5-Fluorouracil Encapsulated Chitosan-Cellulose Fiber Bionanocomposites: Synthesis, Characterization and In Vitro Analysis towards Colorectal Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
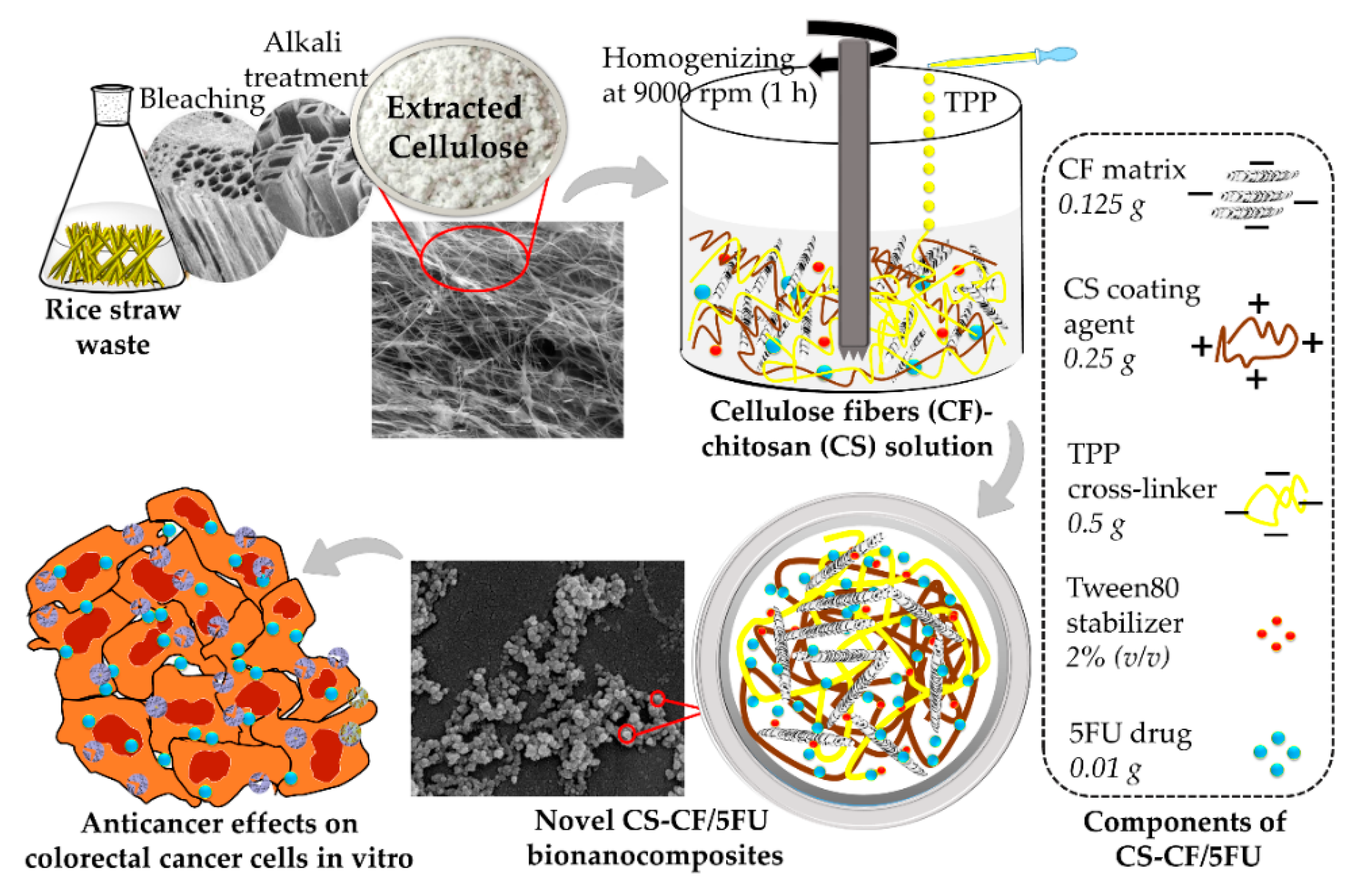
2.2.1. Extraction of Cellulose Fibers from Rice Straw Waste
2.2.2. Synthesis of Chitosan-Cellulose Fiber Bionanocomposites to Encapsulate 5-Fluorouracil
2.3. Characterization
2.3.1. Physicochemical Analysis
2.3.2. Encapsulation Efficiency Study of 5-Fluorouracil
2.3.3. A Comparative Study of In Vitro Release of 5-Fluorouracil Drug from CS-CF/5FU BNCs
2.3.4. Cell Lines and Reagents
2.3.5. In Vitro Cytotoxicity Assay
2.3.6. Statistical Analysis
3. Results and Discussion
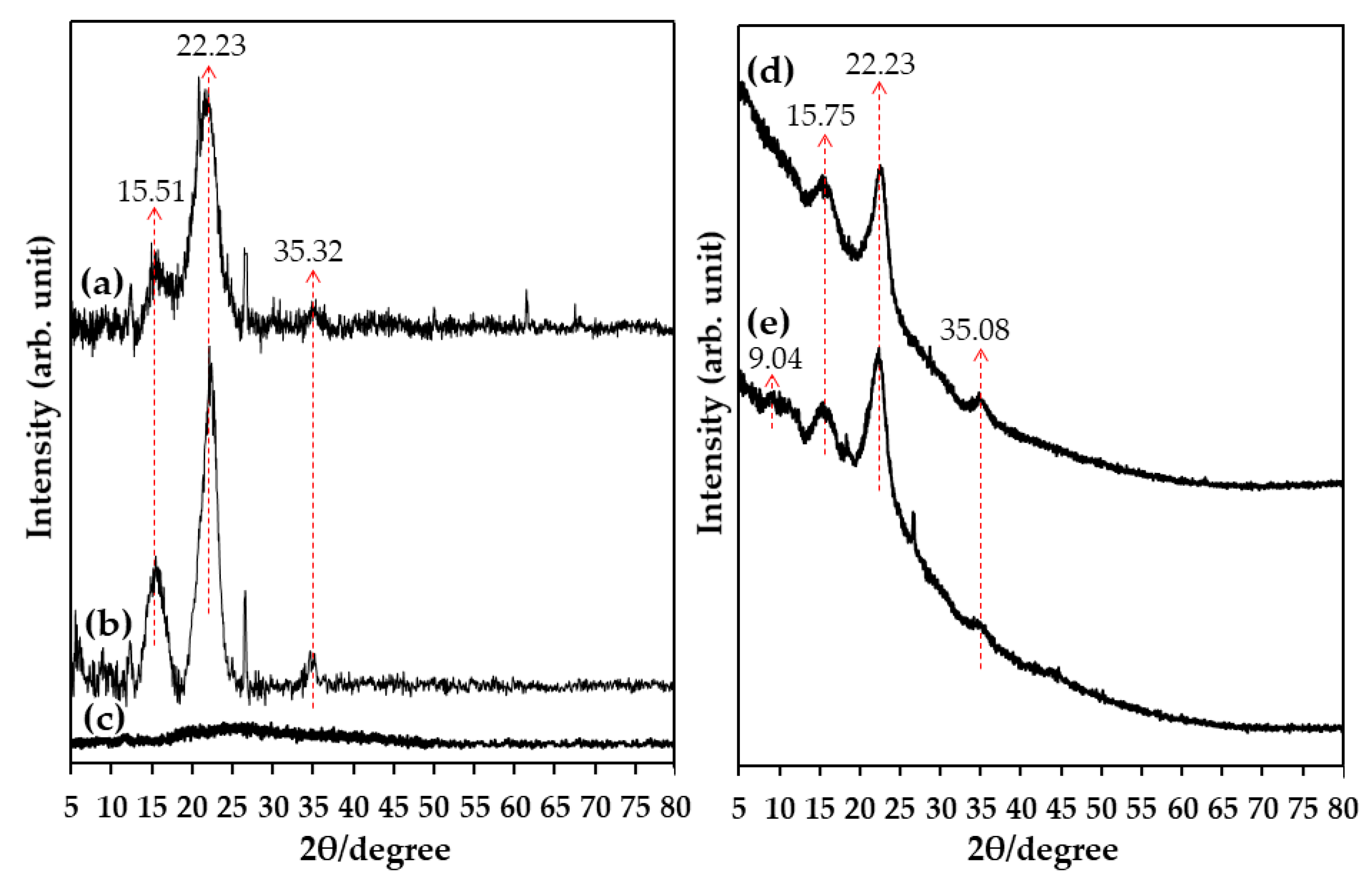
3.1. Physicochemical Characterization of CS-CF/5FU BNCs Using X-ray Powder Diffraction
3.2. Physicochemical Characterization of CS-CF/5FU BNCs Using Scanning Electron Microscopy and Energy Dispersive X-ray Analyses
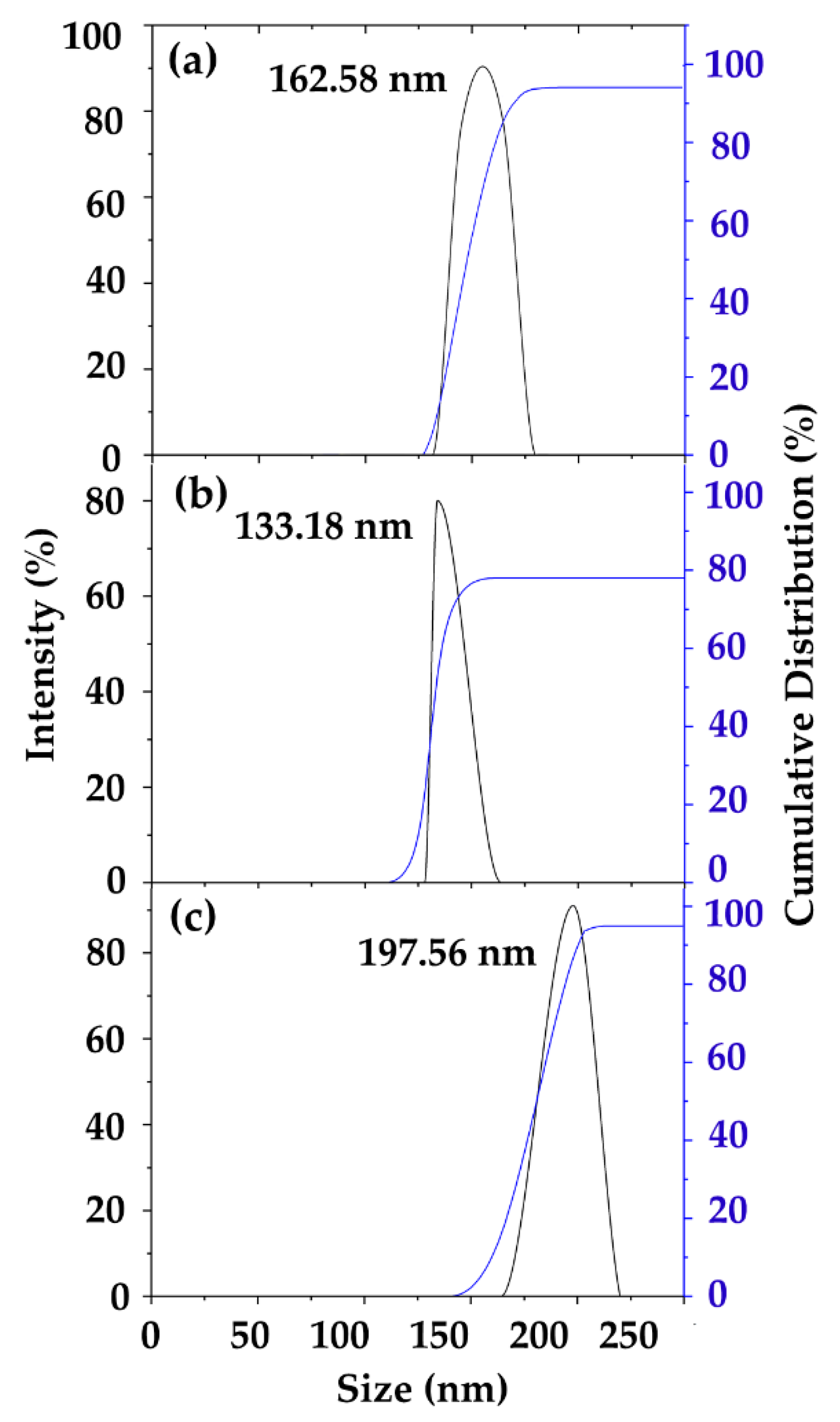
3.3. Physicochemical Characterization of CS-CF/5FU BNCs Using Dynamic Light Scattering
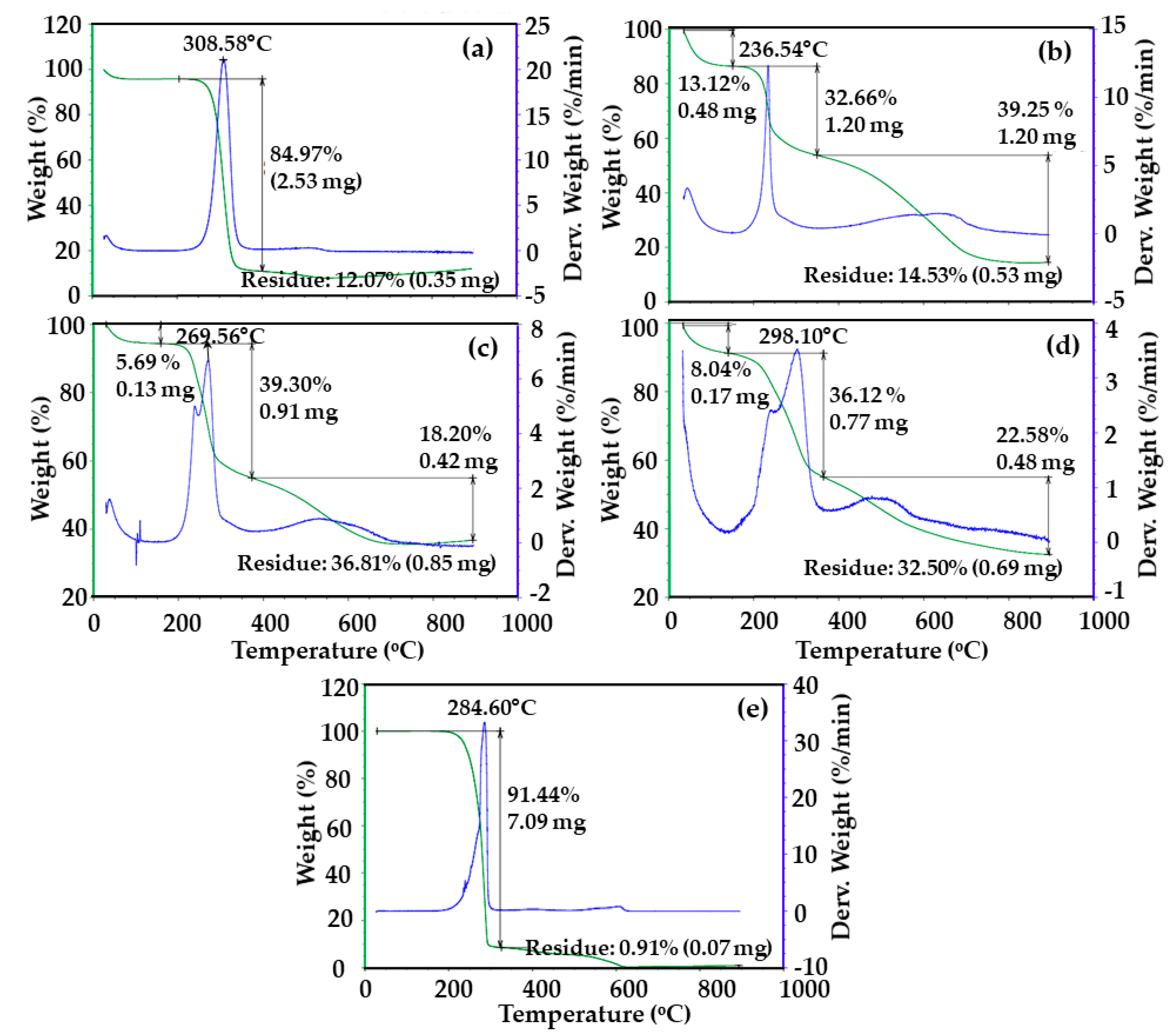
3.4. Physicochemical Characterization of CS-CF/5FU BNCs Using Thermal Analysis
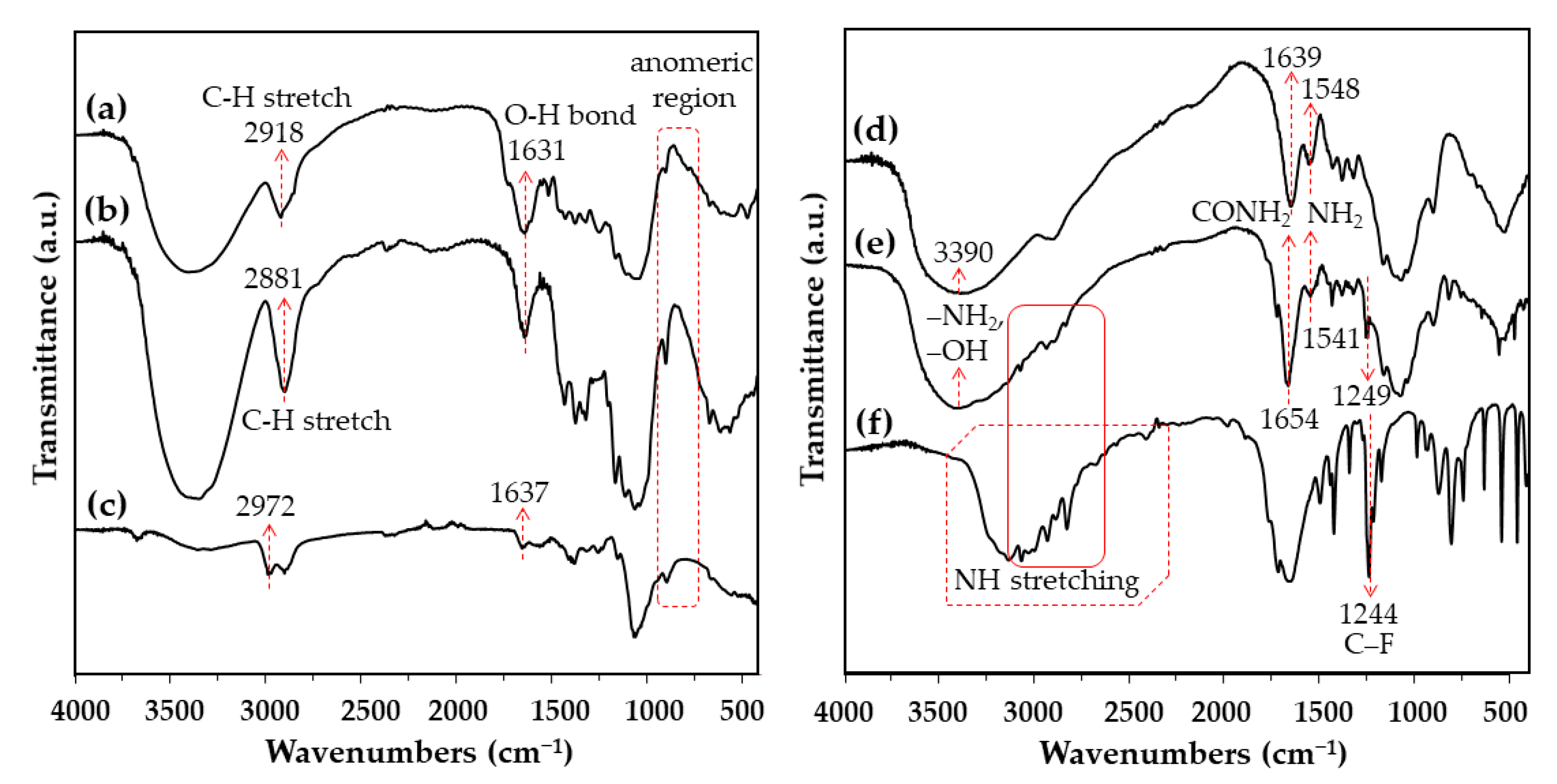
3.5. Physicochemical Characterization of CS-CF/5FU BNCs Using Fourier-Transform Infrared Spectroscopy
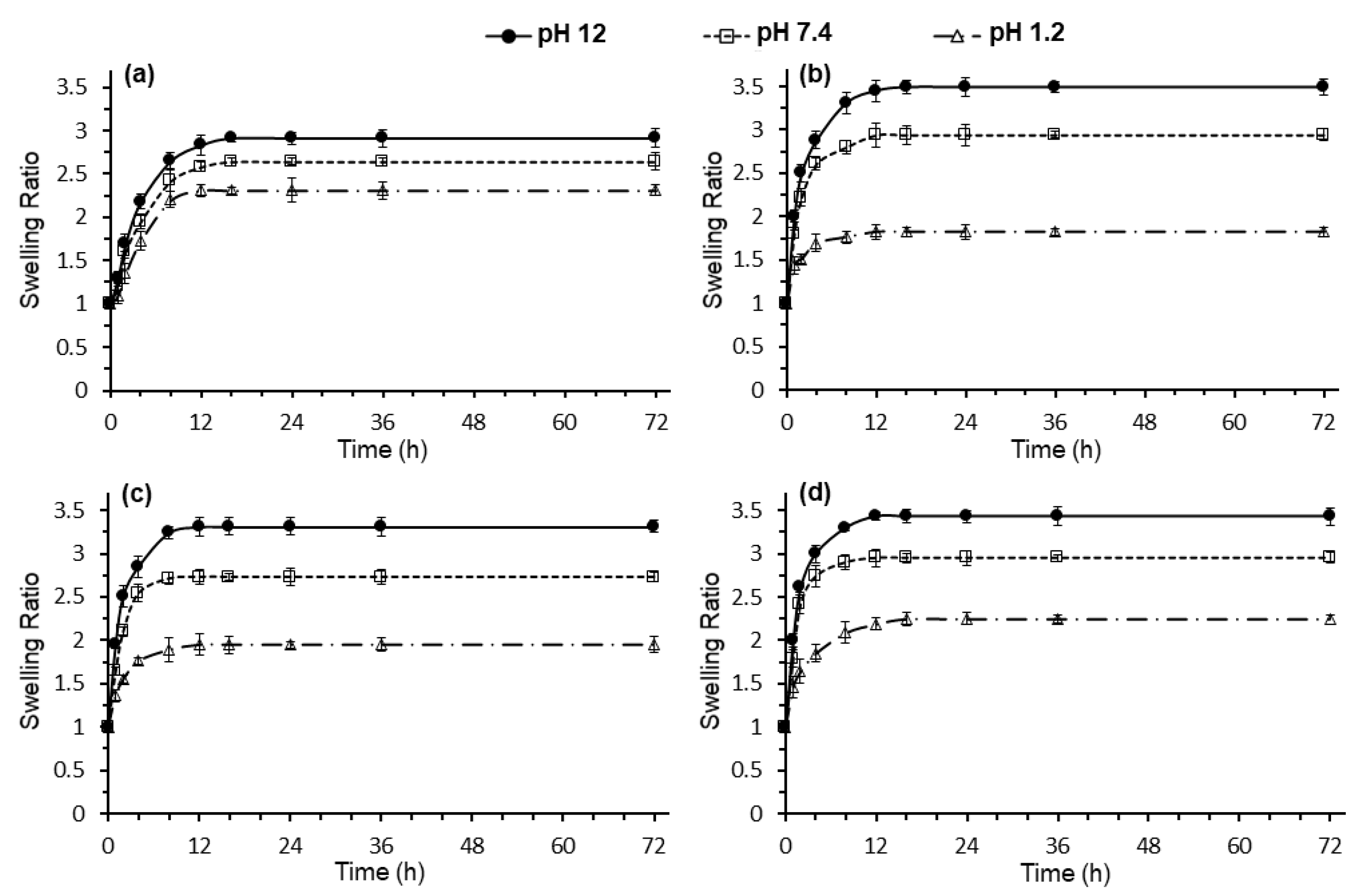
3.6. Swelling Analysis of CS-CF/5FU BNCs
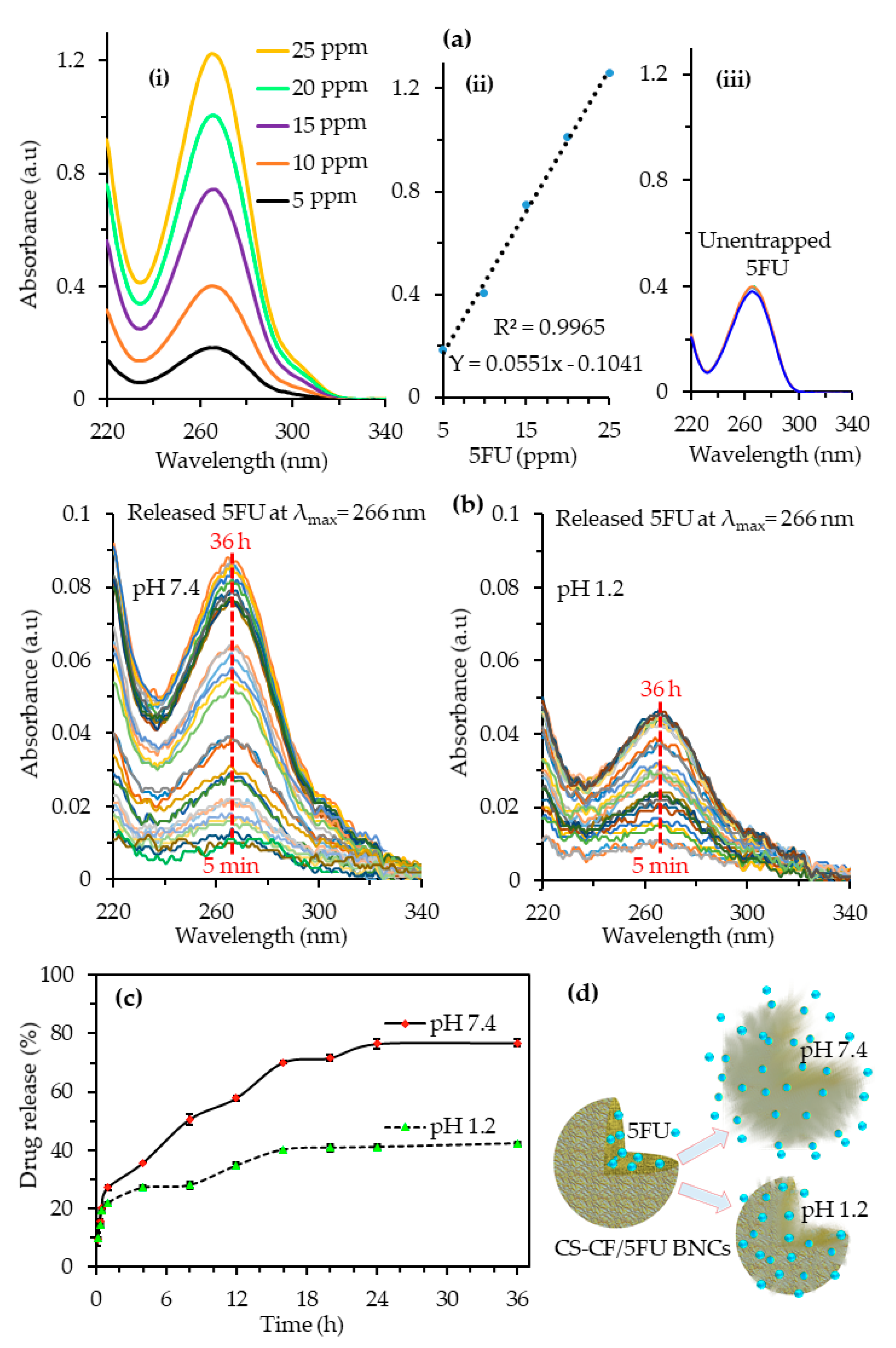
3.7. Drug Loading and Encapsulation Efficiency Percentage of CS-CF/5FU BNCs
3.8. In Vitro Drug Release of CS-CF/5FU BNCs
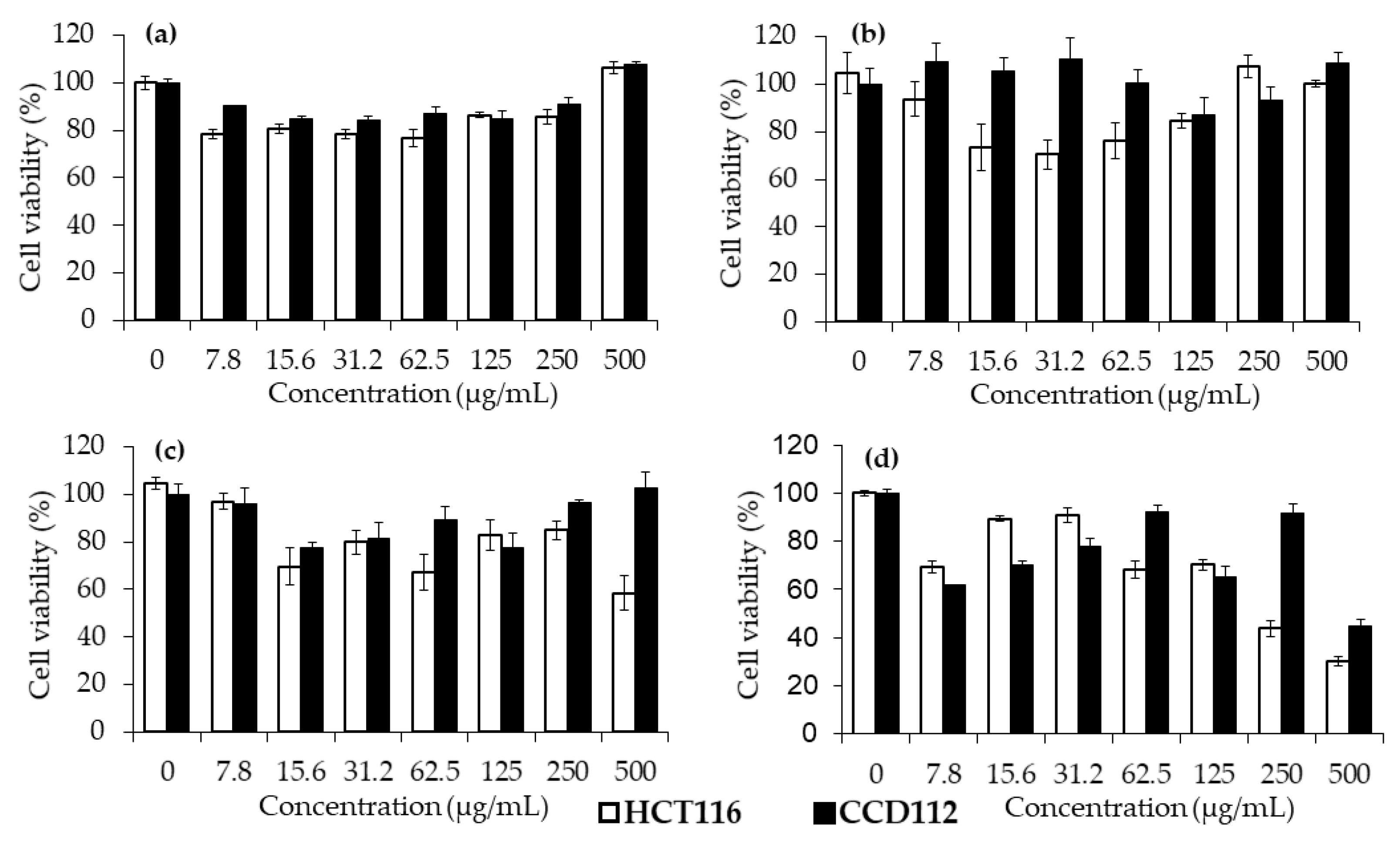
3.9. In Vitro Cytotoxicity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yusefi, M.; Yee, O.S.; Shameli, K. Bio-mediated production and characterisation of magnetic nanoparticles using fruit peel extract. J. Res. Nanosci. Nanotechnol. 2021, 1, 53–61. [Google Scholar] [CrossRef]
- Perumal, A.B.; Sellamuthu, P.S.; Nambiar, R.B.; Sadiku, E.R. Development of polyvinyl alcohol/chitosan bio-nanocomposite films reinforced with cellulose nanocrystals isolated from rice straw. Appl. Surf. Sci. 2018, 449, 591–602. [Google Scholar] [CrossRef]
- Chen, X.; Yu, J.; Zhang, Z.; Lu, C. Study on structure and thermal stability properties of cellulose fibers from rice straw. Carbohydr. Polym. 2011, 85, 245–250. [Google Scholar] [CrossRef]
