Experimental and Computational Analysis of MnO2@V2C-MXene for Enhanced Energy Storage
Abstract
:1. Introduction
2. Experimental Methods
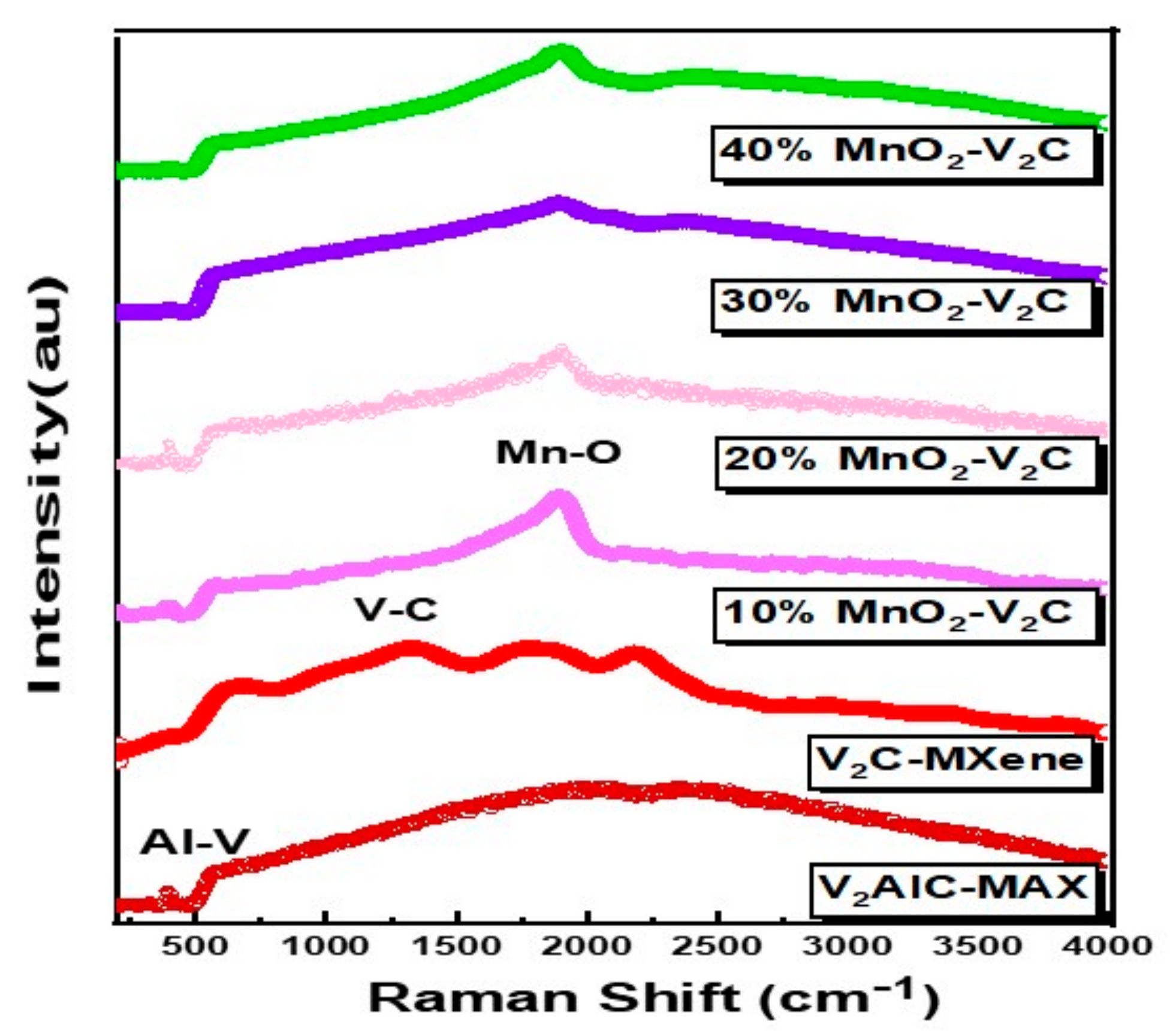
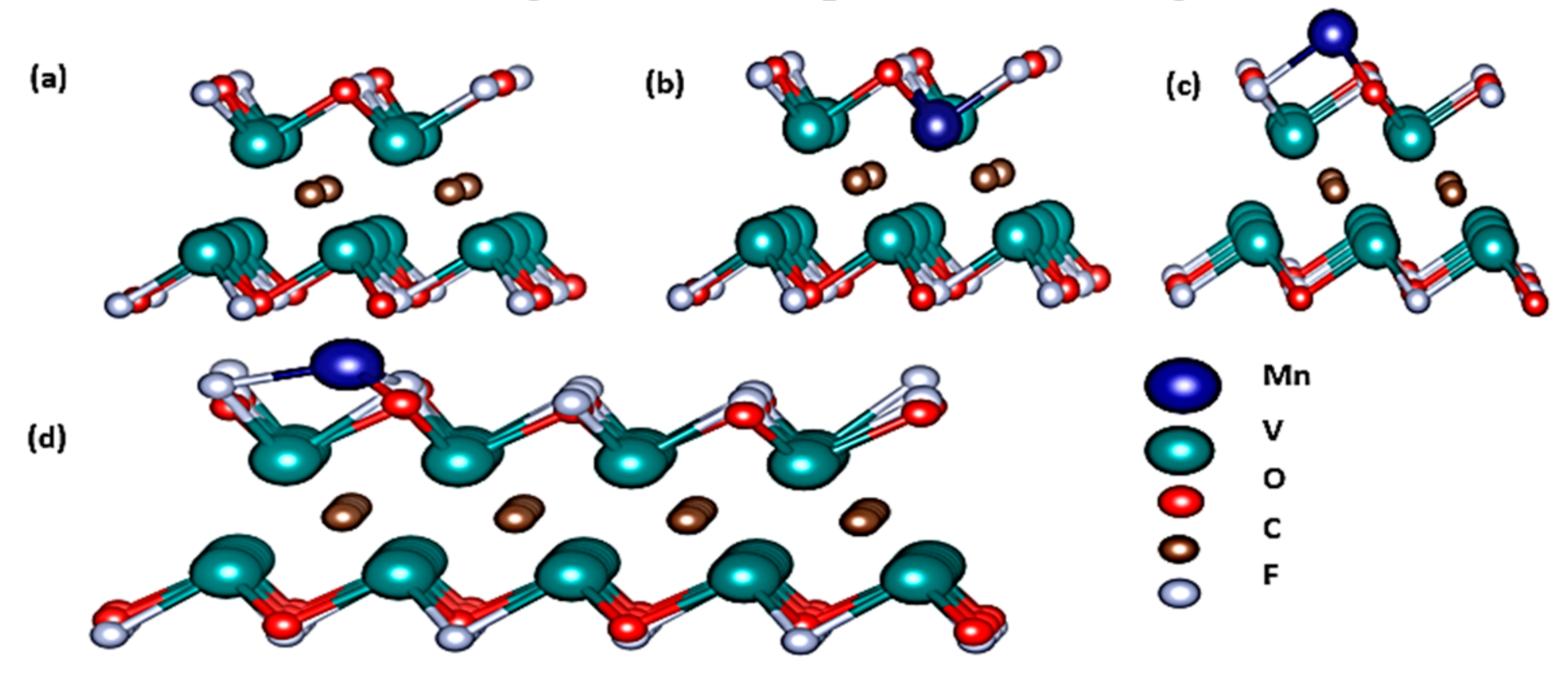
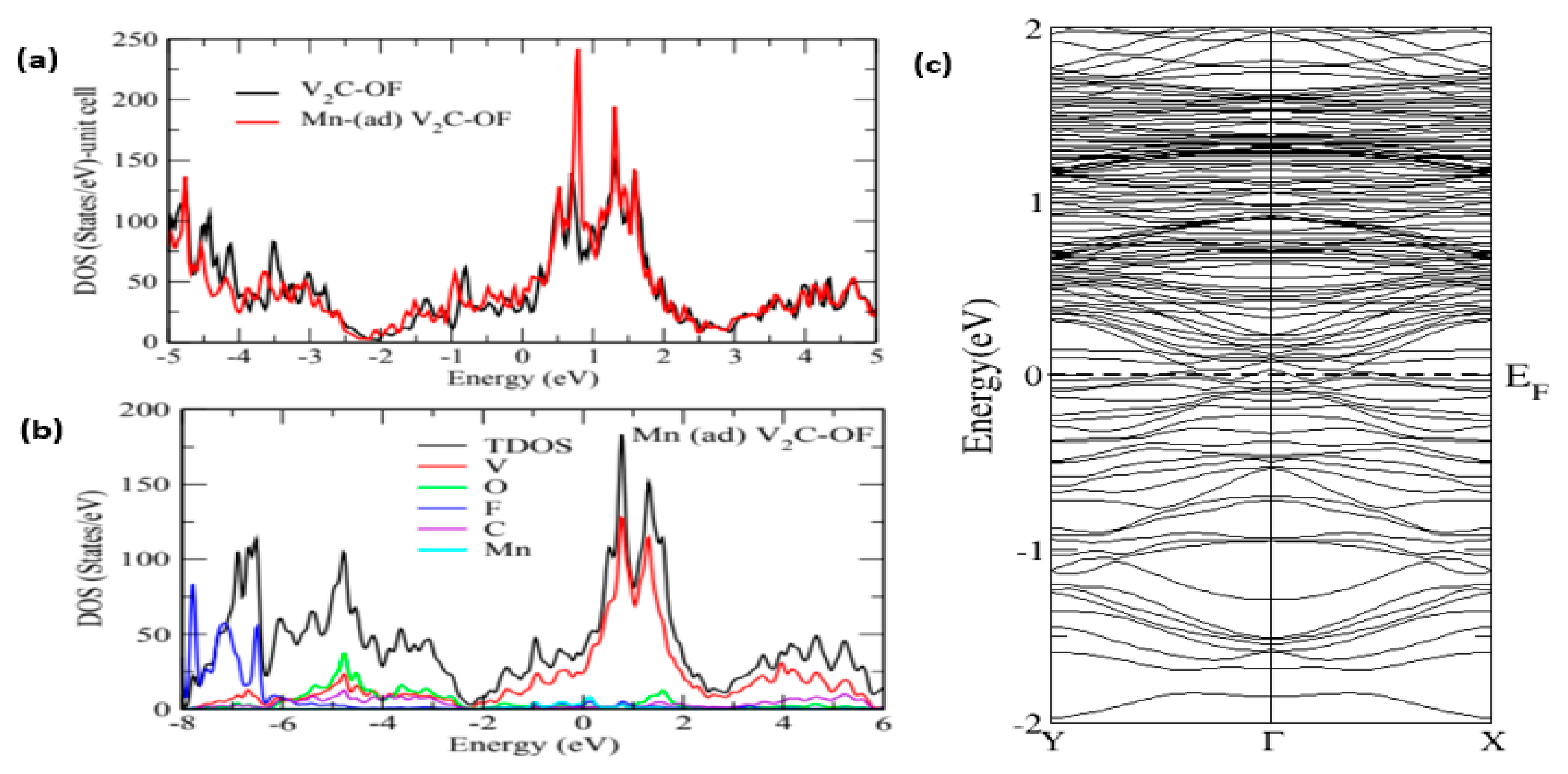
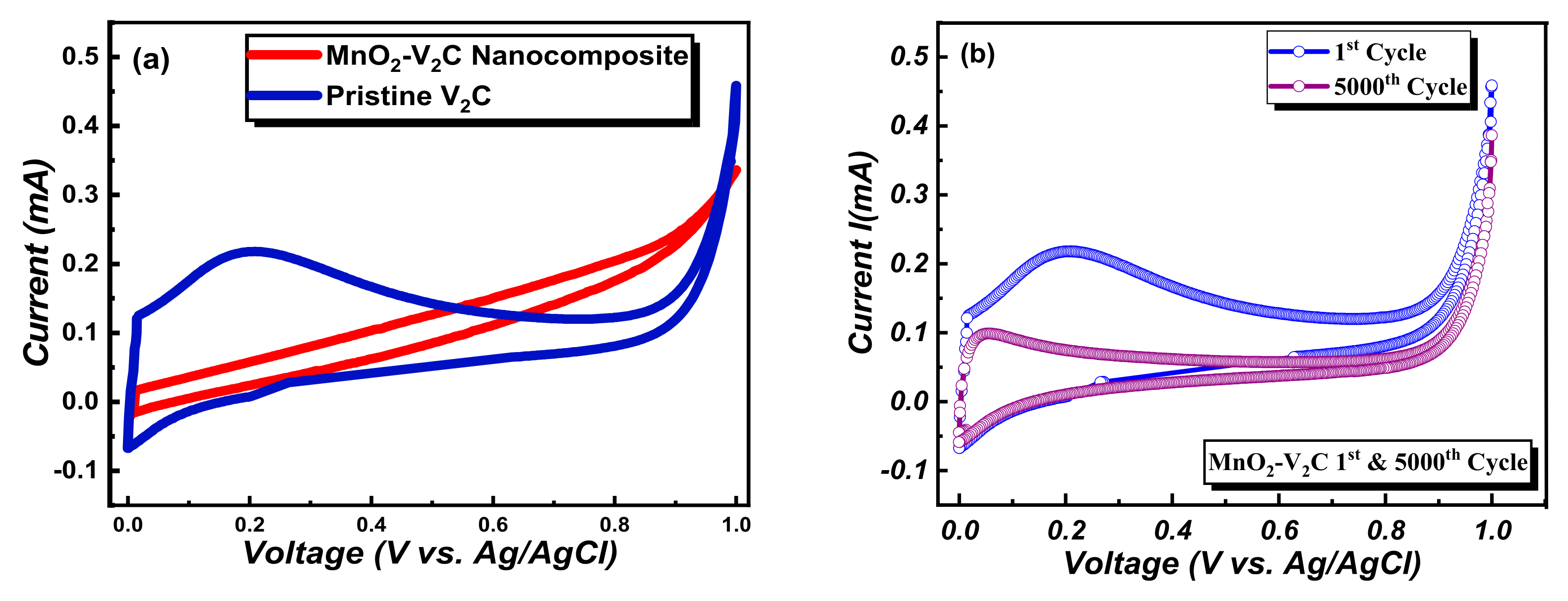
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiori, G.; Bonaccorso, F.; Iannaccone, G.; Palacios, T.; Neumaier, D.; Seabaugh, A.; Banerjee, S.K.; Colombo, L. Electronics based on two-dimensional materials. Nat. Nanotechnol. 2014, 9, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 6139. [Google Scholar] [CrossRef] [Green Version]
- Xia, F.; Wang, H.; Di, X.; Dubey, M.; Ramasubramaniam, A. Two-dimensional material nanophotonics. Nat. Photonics 2014, 8, 899–907. [Google Scholar] [CrossRef]
- Akinwande, D.; Petrone, N.; Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cepellotti, A.; Fugallo, G.; Paulatto, L.; Lazzeri, M.; Mauri, F.; Marzari, N. Phonon hydrodynamics in two-dimensional materials. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Cahangirov, S.; Topsakal, M.; Aktürk, E.; Şahin, H.; Ciraci, S. Two-and one-dimensional honeycomb structures of silicon and germanium. Phys. Rev. Lett. 2009, 102, 236804. [Google Scholar] [CrossRef] [Green Version]
- Lalmi, B.; Oughaddou, H.; Enriquez, H.; Kara, A.; Vizzini, S.; Ealet, B.; Aufray, B. Epitaxial growth of a silicene sheet. Appl. Phys. Lett. 2010, 97, 223109. [Google Scholar] [CrossRef] [Green Version]
- Nowak, I.; Ziolek, M. Niobium compounds: Preparation, characterization, and application in heterogeneous catalysis. Chem. Rev. 1999, 99, 3603–3624. [Google Scholar] [CrossRef]
