First-Principles Study of Au-Doped InN Monolayer as Adsorbent and Gas Sensing Material for SF6 Decomposed Species
Abstract
:1. Introduction
2. Computation Methods
3. Results and Discussion
3.1. Isolated HF, SO2, SOF2, SO2F2 Molecules and Au-InN Monolayer
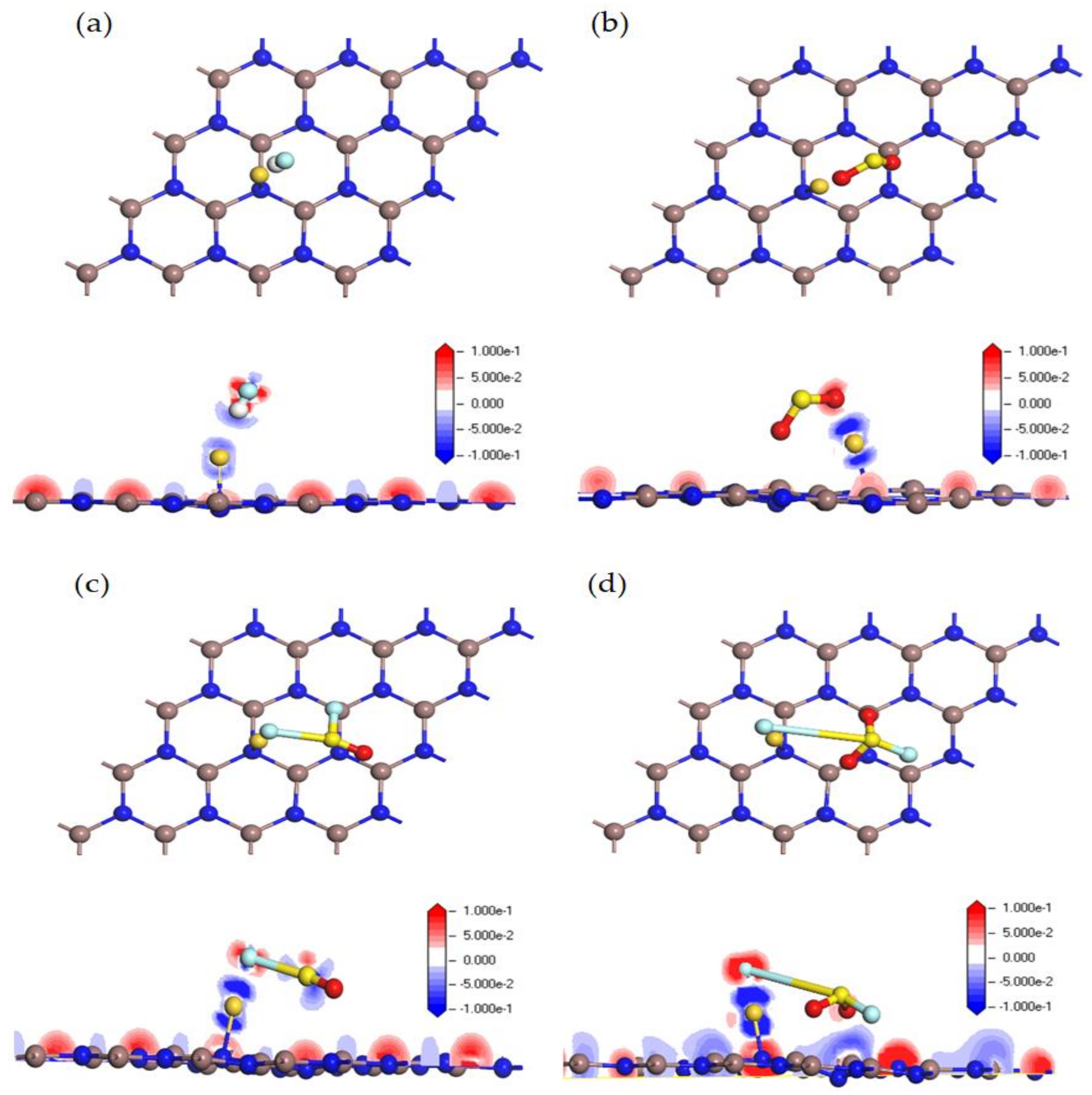
3.2. Adsorption Behavior of HF, SO2, SOF2 and SO2F2 on Au-InN Monolayer
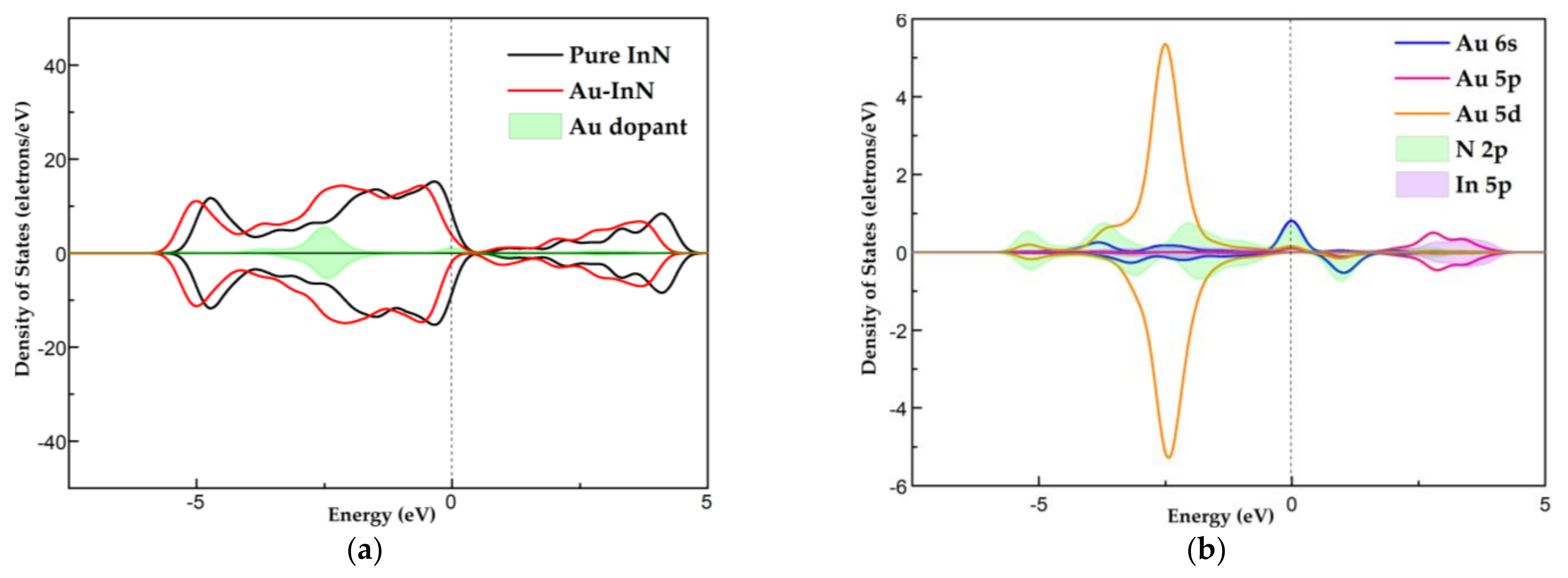