- Ahmad, R.; Deng, Y.; Singh, R.; Hussain, M.; Shah, M.A.A.; Elingarami, S.; He, N.; Sun, Y. Cutting edge protein and carbohydrate-based materials for anticancer drug delivery. J. Biomed. Nanotechnol. 2018, 14, 20–43. [Google Scholar] [CrossRef] [PubMed]
- Aimonen, K.; Suhonen, S.; Hartikainen, M.; Lopes, V.R.; Norppa, H.; Ferraz, N.; Catalán, J. Role of surface chemistry in the in vitro lung response to nanofibrillated cellulose. Nanomaterials 2021, 11, 389. [Google Scholar] [CrossRef]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Cui, X.; Guo, Y.; Zhang, X.; Hongyan, W. Synthesis of chitosan-based nanohydrogels for loading and release of 5-fluorouracil. Colloids Surf. A Phys. Eng. Asp. 2016, 490, 91–97. [Google Scholar] [CrossRef]
- Ruman, U.; Buskaran, K.; Pastorin, G.; Masarudin, M.J.; Fakurazi, S.; Hussein, M.Z. Synthesis and characterization of chitosan-based nanodelivery systems to enhance the anticancer effect of sorafenib drug in hepatocellular carcinoma and colorectal adenocarcinoma cells. Nanomaterials 2021, 11, 497. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Schwaiger, D.; Lohstroh, W.; Müller-Buschbaum, P. Investigation of molecular dynamics of a PTB7: PCBM polymer blend with quasi-elastic neutron scattering. ACS Appl. Polym. Mater. 2020, 2, 3797–3804. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Ogueri, K.S.; Allcock, H.R.; Laurencin, C.T. A regenerative polymer blend composed of glycylglycine ethyl ester-substituted polyphosphazene and poly (lactic-co-glycolic acid). ACS Appl. Polym. Mater. 2020, 2, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Ignacz, G.; Fei, F.; Szekely, G. Ion-stabilized membranes for demanding environments fabricated from polybenzimidazole and its blends with polymers of intrinsic microporosity. ACS Appl. Polym. Mater. 2018, 1, 6349–6356. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-L.; Kan, C.-W.; Wang, W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Christa, J. Multi-polysaccharide based stimuli responsive polymeric network for the in vitro release of 5-fluorouracil and levamisole hydrochloride. New J. Chem. 2017, 41, 11979–11990. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Banerjee, A.; Pathak, S.; Subramanium, V.D.; Dharanivasan, G.; Murugesan, R.; Verma, R.S. Strategies for targeted drug delivery in treatment of colon cancer: Current trends and future perspectives. Drug Discov. Today 2017, 22, 1224–1232. [Google Scholar] [CrossRef]
- Lokich, J. Infusional 5-FU: Historical evolution, rationale, and clinical experience. Oncology 1998, 12, 19–22. [Google Scholar]