- Asghar, M.; Zahra, S.A.; Khan, Z.; Ahmed, M.; Nasir, F.; Iqbal, M.; Mohammad, M.A.; Mahmood, A.; Akinwande, D.; Rizwan, S. Laser-Assisted Fabrication of Nanostructured Substrate Supported Electrodes for Highly Active Supercapacitors. Front. Mater. 2020, 7, 179. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Naguib, M. Multifunctional Pure MXene Fiber from Liquid Crystals of Only Water and MXene. ACS Cent. Sci. 2020, 6, 344–346. [Google Scholar] [CrossRef] [Green Version]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.-Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel electronic and magnetic properties of two-dimensional transition metal carbides and nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Ghidiu, M.; Naguib, M.; Shi, C.; Mashtalir, O.; Pan, L.M.; Zhang, B.; Yang, J.; Gogotsi, Y.; Billinge, S.J.L.; Barsoum, M.W. Synthesis and characterization of two-dimensional Nb4C3 (MXene). Chem. Commun. 2014, 50, 9517–9520. [Google Scholar] [CrossRef]
- Mei, J.; Ayoko, G.A.; Hu, C.; Bell, J.M.; Sun, Z. Two-dimensional fluorine-free mesoporous Mo2C MXene via UV-induced selective etching of Mo2Ga2C for energy storage. Sustain. Mater. Technol. 2020, 25, e00156. [Google Scholar] [CrossRef]
- Wu, M.; Wang, B.; Hu, Q.; Wang, L.; Zhou, A. The synthesis process and thermal stability of V2C MXene. Materials 2018, 11, 2112. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Sun, W.; Ilani-Kashkouli, P.; Tselev, A.; Kent, P.R.; Kabengi, N.; Naguib, M.; Alhabeb, M.; Tsai, W.Y.; Baddorf, A.P.; et al. Tracking ion intercalation into layered Ti3C2 MXene films across length scales. Energy Environ. Sci. 2020, 13, 2549–2558. [Google Scholar] [CrossRef]
- Barsoum, M.W. MAX Phases: Properties of Machinable Ternary Carbides and Nitrides; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Babar, Z.U.D.; Fatheema, J.; Arif, N.; Anwar, M.S.; Gul, S.; Iqbal, M.; Rizwan, S. Magnetic phase transition from paramagnetic in Nb 2 AlC-MAX to superconductivity-like diamagnetic in Nb 2 C-MXene: An experimental and computational analysis. RSC Adv. 2020, 10, 25669–25678. [Google Scholar] [CrossRef]