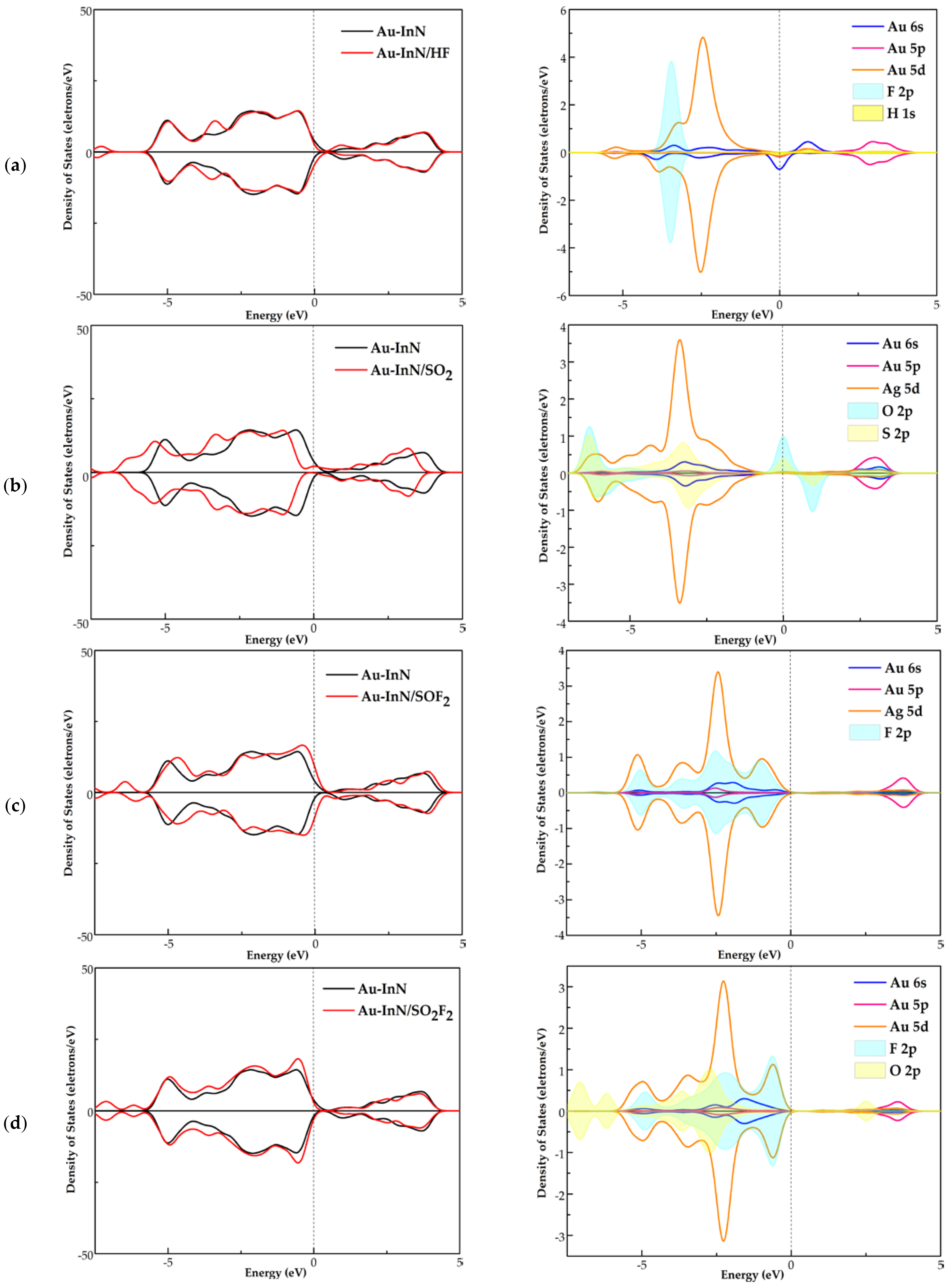
3.3. DOS Analysis of HF, SO2, SOF2 and SO2F2 Adsorption Systems
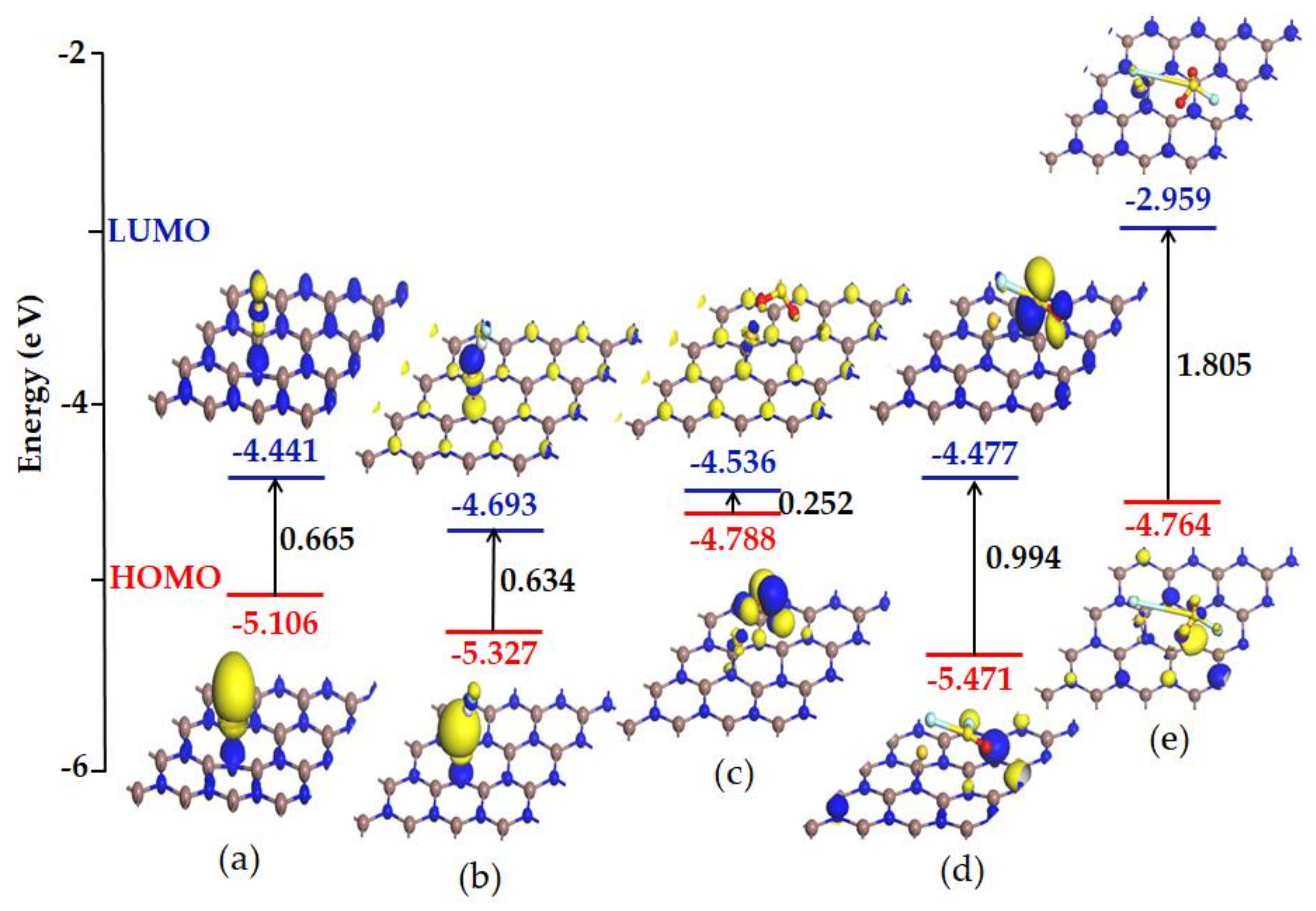
3.4. Frontier Molecular Orbital Analysis
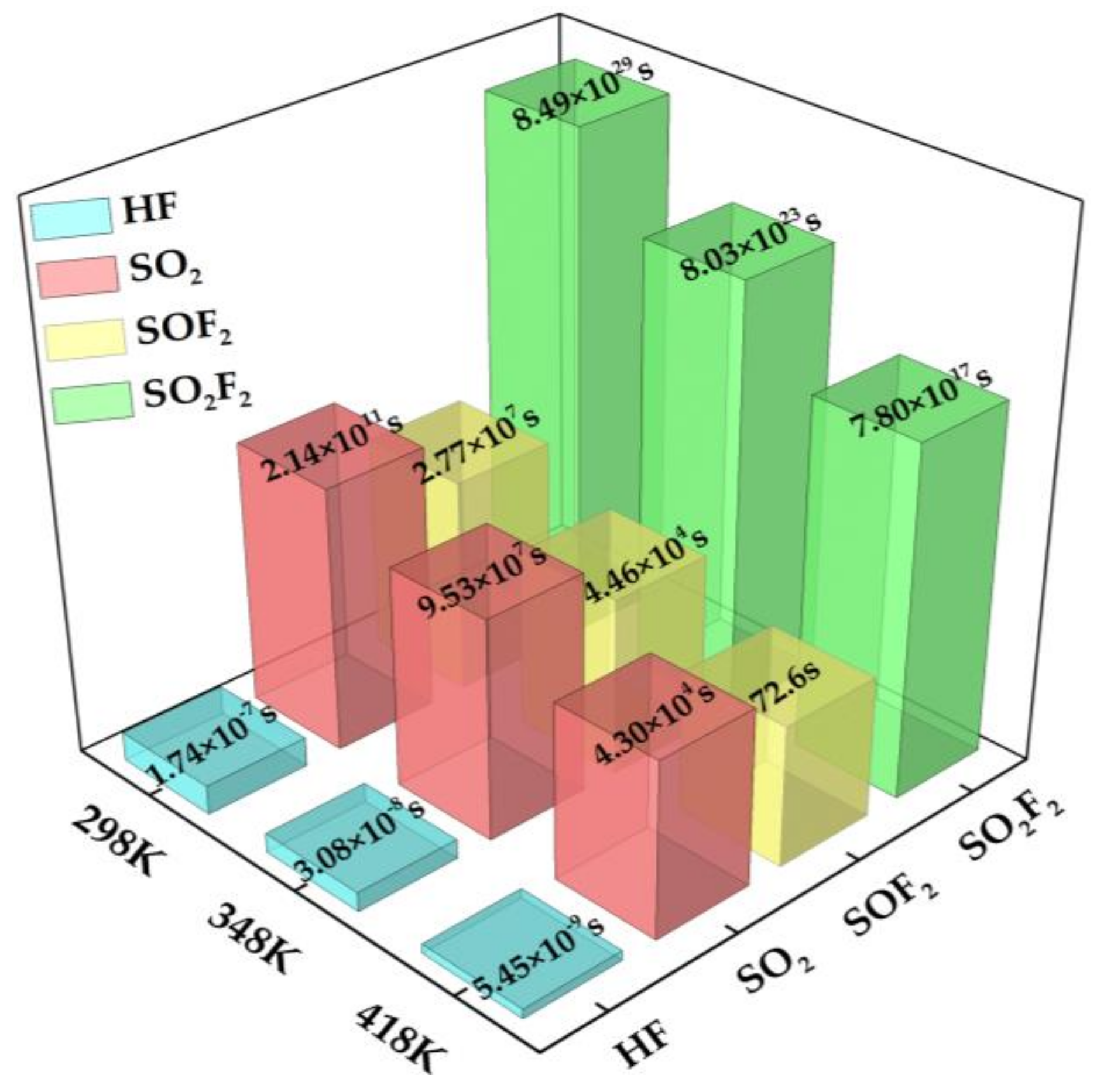