- Anirudhan, T.S.; Binusreejayan, R.S.R. Synthesis and characterization of chitosan based multilayer and pH sensitive co-polymeric system for the targeted delivery of 5-fluorouracil, an in vitro study. Int. J. Polym. Mater 2014, 63, 539–548. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Nima, J.; Divya, P.L. Synthesis, characterization and in vitro cytotoxicity analysis of a novel cellulose based drug carrier for the controlled delivery of 5-fluorouracil, an anticancer drug. Appl. Surf. Sci. 2015, 355, 64–73. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Jahangirian, H.; Teow, S.-Y.; Umakoshi, H.; Saleh, B.; Rafiee-Moghaddam, R.; Webster, T.J. The potential anticancer activity of 5-fluorouracil loaded in cellulose fibers isolated from rice straw. Int. J. Nanomed. 2020, 15, 5417–5432. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-L.; Kan, C.-W. Thermoresponsive hydrogels and their biomedical applications: Special insight into their applications in textile based transdermal therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Yusefi, M.; Shameli, K.; Jumaat, A.F. Preparation and properties of magnetic iron oxide nanoparticles for biomedical applications: A brief review. J. Adv. Res. Mater. Sci. 2020, 75, 10–18. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K. Nanocellulose as a Vehicle for Drug Delivery and Efficiency of Anticancer Activity: A Short-Review. J. Res. Nanosci. Nanotechnol. 2021, 1, 30–43. [Google Scholar] [CrossRef]
- Topuz, F.; Kilic, M.E.; Durgun, E.; Szekely, G. Fast-dissolving antibacterial nanofibers of cyclodextrin/antibiotic inclusion complexes for oral drug delivery. J. Colloid Interface Sci. 2021, 585, 184–194. [Google Scholar] [CrossRef]
- Shehabeldine, A.; El-Hamshary, H.; Hasanin, M.; El-Faham, A.; Al-Sahly, M. Enhancing the antifungal activity of griseofulvin by incorporation a green biopolymer-based nanocomposite. Polymers 2021, 13, 542. [Google Scholar] [CrossRef]
- Bae, J.; Park, J.W. Preparation of an injectable depot system for long-term delivery of alendronate and evaluation of its anti-osteoporotic effect in an ovariectomized rat model. Int. J. Pharm. 2015, 480, 37–47. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef]