- Fatima, M.; Fatheema, J.; Monir, N.B.; Siddique, A.H.; Khan, B.; Islam, A.; Akinwande, D.; Rizwan, S. Nb-Doped MXene With Enhanced Energy Storage Capacity and Stability. Front. Chem. 2020, 8, 168. [Google Scholar] [CrossRef]
- Fatheema, J.; Fatima, M.; Monir, N.B.; Khan, A.S.; Rizwan, S. A comprehensive computational and experimental analysis of stable ferromagnetism in layered 2D Nb-doped Ti3C2 MXene. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 124, 114253. [Google Scholar] [CrossRef]
- Jakubowicz, J.; Sopata, M.; Adamek, G.; Siwak, P.; Kachlicki, T. Formation and Properties of the Ta-Y2O3, Ta-ZrO2, and Ta-TaC Nanocomposites. Adv. Mater. Sci. Eng. 2018, 2018, 2085368. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Gogotsi, Y. Synthesis of two-dimensional materials by selective extraction. Acc. Chem. Res. 2015, 48, 128–135. [Google Scholar] [CrossRef]
- Naguib, M.; Tang, W.; Browning, K.L.; Veith, G.M.; Maliekkal, V.; Neurock, M.; Villa, A. Catalytic Activity of Ti-based MXenes for the Hydrogenation of Furfural. ChemCatChem 2020, 12, 5733–5742. [Google Scholar] [CrossRef]
- Mohammadi, A.V.; Hadjikhani, A.; Shahbazmohamadi, S.; Beidaghi, M. Two-Dimensional Vanadium Carbide (MXene) as a High-Capacity Cathode Material for Rechargeable Aluminum Batteries. ACS Nano 2017, 11, 11135–11144. [Google Scholar]
- Yoon, Y.; Tiwari, A.P.; Choi, M.; Novak, T.G.; Song, W.; Chang, H.; Zyung, T.; Lee, S.S.; Jeon, S.; An, K.-S. Precious-Metal-Free Electrocatalysts for Activation of Hydrogen Evolution with Nonmetallic Electron Donor: Chemical Composition Controllable Phosphorous Doped Vanadium Carbide MXene. Adv. Funct. Mater. 2019, 29, 1903443. [Google Scholar] [CrossRef]
