Investigation of Hybrid Methods for Elimination of Brilliant Blue Dye from Water Phase Using Various Nanomaterials Combined with Activated Sludge and Duckweed
Abstract
:1. Introduction
2. Materials and Methods
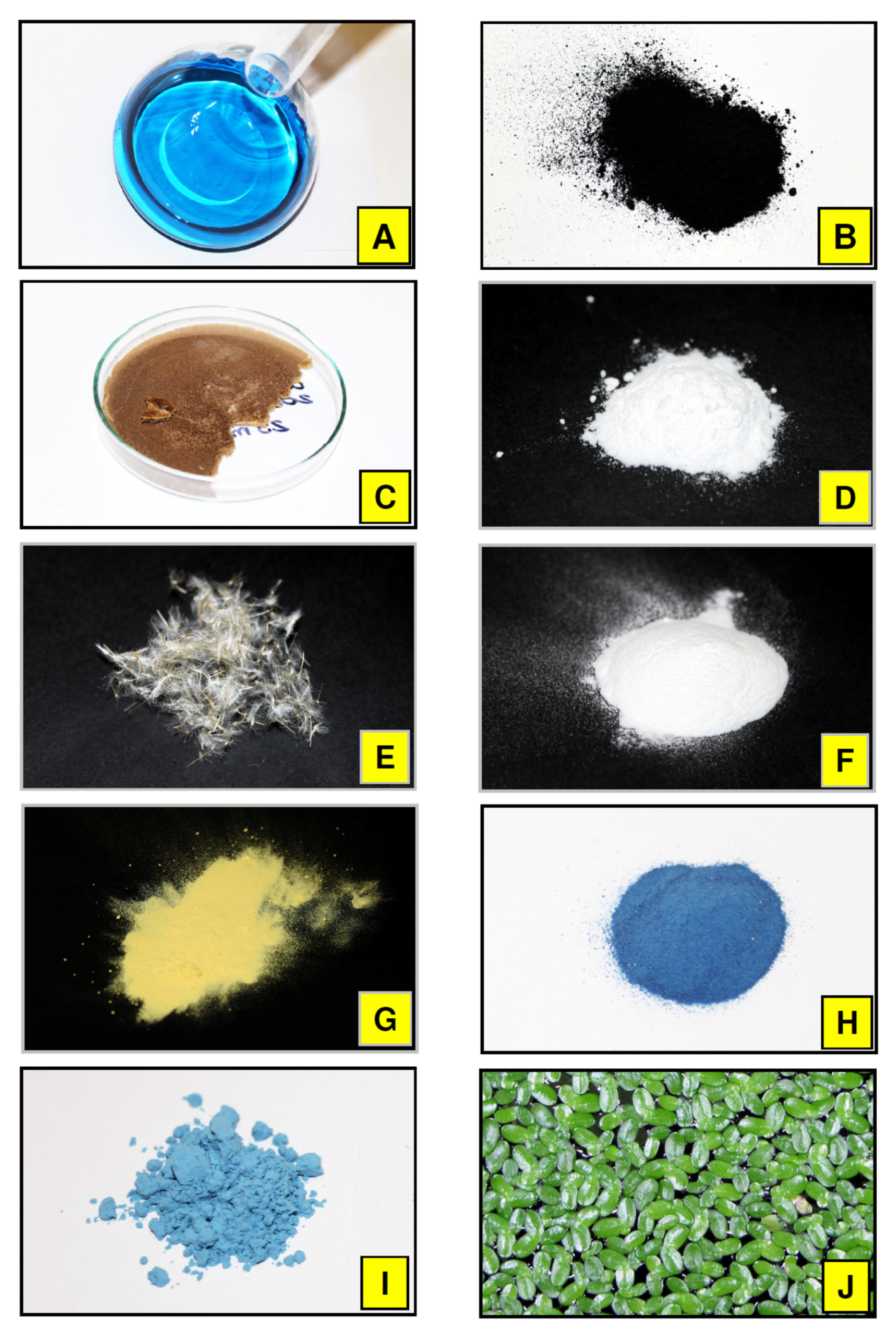
2.1. Chemicals
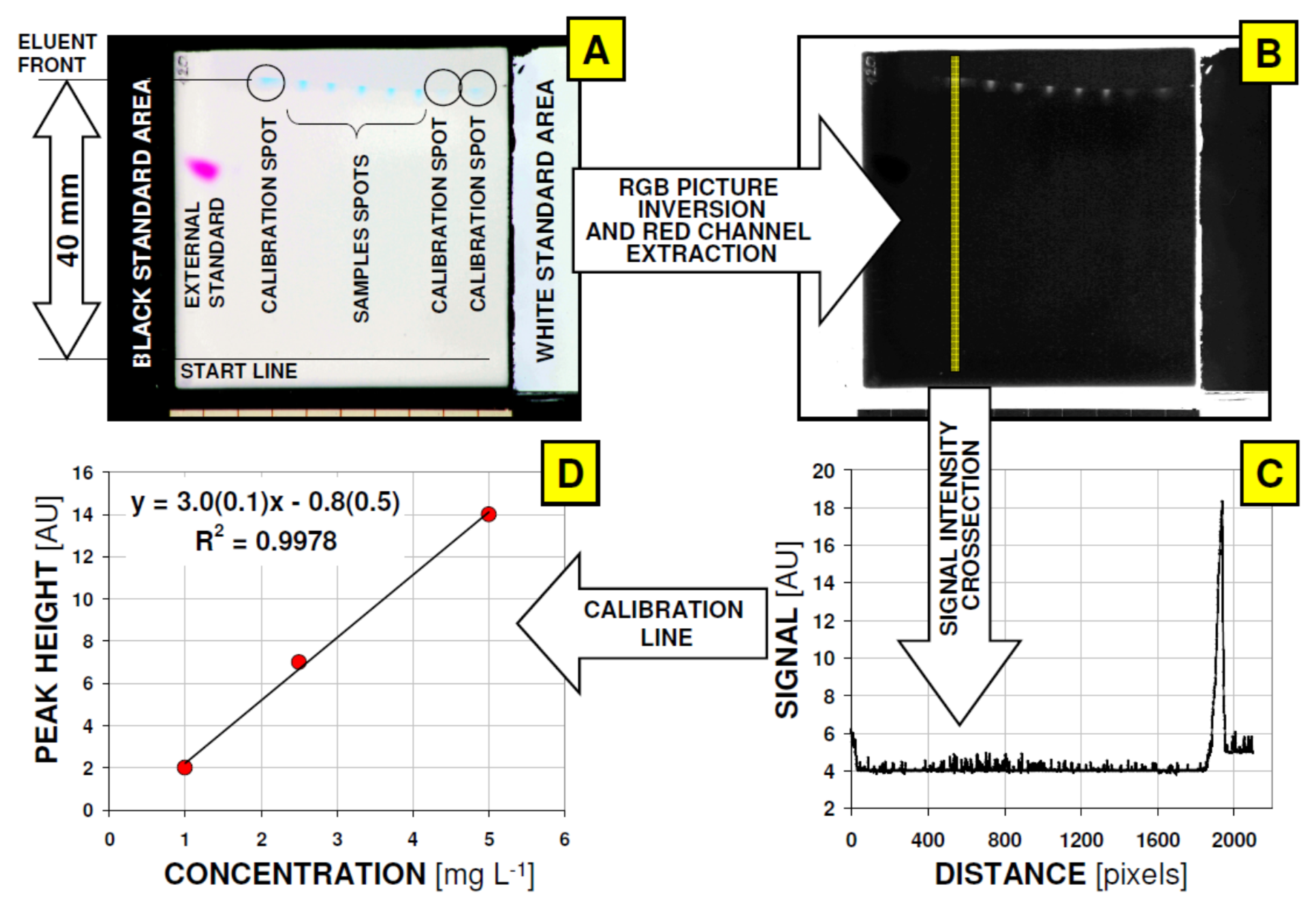
2.2. Quantitative Micro-TLC Chromatography
2.3. Biological Materials
2.4. Synthesis of Egyptian Blue Pigment
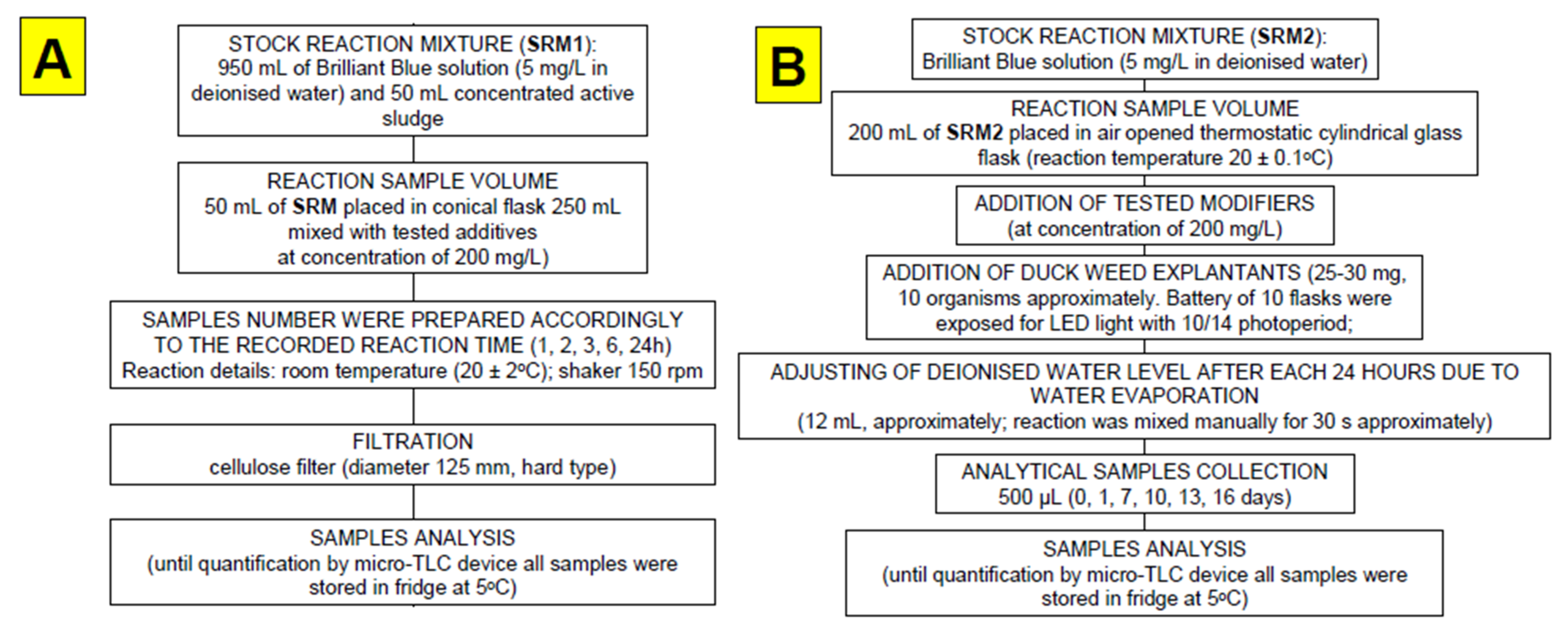
2.5. Dye Removal Test and Measurements
2.6. Data Acquisition and Analysis
3. Results and Discussion
3.1. Problem Overview and Experiment Concept
3.2. Detailed Results and Multivariate Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atta-ur-Rahman (Ed.) Studies in Natural Product Chemistry. In Bioactive Natural Products; Elsevier Science Publishers: Amsterdam, The Netherlands, 2016; Volume 48, ISBN 978-0-444-63602-7. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Holban, A.M. (Eds.) Handbook of Food Bioengineering. In Ingredients Extraction by Physico-Chemical Method in Food; Academic Press/Elsevier: London, UK, 2017; Volume 4, ISBN 978-0-12-811521-3. [Google Scholar] [CrossRef]
- Grumezescu, A.M. (Ed.) Handbook of Food Bioengineering. In Role of Material Science in Food Bioengineering; Academic Press/Elsevier: Cambridge, MA, USA, 2018; Volume 19, ISBN 978-0-12-811448-3. [Google Scholar] [CrossRef]
- Zarzycki, P.K. Pure and Functionalized Carbon Based Nanomaterials, Analytical, Biomedical, Civil and Environmental Engineering Applications, 1st ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 1–374. [Google Scholar]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye removal from water and wastewater using various physical, chemical and biological processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef]
- Piaskowski, K.; Zarzycki, P.K. Carbon-Based Nanomaterials as Promising Material for Wastewater Treatment Processes. Int. J. Environ. Res. Public Health 2020, 17, 5862. [Google Scholar] [CrossRef]
- Flury, M.; Flühler, H. Tracer Characteristics of Brilliant Blue FCF. SSSA 1995, 59, 22–27. [Google Scholar] [CrossRef]