- Ntoutoume, G.M.N.; Granet, R.; Mbakidi, J.P.; Brégier, F.; Léger, D.Y.; Fidanzi-Dugas, C.; Lequart, V.; Joly, N.; Liagre, B.; Chaleix, V. Development of curcumin–cyclodextrin/cellulose nanocrystals complexes: New anticancer drug delivery systems. Bioorganic Med. Chem. Lett. 2016, 26, 941–945. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhang, L.; Xu, Z.; Dai, H.; Wu, W. Nanocellulose/gelatin composite cryogels for controlled drug release. Chem. Eng. 2019, 7, 6381–6389. [Google Scholar] [CrossRef]
- Mohan, D.; Khairullah, N.F.; How, Y.P.; Sajab, M.S.; Kaco, H. 3D printed laminated CaCO3-nanocellulose films as controlled-release 5-fluorouracil. Polymers 2020, 12, 986. [Google Scholar] [CrossRef]
- Latifi, N.; Asgari, M.; Vali, H.; Mongeau, L. A tissue-mimetic nano-fibrillar hybrid injectable hydrogel for potential soft tissue engineering applications. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef]
- Bullo, S.; Buskaran, K.; Baby, R.; Dorniani, D.; Fakurazi, S.; Hussein, M.Z. Dual drugs anticancer nanoformulation using graphene oxide-PEG as nanocarrier for protocatechuic acid and chlorogenic acid. Pharm. Res. 2019, 36, 91. [Google Scholar] [CrossRef] [PubMed]
- Yew, Y.P.; Shameli, K.; Mohamad, S.E.; Lee, K.X.; Teow, S.-Y. Green synthesized montmorillonite/carrageenan/Fe3O4 nanocomposites for ph-responsive release of protocatechuic acid and its anticancer activity. Int. J. Mol. Sci. 2020, 21, 4851. [Google Scholar] [CrossRef] [PubMed]
- Yusefi, M.; Shameli, K.; Ali, R.R.; Pang, S.-W.; Teow, S.-Y. Evaluating anticancer activity of plant-mediated synthesized iron oxide nanoparticles using Punica Granatum fruit peel extract. J. Mol. Struct. 2020, 1204, 127539. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Yee, O.S.; Teow, S.-Y.; Hedayatnasab, Z.; Jahangirian, H.; Webster, T.J.; Kuča, K. Green synthesis of Fe3O4 nanoparticles stabilized by a Garcinia mangostana fruit peel extract for hyperthermia and anticancer activities. Int. J. Nanomed. 2021, 16, 2515. [Google Scholar] [CrossRef]
- Sukri, S.N.A.M.; Shameli, K.; Wong, M.M.-T.; Teow, S.-Y.; Chew, J.; Ismail, N.A. Cytotoxicity and antibacterial activities of plant-mediated synthesized zinc oxide (ZnO) nanoparticles using Punica granatum (pomegranate) fruit peels extract. J. Mol. Struct. 2019, 1189, 57–65. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Hedayatnasab, Z.; Teow, S.-Y.; Ismail, U.N.; Azlan, C.A.; Ali, R.R. Green synthesis of Fe3O4 nanoparticles for hyperthermia, magnetic resonance imaging and 5-fluorouracil carrier in potential colorectal cancer treatment. Res. Chem. Intermed. 2021, 1–20. [Google Scholar] [CrossRef]