- Shan, Q.; Mu, X.; Alhabeb, M.; Shuck, C.E.; Di, P.; Zhao, X.; Chu, X.-F.; Wei, Y.; Du, F.; Chen, G. Two-dimensional vanadium carbide (V2C) MXene as electrode for supercapacitors with aqueous electrolytes. Electrochem. Commun. 2018, 96, 103–107. [Google Scholar] [CrossRef]
- Hu, J.; Xu, B.; Ouyang, C.; Yang, S.A.; Yao, Y. Investigations on V2C and V2CX2 (X = F, OH) monolayer as a promising anode material for Li ion batteries from first-principles calculations. J. Phys. Chem. C 2014, 118, 24274–24281. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Qiao, X.; Zhang, N.; Zhang, C.; Ma, Z.; Wang, H. Lithium-Ion-Engineered Interlayers of V2C MXene as Advanced Host for Flexible Sulfur Cathode with Enhanced Rate Performance. J. Phys. Chem. Lett. 2020, 11, 885–890. [Google Scholar] [CrossRef]
- Lee, E.; VahidMohammadi, A.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Two-Dimensional Vanadium Carbide MXene for Gas Sensors with Ultrahigh Sensitivity Toward Nonpolar Gases. ACS Sens. 2019, 4, 1603–1611. [Google Scholar] [CrossRef]
- Gao, G.; Ding, G.; Li, J.; Yao, K.; Wu, M.; Qian, M. Monolayer MXenes: Promising half-metals and spin gapless semiconductors. Nanoscale 2016, 8, 8986–8994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaha, P.; Schwarz, K.; Madsen, G.; Kvasnicka, D.; Luitz, J. WIEN2k: An Augmented Plane Wave Plus Local Orbitals Program for Calculating Crystal Properties; Vienna University of Technology, Institute of Materials Chemistry: Vienna, Austria, 2001. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimmer, E.; Krakauer, H.; Weinert, M.; Freeman, A.J. Full-potential self-consistent linearized-augmented-plane-wave method for calculating the electronic structure of molecules and surfaces: O_2 molecule. Phys. Rev. B 1981, 24, 864–875. [Google Scholar] [CrossRef]