3.5. Recovery Property of Au-InN Monolayer upon HF, SO2, SOF2 and SO2F2
4. Conclusions
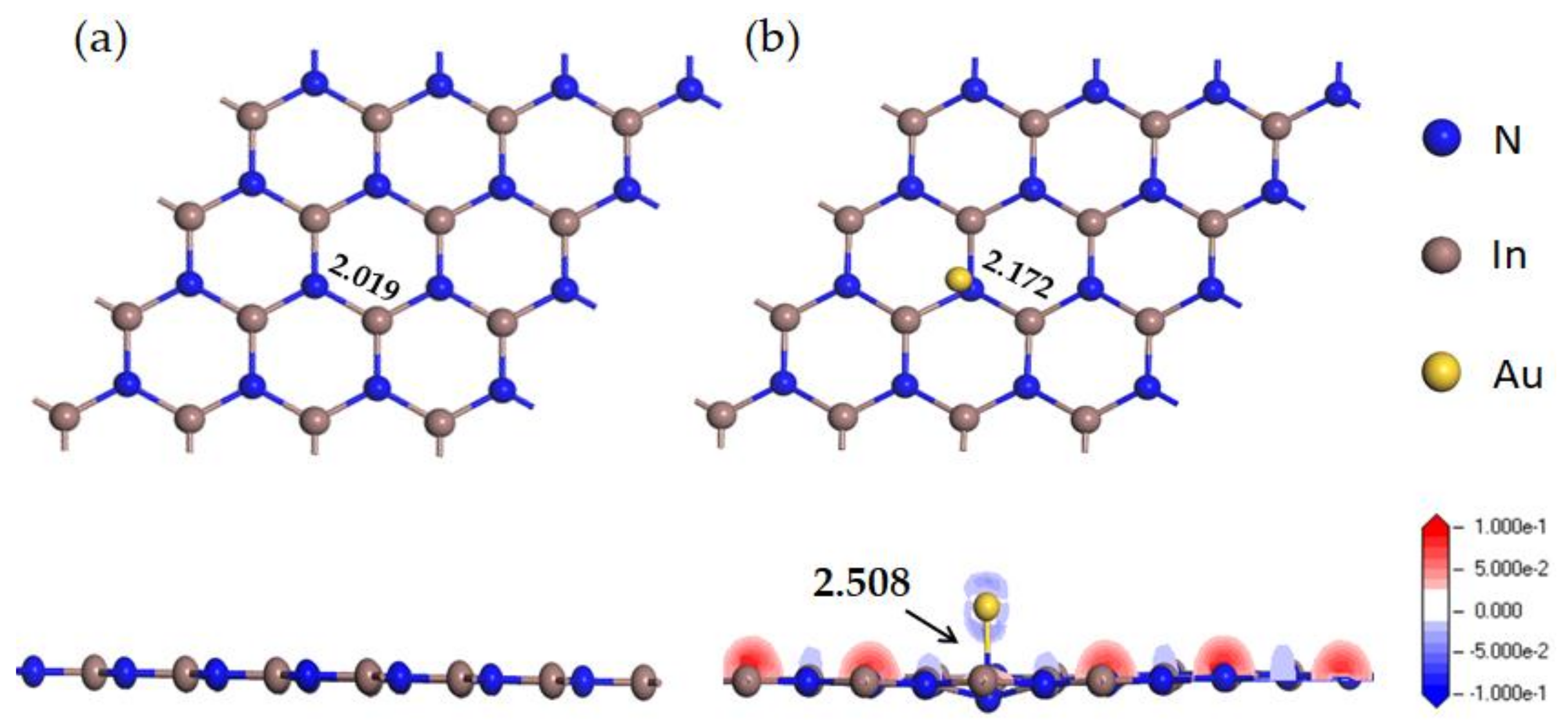
- When the Au atom is doped at the TN site in InN, the doping system is the most stable.
