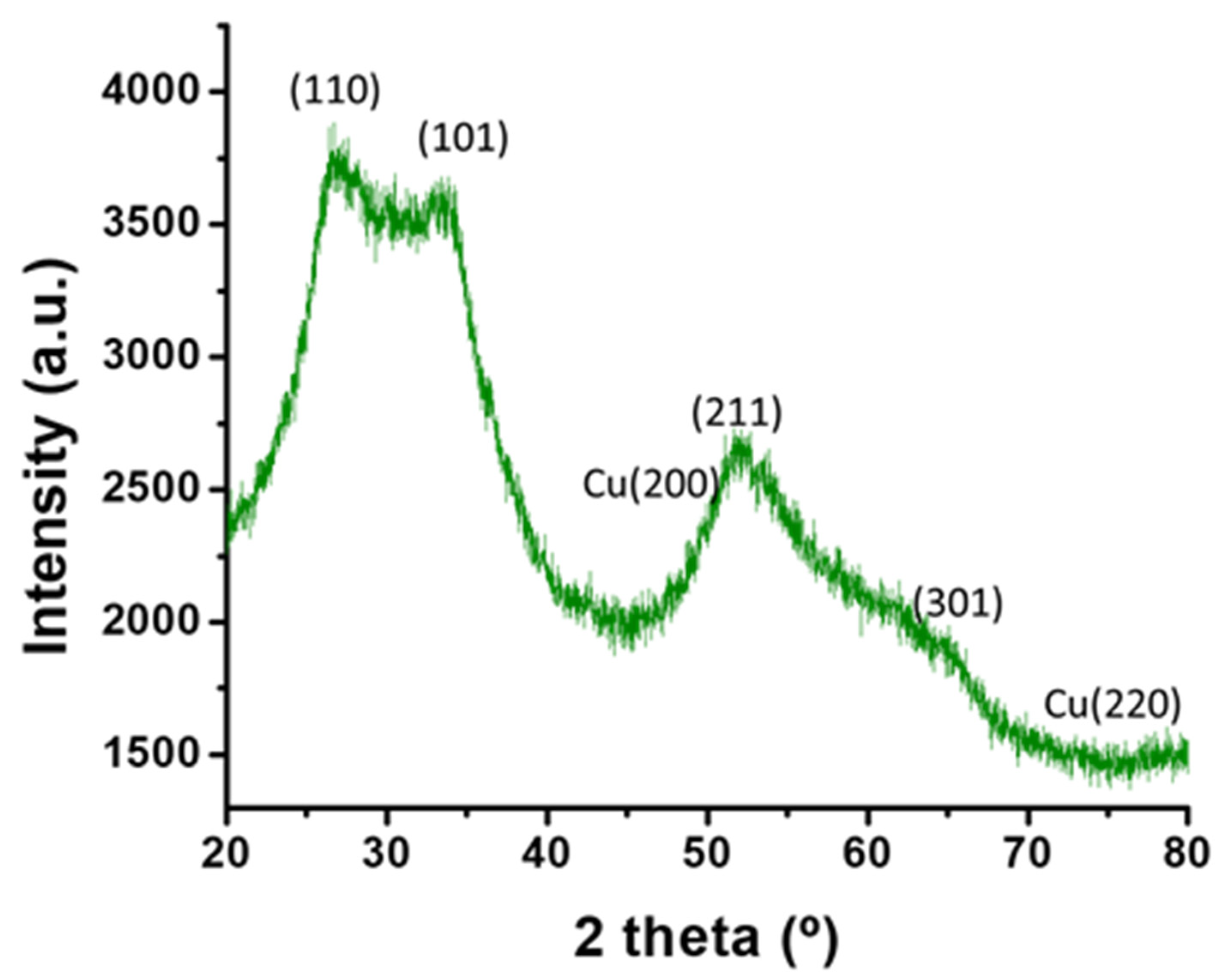
3.5. X-ray Diffraction (XRD) Analysis
3.6. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.7. Enzyme Mimetic Activity
3.8. Effects of Buffer pH and Concentration
3.9. Effect of Incubation Temperature
3.10. Effect of Incubation Time
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tripathi, R.; Chung, S.J. Reclamation of hexavalent chromium using catalytic activity of highly recyclable biogenic pd (0) nanoparticles. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, N.; Tripathi, R.M.; Zafar, F.; Singh, M.P. Catalytic degradation of dichlorvos using biosynthesized zero valent iron nanoparticles. IEEE Trans. Nanobiosci. 2017, 16, 280–286. [Google Scholar] [CrossRef]
- Sharma, P.; Shin, J.B.; Park, B.C.; Lee, J.-W.; Byun, S.W.; Jang, N.-Y.; Kim, Y.J.; Kim, Y.; Kim, Y.K.; Cho, N.-H. Application of radially grown zno nanowires on poly-l-lactide microfibers complexed with a tumor antigen for cancer immunotherapy. Nanoscale 2019, 11, 4591–4600. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, N.; Tripathi, R.M. Short interfering rna therapeutics: Nanocarriers, prospects and limitations. IET Nanobiotechnol. 2015, 9, 386–395. [Google Scholar] [CrossRef]
- Tripathi, R.; Shrivastav, A.; Shrivastav, B. Biogenic gold nanoparticles: As a potential candidate for brain tumor directed drug delivery. Artif. Cells Nanomed. Biotechnol. 2015, 43, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Park, S.H.; Kim, G.; Kim, D.-H.; Ahn, D.; Kim, Y.M.; Kwon, S.J.; Yoon, S.-Y.; Kang, H.J.; Chung, S.J. Metal-induced redshift of optical spectra of gold nanoparticles: An instant, sensitive, and selective visual detection of lead ions. Int. Biodeterior. Biodegrad. 2019, 144, 104740. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Shrivastav, B.R.; Shrivastav, A. Antibacterial and catalytic activity of biogenic gold nanoparticles synthesised by trichoderma harzianum. IET Nanobiotechnol. 2018, 12, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.M.; Pudake, R.N.; Shrivastav, B.; Shrivastav, A. Antibacterial activity of poly (vinyl alcohol)—Biogenic silver nanocomposite film for food packaging material. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025020. [Google Scholar] [CrossRef]
- Das, S.; Srivastava, V.C. An overview of the synthesis of cuo-zno nanocomposite for environmental and other applications. Nanotechnol. Rev. 2018, 7, 267–282. [Google Scholar] [CrossRef]
- Yang, H.; Yu, S.; Liang, H.; Lau, S.; Pramana, S.; Ferraris, C.; Cheng, C.; Fan, H. Ultraviolet electroluminescence from randomly assembled n-sno2 nanowires p-gan: Mg heterojunction. ACS Appl. Mater. Interfaces 2010, 2, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Zhang, X.; Gao, J.; Liu, X.; Liu, J. Peroxidase-like catalytic activity of copper ions and its application for highly sensitive detection of glypican-3. Anal. Chim. Acta 2016, 941, 87–93. [Google Scholar] [CrossRef]