- Abid, M.F.; Zablouk, M.A.; Abid-Alameer, A.M. Experimental study of dye removal from industrial wastewater by membrane technologies of reverse osmosis and nanofiltration. J. Environ. Health Sci. Eng. 2012, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Siti Zuraida, M.; Nurhaslina, C.R.; Ku Halim, K.H. Adsorption of colour from Batik effluent by bacterial, Lactobacillus Delbruckii and its growth. BEIAC 2013, 2, 574–579. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.; Kumar Sharma, D.; Kishore, K. Chapter 3—Toxicity of Food Additives. In Food Safety and Human Health; Ram, L.S., Sukanta, M., Eds.; Elsevier: Oxford, UK, 2019; pp. 67–98. [Google Scholar] [CrossRef]
- Dellamatrice, P.M.; Silva-Stenico, M.E.; Beraldo de Moraes, L.A.; Fiore, M.F.; Rosim Monteiro, R.T. Degradation of textile dyes by cyanobacteria. Braz. J. Microbiol. 2017, 48, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Janović, B.S.; Collins, A.R.; Vujčić, Z.M.; Vujčić, M.T. Acidic Horseradish Peroxidase Activity Abolishes Genotoxicity of Common Dyes. J. Hazard. Mater. 2017, 321, 576–585. [Google Scholar] [CrossRef]
- Hernández-Hernández, K.-A.; Solache-Ríos, M.; Díaz-Nava, M.C. Removal of Brilliant Blue FCF from Aqueous Solutions Using an Unmodified and Iron-Modified Bentonite and the Thermodynamic Parameters of the Process. Water Air. Soil Pollut. 2013, 224, 1562. [Google Scholar] [CrossRef]
- Świderska-Dąbrowska, R.; Piaskowski, K.; Zarzycki, P.K. Preliminary studies of synthetic dyes adsorption on iron sludge and activated carbons. J. AOAC Int. 2018, 101, 1429–1436. [Google Scholar] [CrossRef]
- Flury, M.; Flühler, H. Brilliant Blue FCF as a Dye Tracer for Solute Transport Studies—A Toxicological Overview. J. Environ. Qual. 1994, 23, 1108–1112. [Google Scholar] [CrossRef]
- Gosetti, F.; Gianotti, V.; Angioi, S.; Polati, S.; Marengo, E.; Gennaro, M.C. Oxidative degradation of food dye E133 Brilliant BlueFCF: Liquid chromatography–electrospray mass spectrometry identification of the degradation pathway. J. Chromatogr. A 2004, 1054, 379–387. [Google Scholar] [PubMed]