- The Au-InN monolayer has strong adsorption capacity toward three SF6 decomposed species except HF, and the adsorption can be identified as chemisorption. These results indicate that Au-InN can be a promising scavenger for SO2, SOF2 and SO2F2.
- Compared with Cu atom doping, Au atom doping can improve the adsorption capacity of InN to HF, SOF2 and SO2F2 more significantly.
- Combined with the analysis of DOS, the strong orbital hybridization between the atoms of excited SO2, SOF2, SO2F2 and the Au atom can be observed, which not only makes the adsorption configuration more stable but also reflects the good electron mobility of the Au dopant.
- It can be obtained from the analysis of the frontier molecular orbital and recovery properties that Au-InN has broad application prospects as SO2, SOF2 and SO2F2 scavenger and resistive SOF2 sensors, which is of extraordinary importance to ensure the safe operation of power systems.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Glushkov, D.A.; Khalyasmaa, A.I.; Dmitriev, S.A.; Kokin, S.E. Electrical strength analysis of SF6 gas circuit breaker element. AASRI Proc. 2014, 7, 57–61. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.S.; Lee, J.E.; Park, S.; Ahn, C.-K.; Kim, G.-H. Status of SF6 separation/refining technology development for electric industry in Korea. Sep. Purif. Technol. 2018, 200, 29–35. [Google Scholar] [CrossRef]
- Yin, X.; Wu, H.; Dong, L.; Ma, W.; Zhang, L.; Yin, W.; Xiao, L.; Jia, S.; Tittel, F.K. Ppb-level photoacoustic sensor system for saturation-free CO detection of SF6 decomposition by use of a 10 W fiber-amplified near-infrared diode laser. Sensors Actuators B Chem. 2019, 282, 567–573. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, Q.; Wei, Z.; Xu, L.; Peng, S.; Zeng, W. Synthesis of Hollow Nanofibers and Application on Detecting SF6 Decomposing Products. Front. Mater. 2019, 6, 00183. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.X.; Yu, L.; Gui, Y.G.; Hu, W.H. First-principles study of SF6 decomposed gas adsorbed on Au-decorated grapheme. Appl. Surf. Sci. 2016, 367, 259–269. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, Z.; Cui, H.; Pi, S. Theoretical Study of Monolayer PtSe2 as Outstanding Gas Sensor to Detect SF6 Decompositions. IEEE Electron. Device Lett. 2018, 39, 1405–1408. [Google Scholar] [CrossRef]
- Zeng, F.P.; Wu, S.Y.; Lei, Z.C.; Li, C.; Tang, J.; Yao, Q.; Miao, Y.L. SF6 fault decomposition feature component extraction and triangle fault diAunosis method. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 581–589. [Google Scholar] [CrossRef]
- Andersen, M.P.S.; Kyte, M.; Andersen, S.T.; Nielsen, C.; Nielsen, O.J. Atmospheric Chemistry of (CF3)2CF–C≡N: A Replacement Compound for the Most Potent Industrial Greenhouse Gas, SF6. Environ. Sci. Technol. 2017, 51, 1321–1329. [Google Scholar] [CrossRef]
- Chu, J.; Wang, X.; Wang, D.; Yang, A.; Lv, P.; Wu, Y.; Rong, M.; Gao, L. Highly selective detection of sulfur hexafluoride decomposition components H2S and SOF2 employing sensors based on tin oxide modified reduced graphene oxide. Carbon 2018, 135, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhou, Q.; Zhang, Q.; Hong, C.; Xu, L.; Jin, L.; Chen, W. Synthesis, Characterization and Enhanced Sensing Properties of a NiO/ZnO p–n Junctions Sensor for the SF6 Decomposition Byproducts SO2, SO2F2, and SOF. Sensors 2017, 17, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, M.G.; Messina, E.; Foti, A.; Smart, T.J.; Jones, P.H.; Iatì, M.A.; Saija, R.; Gucciardi, P.G.; Marago, O.M. Optical trapping and optical force positioning of two-dimensional materials. Nanoscale 2018, 10, 1245–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, A.; Sardroodi, J.J. The adsorption of sulfur trioxide and ozone molecules on stanene nanosheets investigated by DFT: Applications to gas sensor devices. Phys. E Low Dimens. Syst. Nanostructures 2019, 108, 382–390. [Google Scholar] [CrossRef]