- Abe, K.; Yano, H. Comparison of the characteristics of cellulose microfibril aggregates of wood, rice straw and potato tuber. Cellulose 2009, 16, 1017–1023. [Google Scholar] [CrossRef]
- Srasri, K.; Thongroj, M.; Chaijiraaree, P.; Thiangtham, S.; Manuspiya, H.; Pisitsak, P.; Ummartyotin, S. Recovery potential of cellulose fiber from newspaper waste: An approach on magnetic cellulose aerogel for dye adsorption material. Int. J. Biol. Macromol. 2018, 119, 662–668. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Cai, M.; Takagi, H.; Nakagaito, A.N.; Katoh, M.; Ueki, T.; Waterhouse, G.I.; Li, Y. Influence of alkali treatment on internal microstructure and tensile properties of abaca fibers. Ind. Crop. Prod. 2015, 65, 27–35. [Google Scholar] [CrossRef]
- Kunjachan, S.; Jose, S.; Lammers, T. Understanding the mechanism of ionic gelation for synthesis of chitosan nanoparticles using qualitative techniques. Asian J. Pharm. 2014, 4. [Google Scholar] [CrossRef]
- Hps, A.K.; Saurabh, C.K.; Adnan, A.; Fazita, M.N.; Syakir, M.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.; Haafiz, M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar] [CrossRef]
- Bozoğlan, B.K.; Duman, O.; Tunç, S. Preparation and characterization of thermosensitive chitosan/carboxymethylcellulose/scleroglucan nanocomposite hydrogels. Int. J. Biol. Macromol. 2020, 162, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, J.; Zhang, L.; Lindman, B.; Edlund, H.; Norgren, M. Spherical nanocomposite particles prepared from mixed cellulose–chitosan solutions. Cellulose 2016, 23, 3105–3115. [Google Scholar] [CrossRef]
- Samy, M.; Abd El-Alim, S.H.; Amin, A.; Ayoub, M.M. Formulation, characterization and in vitro release study of 5-fluorouracil loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 156, 783–791. [Google Scholar] [CrossRef]
- Nguyen, D.H. Potential 5-fluorouracil encapsulated mPEG-Chitosan nanogels for controlling drug release. JAMPS 2017, 1–7. [Google Scholar] [CrossRef]
- Hosokawa, J.; Nishiyama, M.; Yoshihara, K.; Kubo, T.; Terabe, A. Reaction between chitosan and cellulose on biodegradable composite film formation. Ind. Eng. Chem. Res. 1991, 30, 788–792. [Google Scholar] [CrossRef]
- Jiang, F.; Han, S.; Hsieh, Y.-L. Controlled defibrillation of rice straw cellulose and self-assembly of cellulose nanofibrils into highly crystalline fibrous materials. RSC Adv. 2013, 3, 12366–12375. [Google Scholar] [CrossRef]