- Zarzycki, P.K.; Mitura, K.; Piaskowski, K.; Lewandowska, L.; Świderska-Dąbrowska, R.; Baran, M. Carbon and related nanomaterials as active media for analytical, pharmaceutical, biomedical and wastewater processing applications. In Pure and Functionalized Carbon Based Nanomaterials: Analytical, Biomedical, Civil. and Environmental Engineering Applications, 1st ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 326–363. [Google Scholar]
- Mozzocchin, G.A.; Rudello, D.; Bragato, C.; Agnoli, F. A short note on Egyptian blue. J. Cult. Herit. 2004, 5, 129–133. [Google Scholar] [CrossRef]
- Zarzycki, P.K. Simple horizontal chamber for thermostated micro-thin-layer chromatography. J. Chromatogr. A 2008, 1187, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zarzycki, P.K.; Włodarczyk, E.; Baran, M.J. Determination of endocrine disrupting compounds using temperature-dependent inclusion chromatography II. Fast screening of free steroids and related low-molecular-mass compounds fraction in the environmental samples derived from surface waters, treated and untreated sewage waters as well as activated sludge material. J. Chromatogr. A 2009, 1216, 7612–7622. [Google Scholar] [CrossRef]
- Kaleniecka, A.; Zarzycki, P.K. Degradation Studies of Selected Bisphenols in the Presence of β-Cyclodextrin and/or DuckweedWater Plant. J. AOAC Int. 2020, 103, 439–448. [Google Scholar] [CrossRef]
- Ternes, T.A.; Kreckel, P.; Mueller, J. Behaviour and occurrence of estrogens in municipal sewage treatment plants-II. Aerobic batch experiments with activated sludge. Sci. Total Environ. 1999, 225, 91–99. [Google Scholar] [CrossRef]
- Ternes, T.A.; Stumpf, M.; Mueller, J.; Haberer, K.; Wilken, R.-D.; Servos, M. Behavior and occurrence of estrogens in municipal sewage treatment plants -I. Investigations in Germany, Canada and Brazil. Sci. Total Environ. 1999, 225, 81–90. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Włodarczyk, E.; Baran, M.J. Determination of endocrine disrupting compounds using temperature-dependent inclusion chromatography I. Optimization of separation protocol. J. Chromatogr. A 2009, 1216, 7602–7611. [Google Scholar] [CrossRef] [PubMed]