- Cesca, T.; Michieli, N.; Kalinic, B.; Balasa, I.G.; Rangel-Rojo, R.; Reyes-Esqueda, J.A.; Mattei, G. Bidimensional ordered plasmonic nanoarrays for nonlinear optics, nanophotonics and biosensing applications. Mater. Sci. Semicond. Process. 2019, 92, 2–9. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, Z.; Cui, H. Pristine and Cu decorated hexagonal InN monolayer, a promising candidate to detect and scavenge SF6 decompositions based on first-principle study. J. Hazard. Mater. 2019, 363, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhou, Q.; Wang, J.; Xu, L.; Zeng, W. Adsorption of SO2 molecule on Ni-doped and Pd-doped graphene based on first-principle study. Appl. Surf. Sci. 2020, 517, 146180. [Google Scholar] [CrossRef]
- Ma, P.; Salamin, Y.; Baeuerle, B.; Josten, A.; Heni, W.; Emboras, A.; Leuthold, J. Plasmonically Enhanced Graphene Photodetector Featuring 100 Gbit/s Data Reception, High Responsivity, and Compact Size. ACS Photon. 2018, 6, 154–161. [Google Scholar] [CrossRef]
- Idress, M.; Batool, S.; Kong, J.; Zhuang, Q.; Liu, H.; Shao, Q.; Lu, N.; Feng, F.N.; Wujcik, E.K.; Gao, Q.; et al. Polyborosilazane derived ceramics-Nitrogen sulfur dual doped grapheme nanocomposite anode for enhanced lithium ion batteries. Electrochim. Acta 2019, 296, 925–937. [Google Scholar] [CrossRef]
- Sun, Z.; Chang, H. Graphene and Graphene-like Two-Dimensional Materials in Photodetection: Mechanisms and Methodology. ACS Nano 2014, 8, 4133–4156. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Li, Y.; Chen, D.; Zhang, Y. First-principles insight into Ni-doped InN monolayer as a noxious gases scavenger. Appl. Surf. Sci. 2019, 494, 859–866. [Google Scholar] [CrossRef]
- Sarmazdeh, M.M.; Mendi, R.T.; Zelati, A.; Boochani, A.; Nofeli, F. First-principles study of optical properties of InN nanosheet. Int. J. Mod. Phys. B 2016, 30, 1650117. [Google Scholar] [CrossRef]
- Dos Santos, R.B.; Mota, F.D.; Rivelino, R.; Kakanakova-Georgieva, A.; Gueorguiev, G.K. Van der Waals stacks of few-layer h-AlN with graphene: An ab initio study of structural, interaction and electronic properties. Nanotechnology 2016, 27, 145601. [Google Scholar] [CrossRef] [Green Version]
- Caliskan, S.; Hazar, F. First principles study on the spin unrestricted electronic structure properties of transition metal doped InN nanoribbons. Superlattices Microstruct. 2015, 84, 170–180. [Google Scholar] [CrossRef]
- Maleyre, B.; Briot, O.; Ruffenach, S.; Gil, B. Optical investigations on Si-doped InN films. Phys. Status Solidi 2005, 2, 1379–1383. [Google Scholar] [CrossRef]
- Yu, K.M.; Liliental-Weber, Z.; Walukiewicz, W.; Shan, W.; Ager, J.W.; Li, S.X.; Jones, R.E.; Haller, E.E.; Lu, H.; Schaff, W.J. On the crystalline structure, stoichiometry and band gap of InN thin films. Appl. Phys. Lett. 2005, 86, 071910. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Sun, Y.E.; Jiang, C.X.; Yao, Y.; Wang, D.Y.; Zhang, Y. Room-temperature highly sensitive CO gas sensor based on Au-loaded zinc oxide/molybdenum disulfide ternary nanocomposite and its sensing properties. Sens. Actuator B Chem. 2017, 253, 1120–1128. [Google Scholar] [CrossRef]
- Wang, C.; Rong, Q.; Zhang, Y.M.; Hu, J.C.; Zi, B.Y.; Zhu, Z.Q.; Zhang, J.; Liu, Q.J. Molecular imprinting Au-LaFeO3 spheres for highly sensitive acetone gas detection. Mater. Res. Bull. 2019, 109, 265–272. [Google Scholar] [CrossRef]