- Ali, M.E.A.; Aboelfadl, M.M.S.; Selim, A.M.; Khalil, H.F.; Elkady, G.M. Chitosan nanoparticles extracted from shrimp shells, application for removal of Fe (II) and Mn (II) from aqueous phases. Sep. Sci. Technol. 2018, 53, 2870–2881. [Google Scholar] [CrossRef]
- Nivethaa, E.; Dhanavel, S.; Narayanan, V.; Vasu, C.A.; Stephen, A. An in vitro cytotoxicity study of 5-fluorouracil encapsulated chitosan/gold nanocomposites towards MCF-7 cells. RSC Adv. 2015, 5, 1024–1032. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Wang, J.; Zheng, X.; Chang, D.; Wang, S.; Jiang, T. A novel nanogel delivery of poly-α, β-polyasparthydrazide by reverse microemulsion and its redox-responsive release of 5-Fluorouridine. Asian J. Pharm. Sci. 2016, 11, 735–743. [Google Scholar] [CrossRef][Green Version]
- Zhu, W.; Wan, L.; Zhang, C.; Gao, Y.; Zheng, X.; Jiang, T.; Wang, S. Exploitation of 3D face-centered cubic mesoporous silica as a carrier for a poorly water soluble drug: Influence of pore size on release rate. Mater. Sci. Eng. C 2014, 34, 78–85. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Zainol Hilmi, N.H.; Jeffery Daim, L.D. Preparation of chitosan–hexaconazole nanoparticles as fungicide nanodelivery system for combating Ganoderma disease in oil palm. Molecules 2019, 24, 2498. [Google Scholar] [CrossRef]
- De Mesquita, J.P.; Donnici, C.L.; Teixeira, I.F.; Pereira, F.V. Bio-based nanocomposites obtained through covalent linkage between chitosan and cellulose nanocrystals. Carbohydr. Polym. 2012, 90, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Katas, H.; Amin, M.C.I.M.; Kumolosasi, E.; Buang, F.; Sahudin, S. Self-assembled polymeric nanoparticles for percutaneous co-delivery of hydrocortisone/hydroxytyrosol: An ex vivo and in vivo study using an NC/Nga mouse model. Int. J. Pharm. 2013, 444, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Pech-Cohuo, S.-C.; Canche-Escamilla, G.; Valadez-González, A.; Fernández-Escamilla, V.V.A.; Uribe-Calderon, J. Production and modification of cellulose nanocrystals from Agave tequilana weber waste and its effect on the melt rheology of PLA. Int. J. Polym. Sci. 2018, 2018. [Google Scholar] [CrossRef]
- Santmartí, A.; Lee, K.-Y. Crystallinity and Thermal Stability of Nanocellulose. In Nanocellulose Sustainability. Production, Properties, Applications and Case Studies; CRC Press: Boca Raton, FL, USA, 2018; pp. 67–86. [Google Scholar]