- Suszyński, Z.; Zarzycki, P.K. New approach for sensitive photothermal detection of C60 and C70 fullerenes on micro-TLC plates. Anal. Chim. Acta 2015, 863, 70–77. [Google Scholar] [CrossRef]
- Głód, B.K.; Wantusiak, P.M.; Piszcz, P.; Lewczuk, E.; Zarzycki, P.K. Application of micro-TLC to the total antioxidant potential (TAP) measurement. Food Chem. 2015, 173, 749–754. [Google Scholar] [CrossRef]
- Włodarczyk, E.; Baran, M.; Slączka, M.M.; Portka, J.K.; Zarzycki, P.K. Fingerprinting of soot dust materials using micro-TLC. J. Liquid Chromatogr. Relat. Technol. 2014, 37, 2846–2856. [Google Scholar] [CrossRef]
- Lisowski, P.; Zarzycki, P.K. Microfluidic Paper-Based Analytical Devices (μPADs) and Micrototal Analysis Systems (μTAS) -Development, Applications and Future Trends. Chromatographia 2013, 76, 1201–1214. [Google Scholar] [CrossRef] [Green Version]
- Zarzycki, P.K.; Slączka, M.M.; Zarzycka, M.B.; Bartoszuk, M.A.; Włodarczyk, E.; Baran, M.J. Temperature-controlled micro-TLC: A versatile green chemistry and fast analytical tool for separation and preliminary screening of steroids fraction from biological and environmental samples. J. Steroid Biochem. Mol. Biol. 2011, 127, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Zarzycki, P.K.; Zarzycka, M.B. Application of temperature-controlled micro planar chromatography for separation and quantification of testosterone and its derivatives. Anal. Bioanal. Chem. 2008, 391, 2219–2225. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Zarzycka, M.B.; Clifton, V.L.; Adamski, J.; Głód, B.K. Low parachor solvents extraction and thermostated micro-TLC separation for fast screening and classification of spirulina from pharmaceutical formulations and food samples. J. Chromatograf. A 2011, 1218, 5694–5704. [Google Scholar] [CrossRef]
- Swiderska, R.; Anielak, A.M. The significance of Electrokinetic potential in the adsorption process of humic substances. Rocz. Ochr. Sr. 2004, 6, 31–49. [Google Scholar]