- Yan, M.; Huang, Z.-Q.; Zhang, Y.; Chang, C.-R. Trends in water-promoted oxygen dissociation on the transition metal surfaces from first principles. Phys. Chem. Chem. Phys. 2016, 19, 2364–2371. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Wu, W.; Liu, Y.; Zhou, Z. Transition metal (Pd, Pt, Ag, Au) decorated InN monolayer and their adsorption properties towards NO2: Density functional theory study. Appl. Surf. Sci. 2018, 455, 106–114. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhou, Q.; Lu, Z.R.; Gui, Y.G.; Zeng, W. Adsorption of H2O molecule on TM (Ag, Au) doped-MoS2 monolayer: A first-principles study. Phys. E 2019, 113, 72–78. [Google Scholar] [CrossRef]
- Delley, B.; Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, C.; Li, L.; Zeng, H.; Li, C.; Mitome, M.; Golberg, D.; Bando, Y. “Chemical Blowing” of Thin-Walled Bubbles: High-Throughput Fabrication of Large-Area, Few-Layered BN and Cx-BN Nanosheets. Adv. Mater. 2011, 23, 4072–4076. [Google Scholar] [CrossRef] [PubMed]
- Tamijani, A.A.; Salam, A.; De Lara-Castells, M.P. Adsorption of Noble-Gas Atoms on the TiO2 Surface: An Ab Initio-Assisted Study with van der Waals-Corrected DFT. J. Phys. Chem. C 2016, 120, 18126–18139. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Delley, B. Hardness conserving semilocal pseudopotentials. Phys. Rev. B 2002, 66, 155125. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Li, B.; Zhou, Q.; Peng, R.; Liao, Y.; Zeng, W. Adsorption of SF6 decomposition gases (H2S, SO2, SOF2 and SO2F2) on Sc-doped MoS2 surface: A DFT study. Appl. Surf. Sci. 2021, 549, 149271. [Google Scholar] [CrossRef]
- Ju, W.; Li, T.; Su, X.; Li, H.; Li, X.; Ma, D. Au cluster adsorption on perfect and defective MoS2 monolayers: Structural and electronic properties. Phys. Chem. Chem. Phys. 2017, 19, 20735–20748. [Google Scholar] [CrossRef]
- Wu, P.; Yin, N.; Li, P.; Cheng, W.; Huang, M. The adsorption and diffusion behavior of noble metal adatoms (Pd, Pt, Cu, Ag and Au) on a MoS2 monolayer: A first-principles study. Phys. Chem. Chem. Phys. 2017, 19, 20713–20722. [Google Scholar] [CrossRef]
- Saoud, F.S.; Plenet, J.C.; Henini, M. Structural, electronic and vibrational properties of InN under high pressure. Phys. B Condens. Matter 2012, 407, 1008–1013. [Google Scholar] [CrossRef] [Green Version]
- Shokri, A.; Salami, N. Gas sensor based on MoS2 monolayer. Sensors Actuators B Chem. 2016, 236, 378–385. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom frAuments for describing molecular charge-densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.X.; Zhang, J.; Zhang, Y. Nanomaterials-based gas sensors of SF6 decomposed species for evaluating the operation status of high-voltAue insulation devices. High. Voltague 2019, 4, 242–258. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, D.; Cui, H.; Dong, X.; Xiao, S.; Tang, J. Understanding of SF6 decompositions adsorbed on cobalt-doped SWCNT: A DFT study. Appl. Surf. Sci. 2017, 420, 371–382. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, A.; Wang, X.; Murphy, A.; Li, X.; Liu, D.; Wu, Y.; Rong, M. Theoretical study of the neutral decomposition of SF6in the presence of H2O and O2 in discharges in power equipment. J. Phys. D Appl. Phys. 2016, 49, 385203. [Google Scholar] [CrossRef]
- Cui, H.; Zheng, K.; Zhang, Y.; Ye, H.; Chen, X. Superior Selectivity and Sensitivity of C3N Sensor in Probing Toxic Gases NO2 and SO2. IEEE Electron. Device Lett. 2018, 39, 284–287. [Google Scholar] [CrossRef]