- Agarwal, M.; Bhadwal, A.S.; Kumar, N.; Shrivastav, A.; Shrivastav, B.R.; Singh, M.P.; Zafar, F.; Tripathi, R.M. Catalytic degradation of methylene blue by biosynthesised copper nanoflowers using f. Benghalensis leaf extract. IET Nanobiotechnol. 2016, 10, 321–325. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponce, A.A.; Klabunde, K.J. Chemical and catalytic activity of copper nanoparticles prepared via metal vapor synthesis. J. Mol. Catal. A Chem. 2005, 225, 1–6. [Google Scholar] [CrossRef]
- Fukumura, T.; Sambandan, E.; Yamashita, H. Preparation, characterizations, and antibacterial properties of cu/sno 2 nanocomposite bilayer coatings. J. Coat. Technol. Res. 2018, 15, 437–443. [Google Scholar] [CrossRef]
- Ma, M.-Y.; He, Z.-Q.; Xiao, Z.-B.; Huang, K.-L.; Xiong, L.-Z.; Wu, X.-M. Synthesis and electrochemical properties of sno2-cuo nanocomposite powders. Trans. Nonferrous Met. Soc. China 2006, 16, 791–794. [Google Scholar] [CrossRef]
- Tripathi, R.; Chung, S.J. Biogenic nanomaterials: Synthesis, characterization, growth mechanism, and biomedical applications. J. Microbiol. Methods 2019, 157, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.M.; Ahn, D.; Kim, Y.M.; Chung, S.J. Enzyme mimetic activity of zno-pd nanosheets synthesized via a green route. Molecules 2020, 25, 2585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lai, J.; Wu, K.; Huang, X.; Guo, S.; Zhang, L.; Liu, J. Peroxidase-catalyzed chemiluminescence system and its application in immunoassay. Talanta 2018, 180, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Jv, Y.; Li, B.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Commun. 2010, 46, 8017–8019. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Chung, S.J. Phytosynthesis of palladium nanoclusters: An efficient nanozyme for ultrasensitive and selective detection of reactive oxygen species. Molecules 2020, 25, 3349. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Jasuja, N.D.; Sharma, S.; Choudhary, J.; Joshi, S.C. Essential oil and important activities of thuja orientalis and thuja occidentalis. J. Essent. Oil Bear. Plants 2015, 18, 931–949. [Google Scholar] [CrossRef]
- Tripathi, R.; Yoon, S.-Y.; Ahn, D.; Chung, S.J. Facile synthesis of triangular and hexagonal anionic gold nanoparticles and evaluation of their cytotoxicity. Nanomaterials 2019, 9, 1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, R.; Rao, R.P.; Tsuzuki, T. Green synthesis of sulfur nanoparticles and evaluation of their catalytic detoxification of hexavalent chromium in water. RSC Adv. 2018, 8, 36345–36352. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, R.M.; Ranac, D.; Shrivastav, A.; Singh, R.P.; Shrivastav, B.R. Biogenic synthesis of silver nanoparticles using saraca indica leaf extract and evaluation of their antibacterial activity. Nano Biomed. Eng. 2013, 5, 50–56. [Google Scholar] [CrossRef] [Green Version]
- García-Hernández, L.; Flores-Saldivar, J.A.; Ortega, P.R.; Guerrero, M.U.F. Synthesis of colloidal cunps using the extract of thuja orientalis. ECS Trans. 2021, 101, 131. [Google Scholar] [CrossRef]
- Kumar, P.; Andersson, G.; Subhedar, K.M.; Dhami, H.S.; Gupta, G.; Mukhopadhyay, A.K.; Joshi, R.P. Utilization of green reductant thuja orientalis for reduction of go to rgo. Ceram. Int. 2021, 47, 14862–14878. [Google Scholar] [CrossRef]