- Biswas, P.; Bandyopadhyaya, R. Water disinfection using silver nanoparticle impregnated activated carbon: Escherichia coli cell-killing in batch and continuous packed column operation over a long duration. Water Res. 2016, 100, 105–115. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Gong, J.-L.; Zeng, G.-M.; Ou, X.-M.; Song, B.; Guo, M.; Zhang, J.; Liu, H.-Y. Antimicrobial behavior comparison and antimicrobial mechanism of silver coated carbon nanocomposites. Process. Saf. Environ. 2016, 102, 596–605. [Google Scholar] [CrossRef]
- Maddigpu, P.R.; Sawant, B.; Wanjari, S.; Goel, M.D.; Vione, D.; Dhodapkar, R.S.; Rayalu, S. Carbon nanoparticles for solar disinfection of water. J. Hazard. Mater. 2018, 343, 157–165. [Google Scholar] [CrossRef]
- Saya, L.; Gautam, D.; Malik, V.; Singh, W.R.; Hooda, S. Natural Polysaccharide Based Graphene Oxide Nanocomposites for Removal of Dyes from Wastewater: A Review. J. Chem. Eng. Data 2021, 66, 11–37. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Bijelić, L.; Puntaric, D.; Gvozdic, V.; Vidosavljevic, D.; Juric, D.; Lončaric, Z.; Puntaric, A.; Puntaric, E.; Vidosavljevic, M.; Puntaric, I.; et al. Presence of war related elements in dandelion (Taraxacum officinale) as a possible consequence of military activities in east Croatia. A. Agricult. Scandi. Sec. B Soil Plant. Sci. 2018, 68, 264–272. [Google Scholar] [CrossRef]
- Laima, M.; Maksymowska-Brossard, D.; Sauriau, P.G.; Richard, P.; Girard, M.; Gouleau, D.; Joassard, L. Fluffdeposition on intertidalsediments: Effects on benthic biota, ammoniumfluxes and nitrificationrates. Biogeochemistry 2002, 61, 115–133. [Google Scholar] [CrossRef]
- Tauber, F.; Emeis, K.-C. Sediment mobility in the Pomeranian Bight (Baltic Sea): A case study based on side scan-sonar imagesand hydrodynamic modeling. Geo Mar. Lett. 2005, 25, 221–229. [Google Scholar] [CrossRef]
- Virtanem, J.; Pammo, A.; Keskinen, J.; Sarlin, E.; Tuukkanen, S. Pyrolysed cellulose nanofibrils and dandelion pappus in supercapacitor application. Cellulose 2017, 24, 3387–3397. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Piaskowski, K.; Lewandowska, L.; Fenert, B.; Świderska-Dąbrowska, R.; Ślączka-Wilk, M.M.; Pereira, J.C. Portable Micro-Planar Extraction, Separation and Quantification Devices for Bioanalytical and Environmental Engineering Applications. In Micro- and Nanotechnology Enabled Applications for Mobile and Portable Miniaturized Analysis; Sabu, T.M., Ahmadi, T., Anh, N., Abbas, A., Tayyebeh, M., Eds.; Elsevier: Oxford, UK, 2021. (in press) [Google Scholar]