- Dall’Agnese, Y.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Two-Dimensional Vanadium Carbide (MXene) as Positive Electrode for Sodium-Ion Capacitors. J. Phys. Chem. Lett. 2015, 6, 2305–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VahidMohammadi, A.; Mojtabavi, M.; Caffrey, N.M.; Wanunu, M.; Beidaghi, M. 2D MXenes: Assembling 2D MXenes into Highly Stable Pseudocapacitive Electrodes with High Power and Energy Densities. Adv. Mater. 2019, 31, 1970057. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New Two-Dimensional Niobium and Vanadium Carbides as Promising Materials for Li-Ion Batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhu, J.; Yang, C.; Wang, F. Enhanced supercapacitive performance of manganese oxides doped two-dimensional titanium carbide nanocomposite in alkaline electrolyte. J. Alloy. Compd. 2016, 685, 194–201. [Google Scholar] [CrossRef]
- Feng, L.; Xuan, Z.; Zhao, H.; Bai, Y.; Guo, J.; Su, C.-W.; Chen, X. MnO2 prepared by hydrothermal method and electrochemical performance as anode for lithium-ion battery. Nanoscale Res. Lett. 2014, 9, 290. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.-L.; Pung, S.-Y.; Sreekantan, S.; Ismail, A.A. Synthesis of V2O5 Nanoflakes on PET Fiber as Visible-Light-Driven Photocatalysts for Degradation of RhB Dye. J. Catal. 2014, 2014, 370696. [Google Scholar] [CrossRef] [Green Version]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Chen, K.; Tong, D.; Huang, Y.; Zhang, J.; Xue, J.; Huang, Q.; Chen, T. CO2 and temperature dual responsive “Smart” MXene phases. Chem. Commun. 2015, 51, 314–317. [Google Scholar] [CrossRef]