- Jiang, F.; Hsieh, Y.-L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kavaz, D.; Kirac, F.; Kirac, M.; Vaseashta, A. Low releasing mitomycin c molecule encapsulated with chitosan nanoparticles for intravesical installation. J. Biomater. Nanobiotechnol. 2017, 8, 203–219. [Google Scholar] [CrossRef]
- Area, M.C.; Ceradame, H. Paper aging and degradation: Recent findings and research methods. Bioresources 2011, 6, 5307–5337. [Google Scholar]
- Lustriane, C.; Dwivany, F.M.; Suendo, V.; Reza, M. Effect of chitosan and chitosan-nanoparticles on post harvest quality of banana fruits. J. Plant Biotechnol. 2018, 45, 36–44. [Google Scholar] [CrossRef]
- Puchtler, H.; Meloan, S. On Schiff’s bases and aldehyde-Fuchsin: A review from H. Schiff to RD Lillie. Histochemistry 1981, 72, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Zheng, X.-F.; Lian, Q.; Yang, H.; Wang, X. Surface molecularly imprinted polymer of chitosan grafted poly (methyl methacrylate) for 5-fluorouracil and controlled release. Sci. Rep. 2016, 6, 21409. [Google Scholar] [CrossRef]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12, 2021. [Google Scholar] [CrossRef] [PubMed]
- Nugraheni, A.D.; Purnawati, D.; Rohmatillah, A.; Mahardika, D.N.; Kusumaatmaja, A. Swelling of PVA/chitosan/TiO2 nanofibers membrane in different pH. Mater. Sci. Forum. 2020, 990, 220–224. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Yuan, Z.; Han, H.; Li, T.; Li, L.; Guo, X. Chitosan cross-linked poly (acrylic acid) hydrogels: Drug release control and mechanism. Colloids Surf. B 2017, 152, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Fan, D.; Song, P.; Zhang, S.; Liu, X. Preparation and application of pH-responsive composite hydrogel beads as potential delivery carrier candidates for controlled release of berberine hydrochloride. R. Soc. Open Sci. 2020, 7, 200676. [Google Scholar] [CrossRef]
- Kadry, G. Comparison between gelatin/carboxymethyl cellulose and gelatin/carboxymethyl nanocellulose in tramadol drug loaded capsule. Heliyon 2019, 5, e02404. [Google Scholar] [CrossRef]
- Seabra, A.B.; Bernardes, J.S.; Fávaro, W.J.; Paula, A.J.; Durán, N. Cellulose nanocrystals as carriers in medicine and their toxicities: A review. Carbohyd. Polym. 2018, 181, 514–527. [Google Scholar] [CrossRef]