- Włodarczyk, E.; Zarzycki, P.K. Chromatographic behaviour of selected dyes on micro-TLC plates under NP and RP conditions—Potential application as the internal standards substances for chromatographic and/or microfluidic systems. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 5–6. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Fenert, B.; Głód, B.K. 17-Cyclodextrins-based nanocomplexes for encapsulation of bioactive compounds in food, cosmetics, and pharmaceutical products: Principles of supramolecular complexes formation, their influence on the antioxidative properties of target chemi-cals, and recent advances in selected industrial applications. In Encapsulations; Nanotechnology in the Agri-Food Industry Volume 2 Nanotechnology in the Agri-Food Industry; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 17; pp. 717–767. ISBN 978-0-12-804307-3. [Google Scholar]
- Li, Q.; Gluch, J.; Krüger, P.; Gall, M.; Neinhuis Ch Zschech, E. Pollen structure visualization using high-resolution laboratory-based hard X-ray tomography. Biochem. Biophys. Res. Commun. 2016, 479, 272–276. [Google Scholar] [CrossRef]
- Mackenzie, G.; Beckett, S.; Atkin, S.; Diego-Taboada, A. Hollow Pollen Shells to Enhance Drug Delivery. In Pollen and Spore Shells—Nature’s Microcapsules; Anilkumar, G., Gaonkar, N.V., Atul, R.K., Robert, S., Eds.; Elsevier: Oxford, UK, 2014; pp. 283–297. [Google Scholar]
- Prabhakar, A.-K.; Lai, H.Y.; Potroz, M.G.; Corliss, M.K.; Park, J.H.; Mundargi, R.C.; Cho, D.; Bang, S.-I.; Cho, N.-J. Chemical processing strategies to obtain sporopollenin exine capsules from multi-compartmental pine pollen. J. Ind. Eng. Chem. 2017, 53, 375–385. [Google Scholar] [CrossRef]
- National Gallery of Art. Artists’ Pigments: A Handbook of Their History and Characteristics; FitzHugh, E.W., Ed.; Washington Archetype Publications: London, UK, 1997; Volume 3. [Google Scholar]