- Spanier, J.E.; Gupta, S.; Amer, M.; Barsoum, M.W. Vibrational behavior of the ${M}_{n+1}A{X}_{n}$ phases from first-order Raman scattering $(M=\mathrm{Ti},\mathrm{V},\mathrm{Cr},$ $A=\mathrm{Si},$ $X=\mathrm{C},\mathrm{N})$. Phys. Rev. B 2005, 71, 12103. [Google Scholar] [CrossRef] [Green Version]
- Widjaja, E.; Sampanthar, J. The detection of laser-induced structural change of MnO2 using in situ Raman spectroscopy combined with self-modeling curve resolution technique. Anal. Chim. Acta 2007, 585, 241–245. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.; Wu, X.; Han, Q.; Wang, X. Graphene Oxide−MnO2 Nanocomposites for Supercapacitors. ACS Nano 2010, 4, 2822–2830. [Google Scholar] [CrossRef]
- Yorulmaz, U.; Özden, A.; Perkgöz, N.K.; Ay, F.; Sevik, C. Vibrational and mechanical properties of single layer MXene structures: A first-principles investigation. Nanotechnology 2016, 27, 335702. [Google Scholar] [CrossRef]
- Schwarz, K.; Blaha, P.; Madsen, G.K.H. Electronic structure calculations of solids using the WIEN2k package for material sciences. Comput. Phys. Commun. 2002, 147, 71–76. [Google Scholar] [CrossRef]
- Perdew, J.P.; Yang, W.; Burke, K.; Yang, Z.; Gross, E.K.; Scheffler, M.; Scuseria, G.E.; Henderson, T.M.; Zhang, I.Y.; Ruzsinszky, A.; et al. Understanding band gaps of solids in generalized Kohn–Sham theory. Proc. Natl. Acad. Sci. USA 2017, 114, 2801–2806. [Google Scholar] [CrossRef] [Green Version]
- Bowman, A.L.; Wallace, T.C.; Yarnell, J.L.; Wenzel, R.G.; Storms, E.K. The crystal structures of V2C. Acta Crystallogr. 1965, 19, 6–9. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Shen, P. Are MXenes promising anode materials for Li ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) monolayer. J. Am. Chem. Soc. 2012, 134, 16909–16916. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Kang, W.; Xue, J. MXene nanoribbons. J. Mater. Chem. C. 2015, 3, 879–888. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Scheibe, B.; Szuplewska, A.; Rozmysłowska-Wojciechowska, A.; Chudy, M.; Aparicio, C.; Scheibe, M.; Janica, I.; Ciesielski, A.; Otyepka, M.; et al. On the rapid in situ oxidation of two-dimensional V2CTz MXene in culture cell media and their cytotoxicity. Mater. Sci. Eng. C 2021, 119, 111431. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Gkountaras, A.; Chaix-Pluchery, O.; Gélard, I.; Coraux, J.; Chapelier, C.; Barsoum, M.W.; Ouisse, T. Elementary processes governing V 2 AlC chemical etching in HF. RSC Adv. 2020, 10, 25266–25274. [Google Scholar] [CrossRef]
- Ravuri, S.; Kollu, P.; Jeong, S. Synthesis and properties of 2D-Titanium carbide MXene sheets towards electrochemical energy storage applications. Ceram. Int. 2017. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, M.; Zahra, S.A.; Khan, S.A.; Akinwande, D.; Minár, J.; Rizwan, S. Experimental and Computational Analysis of MnO2@V2C-MXene for Enhanced Energy Storage. Nanomaterials 2021, 11, 1707. https://doi.org/10.3390/nano11071707
Fatima M, Zahra SA, Khan SA, Akinwande D, Minár J, Rizwan S. Experimental and Computational Analysis of MnO2@V2C-MXene for Enhanced Energy Storage. Nanomaterials. 2021; 11(7):1707. https://doi.org/10.3390/nano11071707
Chicago/Turabian StyleFatima, Mahjabeen, Syedah Afsheen Zahra, Saleem Ayaz Khan, Deji Akinwande, Jan Minár, and Syed Rizwan. 2021. "Experimental and Computational Analysis of MnO2@V2C-MXene for Enhanced Energy Storage" Nanomaterials 11, no. 7: 1707. https://doi.org/10.3390/nano11071707
APA StyleFatima, M., Zahra, S. A., Khan, S. A., Akinwande, D., Minár, J., & Rizwan, S. (2021). Experimental and Computational Analysis of MnO2@V2C-MXene for Enhanced Energy Storage. Nanomaterials, 11(7), 1707. https://doi.org/10.3390/nano11071707