- Karmaoui, M.; Jorge, A.B.; McMillan, P.F.; Aliev, A.E.; Pullar, R.C.; Labrincha, J.A.; Tobaldi, D.M. One-step synthesis, structure, and band gap properties of sno2 nanoparticles made by a low temperature nonaqueous sol–gel technique. ACS Omega 2018, 3, 13227–13238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Saadi, T.M.; Hussein, B.H.; Hasan, A.B.; Shehab, A. Study the structural and optical properties of cr doped sno2 nanoparticles synthesized by sol-gel method. Energy Procedia 2019, 157, 457–465. [Google Scholar] [CrossRef]
- Chetri, P.; Saikia, B.; Choudhury, A. Structural and optical properties of cu doped sno2 nanoparticles: An experimental and density functional study. J. Appl. Phys. 2013, 113, 233514. [Google Scholar] [CrossRef]
- Speakman, S.A. Introduction to X-Ray Powder Diffraction Data Analysis. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2013. [Google Scholar]
- Shaheen, F.; Aziz, M.H.; Fatima, M.; Khan, M.A.; Ahmed, F.; Ahmad, R.; Ahmad, M.A.; Alkhuraiji, T.S.; Akram, M.W.; Raza, R. In vitro cytotoxicity and morphological assessments of go-zno against the mcf-7 cells: Determination of singlet oxygen by chemical trapping. Nanomaterials 2018, 8, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.M.; Kalathil, S.; Lee, J.-T.; Cho, M.-H. Enhancement in the photocatalytic activity of au@ tio 2 nanocomposites by pretreatment of tio 2 with uv light. Bull. Korean Chem. Soc. 2012, 33, 1753–1758. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yao, Q.; Tang, X.; Zhong, H.; Qiu, P.; Wang, X. A highly selective and sensitive colorimetric detection of uric acid in human serum based on mos 2-catalyzed oxidation tmb. Anal. Bioanal. Chem. 2019, 411, 943–952. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.-L.; Liu, J.-J.; Zhang, H.-Z.; Wang, J. Ultra-small cus nanoparticles as peroxidase mimetics for sensitive and colorimetric detection of uric acid in human serum. Chin. J. Anal. Chem. 2018, 46, e1825–e1831. [Google Scholar] [CrossRef]
- Pan, Y.; Pang, Y.; Shi, Y.; Zheng, W.; Long, Y.; Huang, Y.; Zheng, H. One-pot synthesis of a composite consisting of the enzyme ficin and a zinc (ii)-2-methylimidazole metal organic framework with enhanced peroxidase activity for colorimetric detection for glucose. Microchim. Acta 2019, 186, 213. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, R.M.; Chung, S.J. Eco-Friendly Synthesis of SnO2-Cu Nanocomposites and Evaluation of Their Peroxidase Mimetic Activity. Nanomaterials 2021, 11, 1798. https://doi.org/10.3390/nano11071798
Tripathi RM, Chung SJ. Eco-Friendly Synthesis of SnO2-Cu Nanocomposites and Evaluation of Their Peroxidase Mimetic Activity. Nanomaterials. 2021; 11(7):1798. https://doi.org/10.3390/nano11071798
Chicago/Turabian StyleTripathi, Ravi Mani, and Sang J. Chung. 2021. "Eco-Friendly Synthesis of SnO2-Cu Nanocomposites and Evaluation of Their Peroxidase Mimetic Activity" Nanomaterials 11, no. 7: 1798. https://doi.org/10.3390/nano11071798
APA StyleTripathi, R. M., & Chung, S. J. (2021). Eco-Friendly Synthesis of SnO2-Cu Nanocomposites and Evaluation of Their Peroxidase Mimetic Activity. Nanomaterials, 11(7), 1798. https://doi.org/10.3390/nano11071798