- Errington, B.; Lawson, G.; Lewis, S.W.; Smith, G.D. Micronised Egyptian blue pigment: A novel near-infrared luminescent fingerprint dusting powder. Dyes Pigment. 2016, 132, 310–315. [Google Scholar] [CrossRef]
- Bianchettia, P.; Talaricoa, F.; Viglianoa, M.G.; Ali, M.F. Production and characterization of Egyptian blue and Egyptian green frit. J. Cult. Herit. 2000, 1, 179–188. [Google Scholar] [CrossRef]
- Panagopoulou, A.; Karanasios, K.; Xanthopoulou, G. Ancient Egyptian Blue (CaCuSi4O10) Pigment by Modern Solution Combustion Synthesis Method. EurasianChem. Technol. J. 2016, 18, 31–37. [Google Scholar] [CrossRef] [Green Version]
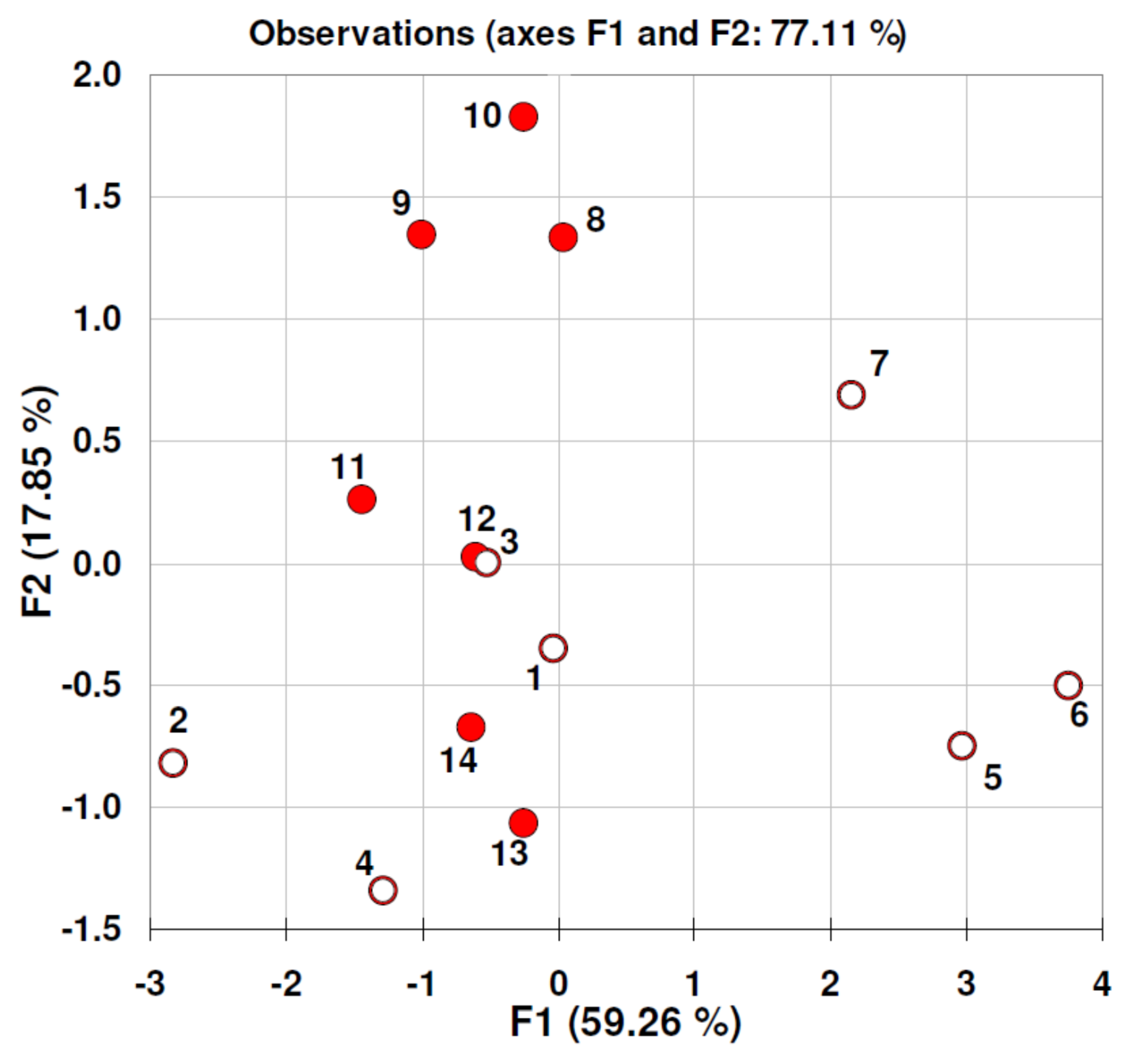
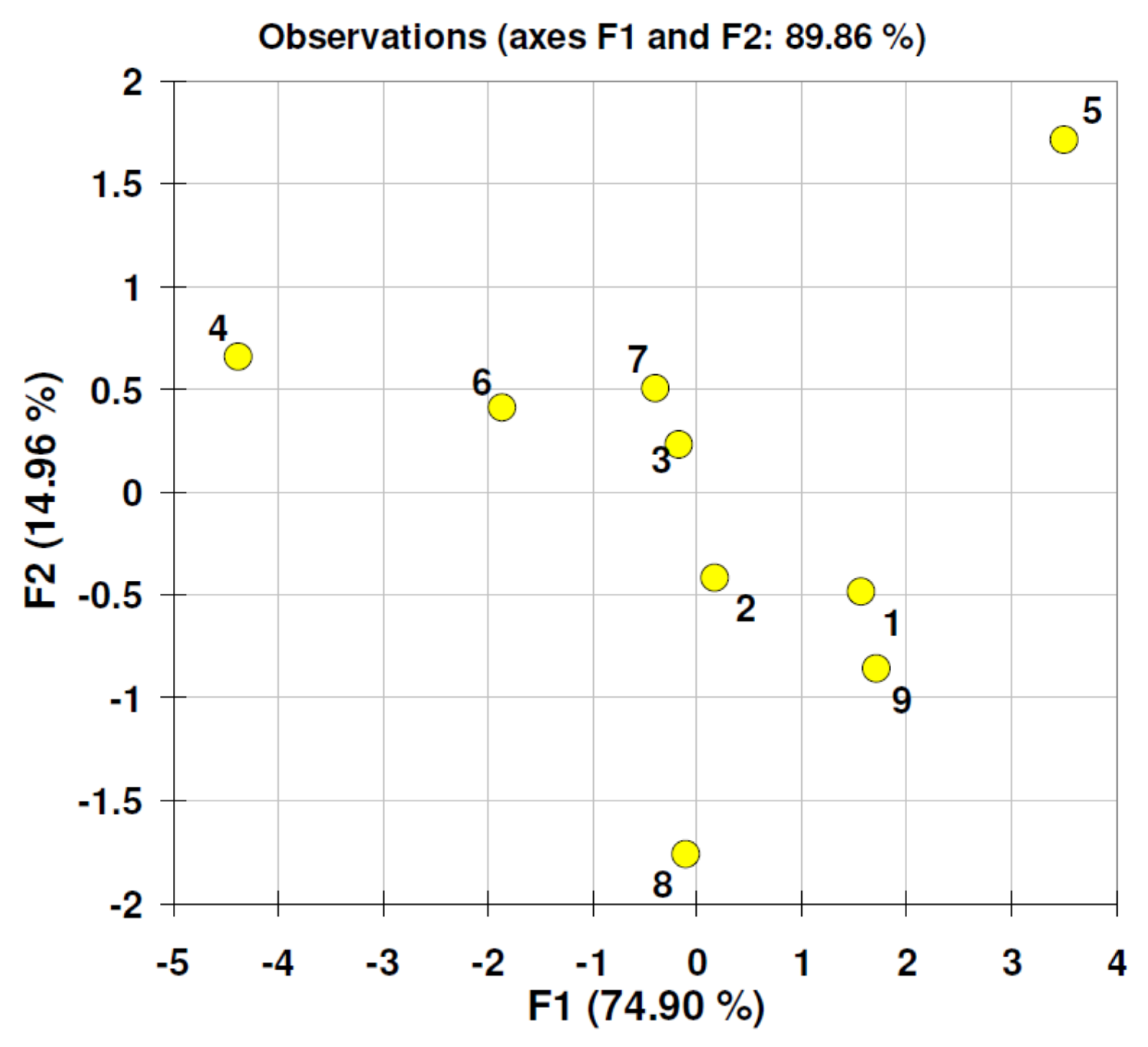
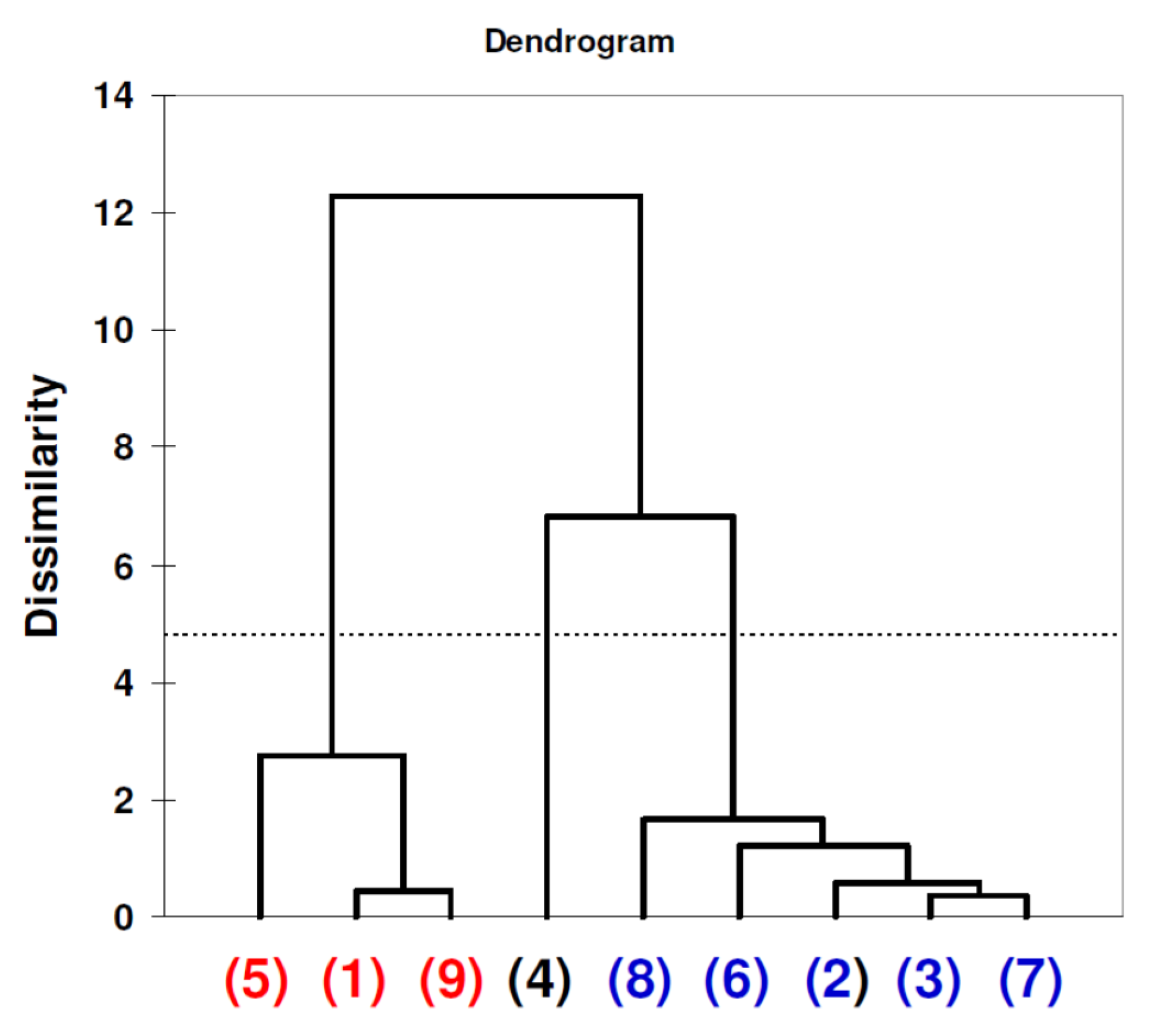
| Material | Particle Size Value [nm] (Standard Deviation) n = 20 | Zeta Potential [mV] (Standard Deviation) n = 24 |
|---|---|---|
| Graphene Oxide | 20.253 (7.064) | −20.6 (2.7) |
| Microcrystalline Cellulose | 32.135 (5.816) | −4.9 (2.1) |
| Pine Pollen | 25–35 μm (approximate dimensions based on SEM measurement) | Not available |
| β-Cyclodextrin | 1.53 * | Not available |
| Dandelion Pappus | Fiber length 3–5 mm Fiber diameter 25 μm (approximate dimensions based on SEM measurement) | Not available |
| Active Carbon Norit SA | 9567 (4632) | −13.3 (2.0) |
| Egyptian Blue EB1 | 34.621 (12.683) | −14.9 (6.0) |
| Egyptian Blue EB2 | 3.458 (942) | −23.3 (6.5) |
| Time (Hour)/Matrix Tested * | 1 | 2 | 3 | 6 | 24 |
|---|---|---|---|---|---|
| BLANK | 4.353 | 5.095 | 4.918 | 4.903 | 5.240 |
| AC | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| GO | 3.859 | 2.076 | 4.021 | 3.059 | 3.303 |
| CD | 4.353 | 5.095 | 4.470 | 4.376 | 4.755 |
| DP | 4.353 | 2.773 | 3.573 | 4.903 | 4.997 |
| MC | 6.211 | 5.899 | 8.351 | 5.382 | 6.952 |
| PP | 7.330 | 5.899 | 7.027 | 7.273 | 6.952 |
| EB2 | 5.651 | 6.347 | 8.351 | 5.854 | 4.396 |
| AS BLANK | 5.479 | 5.833 | 4.921 | 4.611 | 3.427 |
| AS + AC | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| AS + GO | 4.789 | 5.833 | 4.184 | 3.278 | 3.427 |
| AS + CD | 5.479 | 5.833 | 4.184 | 5.278 | 2.536 |
| AS + DP | 4.789 | 4.167 | 5.289 | 2.278 | 3.724 |