- Allian, A.D.; Takanabe, K.; Fujdala, K.L.; Hao, X.; Truex, T.J.; Cai, J.; Buda, C.; Neurock, M.; Iglesia, E. Chemisorption of CO and Mechanism of CO Oxidation on Supported Platinum Nanoclusters. J. Am. Chem. Soc. 2011, 133, 4498–4517. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Qiu, Y.; Zhu, J.; Zhang, Y.; Hu, G. A DFT study of transition metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-embedded monolayer MoS2 for gas adsorption. Comput. Mater. Sci. 2017, 138, 255–266. [Google Scholar] [CrossRef]
- Ma, D.W.; Ma, B.Y.; Lu, Z.W.; He, C.Z.; Tang, Y.N.; Lu, Z.S.; Yang, Z.X. Interaction between H2O, N2,CO, NO, NO2 and N2O molecules and a defective WSe2 monolayer. Phys. Chem. Chem. Phys. 2017, 19, 26022–26033. [Google Scholar] [CrossRef]
- Giovanni, M.; Poh, H.L.; Ambrosi, A.; Zhao, G.J.; Sofer, Z.; Sanek, F.; Khezri, B.; Webster, R.D.; Pumera, M. Noble metal (Pd, Ru, Rh, Pt, Au, Au) doped grapheme hybrids for electrocatalysis. Nanoscale 2012, 4, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, X.; Tang, J.; Cui, H.; Li, Y. Noble metal (Pt or Au)-doped monolayer MoS2 as a promising adsorbent and gas-sensing material to SO2, SOF2 and SO2F2: A DFT study. Appl. Phys. A 2018, 124, 194. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Chen, Y.-B.; Zhou, K.-G.; Liu, C.; Zeng, J.; Zhang, H.-L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.; Cho, K.; Qi, P.; Dai, H. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. [Google Scholar] [CrossRef]
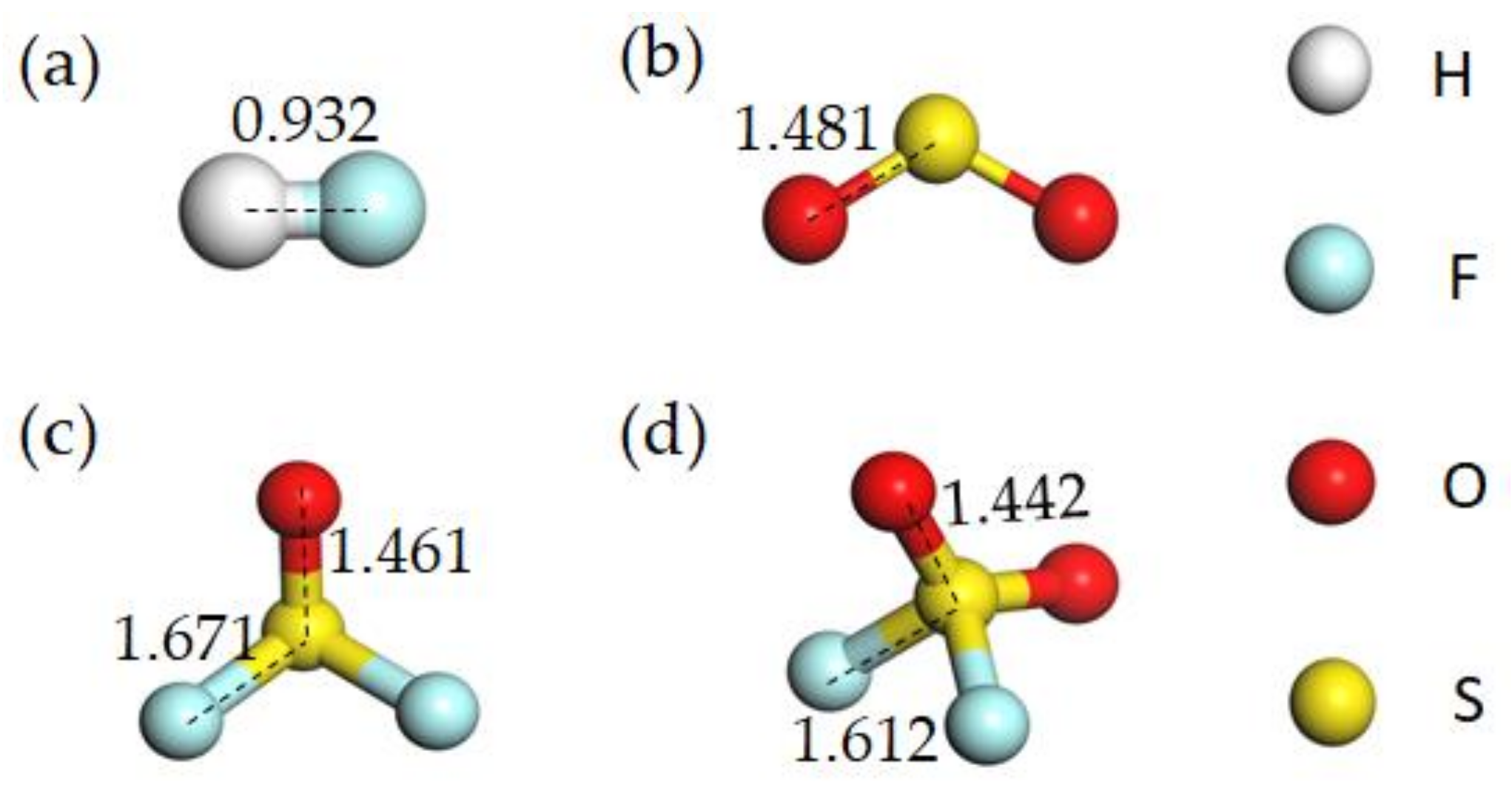
| Gas | Bond Length(Å) | Bond Angle (°) | ||
|---|---|---|---|---|
| HF | H-F | 0.932 | - | - |
| SO2 | S-O | 1.481 | O-S-O | 119.936 |
| SOF2 | S-O | 1.461 | O-S-O | 107.190 |
| S-F | 1.671 | F-S-F | 93.238 | |
| SO2F2 | S-O S-F | 1.442 1.612 | O-S-O | 126.682 |
| F-S-F | 94.408 | |||
| O-S-F | 107.836 | |||
| Gas | H | F | S | O |
|---|---|---|---|---|
| HF | 0.345 | −0.345 | - | - |
| SO2 | - | - | 0.455 | −0.227 |
| SOF2 | - | −0.251 | 0.710 | −0.208 |
| SO2F2 | - | −0.214 | 0.870 | −0.220 |
| System | The Length of Bond (Å) | Adsorption Distance(Å) | Atom | Charge(e) | |
|---|---|---|---|---|---|
| Au-InN+HF | H-F | 0.957 | 2.307 | H | 0.332 |
| Au-N | 2.370 | F | −0.379 | ||
| Au-InN+SO2 | S-O | 1.603 | 2.093 | S | 0.352 |
| Au-N | 2.070 | O1 | −0.434 | ||
| O2 | −0.406 | ||||
| Au-InN+SOF2 | S-F | 2.698 | 2.016 | S | 0.565 |
| O | −0.303 | ||||
| Au-N | 2.081 | F1 | −0.294 | ||
| F2 | −0.537 | ||||
| Au-InN+SO2F2 | S-F | 4.776 | 1.984 | S | 0.593 |
| O1 | −0.384 | ||||
| Au-N | 2.087 | O2 | −0.380 | ||
| F1 | −0.532 | ||||
| F2 | −0.412 | ||||
| Adsorption System | QT (e) | Ead(eV) |
|---|---|---|
| Cu-InN/HF | −0.14 | −0.09 |
| Cu-InN/SO2 | −0.24 | −1.85 |
| Cu-InN/SOF2 | −0.10 | −0.85 |
| Cu-InN/SO2F2 | −0.55 | −1.02 |
| Au-InN/HF | −0.05 | −0.31 |
| Au-InN/SO2 | −0.49 | −1.38 |
| Au-InN/SOF2 | −0.57 | −1.15 |
| Au-InN/SO2F2 | −1.12 | −2.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, R.; Zhou, Q.; Zeng, W. First-Principles Study of Au-Doped InN Monolayer as Adsorbent and Gas Sensing Material for SF6 Decomposed Species. Nanomaterials 2021, 11, 1708. https://doi.org/10.3390/nano11071708
Peng R, Zhou Q, Zeng W. First-Principles Study of Au-Doped InN Monolayer as Adsorbent and Gas Sensing Material for SF6 Decomposed Species. Nanomaterials. 2021; 11(7):1708. https://doi.org/10.3390/nano11071708
Chicago/Turabian StylePeng, Ruochen, Qu Zhou, and Wen Zeng. 2021. "First-Principles Study of Au-Doped InN Monolayer as Adsorbent and Gas Sensing Material for SF6 Decomposed Species" Nanomaterials 11, no. 7: 1708. https://doi.org/10.3390/nano11071708
APA StylePeng, R., Zhou, Q., & Zeng, W. (2021). First-Principles Study of Au-Doped InN Monolayer as Adsorbent and Gas Sensing Material for SF6 Decomposed Species. Nanomaterials, 11(7), 1708. https://doi.org/10.3390/nano11071708