- Illangakoon, U.E.; Yu, D.-G.; Ahmad, B.S.; Chatterton, N.P.; Williams, G.R. 5-Fluorouracil loaded Eudragit fibers prepared by electrospinning. Int. J. Pharm. 2015, 495, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, M.; Shen, J.; He, Z.; Fatehi, P.; Ni, Y. Applications of cellulose-based materials in sustained drug delivery systems. Curr. Med. Chem. 2019, 26, 2485–2501. [Google Scholar] [CrossRef] [PubMed]
- Roman, M. Toxicity of cellulose nanocrystals: A review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Xu, H.; Aguilar, Z.P.; Yang, L.; Kuang, M.; Duan, H.; Xiong, Y.; Wei, H.; Wang, A. Antibody conjugated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood. Biomaterials 2011, 32, 9758–9765. [Google Scholar] [CrossRef] [PubMed]


| Sample | Hydrodynamic Particle Size (nm) | ||
|---|---|---|---|
| pH 1.2 | pH 7.4 | pH 12 | |
| CF | 135.56 ± 2.87 | 174.43 ± 3.28 | 203.17 ± 4.81 |
| CS | 71.61 ± 5.54 | 140.09 ± 4.70 | 246.09 ± 5.10 |
| CS-CF BNCs | 109.03 ± 4.12 | 198 ± 3.25 | 275.34 ± 4.59 |
| CS-CF/5FU BNCs | 112.51 ± 4.09 | 203.52 ± 2.94 | 274.23 ± 5.11 |
| Sample | IC50 (μg/mL) | |
|---|---|---|
| HCT-116 | CCD 112 | |
| CF | >500 | >500 |
| CS NPs, | >500 | >500 |
| CS-CF BNCs | >500 | >500 |
| CS-CF/5FU BNCs | 228.27 | >500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusefi, M.; Chan, H.-Y.; Teow, S.-Y.; Kia, P.; Lee-Kiun Soon, M.; Sidik, N.A.B.C.; Shameli, K. 5-Fluorouracil Encapsulated Chitosan-Cellulose Fiber Bionanocomposites: Synthesis, Characterization and In Vitro Analysis towards Colorectal Cancer Cells. Nanomaterials 2021, 11, 1691. https://doi.org/10.3390/nano11071691
Yusefi M, Chan H-Y, Teow S-Y, Kia P, Lee-Kiun Soon M, Sidik NABC, Shameli K. 5-Fluorouracil Encapsulated Chitosan-Cellulose Fiber Bionanocomposites: Synthesis, Characterization and In Vitro Analysis towards Colorectal Cancer Cells. Nanomaterials. 2021; 11(7):1691. https://doi.org/10.3390/nano11071691
Chicago/Turabian StyleYusefi, Mostafa, Hui-Yin Chan, Sin-Yeang Teow, Pooneh Kia, Michiele Lee-Kiun Soon, Nor Azwadi Bin Che Sidik, and Kamyar Shameli. 2021. "5-Fluorouracil Encapsulated Chitosan-Cellulose Fiber Bionanocomposites: Synthesis, Characterization and In Vitro Analysis towards Colorectal Cancer Cells" Nanomaterials 11, no. 7: 1691. https://doi.org/10.3390/nano11071691
APA StyleYusefi, M., Chan, H.-Y., Teow, S.-Y., Kia, P., Lee-Kiun Soon, M., Sidik, N. A. B. C., & Shameli, K. (2021). 5-Fluorouracil Encapsulated Chitosan-Cellulose Fiber Bionanocomposites: Synthesis, Characterization and In Vitro Analysis towards Colorectal Cancer Cells. Nanomaterials, 11(7), 1691. https://doi.org/10.3390/nano11071691