| AS + MC | 3.544 | 5.197 | 4.285 | 5.779 | 4.241 |
| AS + PP | 4.152 | 3.586 | 6.100 | 5.377 | 4.664 |
| AS + EB2 | 3.848 | 4.553 | 5.737 | 3.771 | 5.086 |
| Time (Day)/Matrix Tested * | 0 | 1 | 7 | 10 | 13 | 16 |
|---|---|---|---|---|---|---|
| BLANK | 4.364 | 5.346 | 5.595 | 4.846 | 4.709 | 4.828 |
| DW | 4.411 | 5.202 | 5.202 | 4.411 | 4.016 | 4.016 |
| DW + AC | 4.873 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| DW + GO | 4.687 | 5.392 | 4.335 | 3.982 | 3.982 | 4.335 |
| DW + CD | 3.838 | 3.648 | 3.369 | 3.075 | 3.046 | 2.606 |
| DW + DP | 5.554 | 5.113 | 5.554 | 5.113 | 5.995 | 5.554 |
| DW + MC | 4.027 | 4.353 | 4.353 | 3.373 | 3.700 | 4.027 |
| DW + PP | 4.438 | 4.774 | 4.774 | 3.767 | 4.103 | 4.438 |
| DW + EB1 | 3.700 | 5.779 | 4.888 | 4.294 | 4.591 | 3.700 |
| DW + EB2 | 4.411 | 5.597 | 5.202 | 5.597 | 4.411 | 4.806 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarzycki, P.K.; Lewandowska, L.; Fenert, B.; Piaskowski, K.; Kobaka, J. Investigation of Hybrid Methods for Elimination of Brilliant Blue Dye from Water Phase Using Various Nanomaterials Combined with Activated Sludge and Duckweed. Nanomaterials 2021, 11, 1747. https://doi.org/10.3390/nano11071747
Zarzycki PK, Lewandowska L, Fenert B, Piaskowski K, Kobaka J. Investigation of Hybrid Methods for Elimination of Brilliant Blue Dye from Water Phase Using Various Nanomaterials Combined with Activated Sludge and Duckweed. Nanomaterials. 2021; 11(7):1747. https://doi.org/10.3390/nano11071747
Chicago/Turabian StyleZarzycki, Paweł K., Lucyna Lewandowska, Bożena Fenert, Krzysztof Piaskowski, and Janusz Kobaka. 2021. "Investigation of Hybrid Methods for Elimination of Brilliant Blue Dye from Water Phase Using Various Nanomaterials Combined with Activated Sludge and Duckweed" Nanomaterials 11, no. 7: 1747. https://doi.org/10.3390/nano11071747
APA StyleZarzycki, P. K., Lewandowska, L., Fenert, B., Piaskowski, K., & Kobaka, J. (2021). Investigation of Hybrid Methods for Elimination of Brilliant Blue Dye from Water Phase Using Various Nanomaterials Combined with Activated Sludge and Duckweed. Nanomaterials, 11(7), 1747. https://doi.org/10.3390/nano11071747






