Recent Advances in the Development of Smart and Active Biodegradable Packaging Materials
Abstract
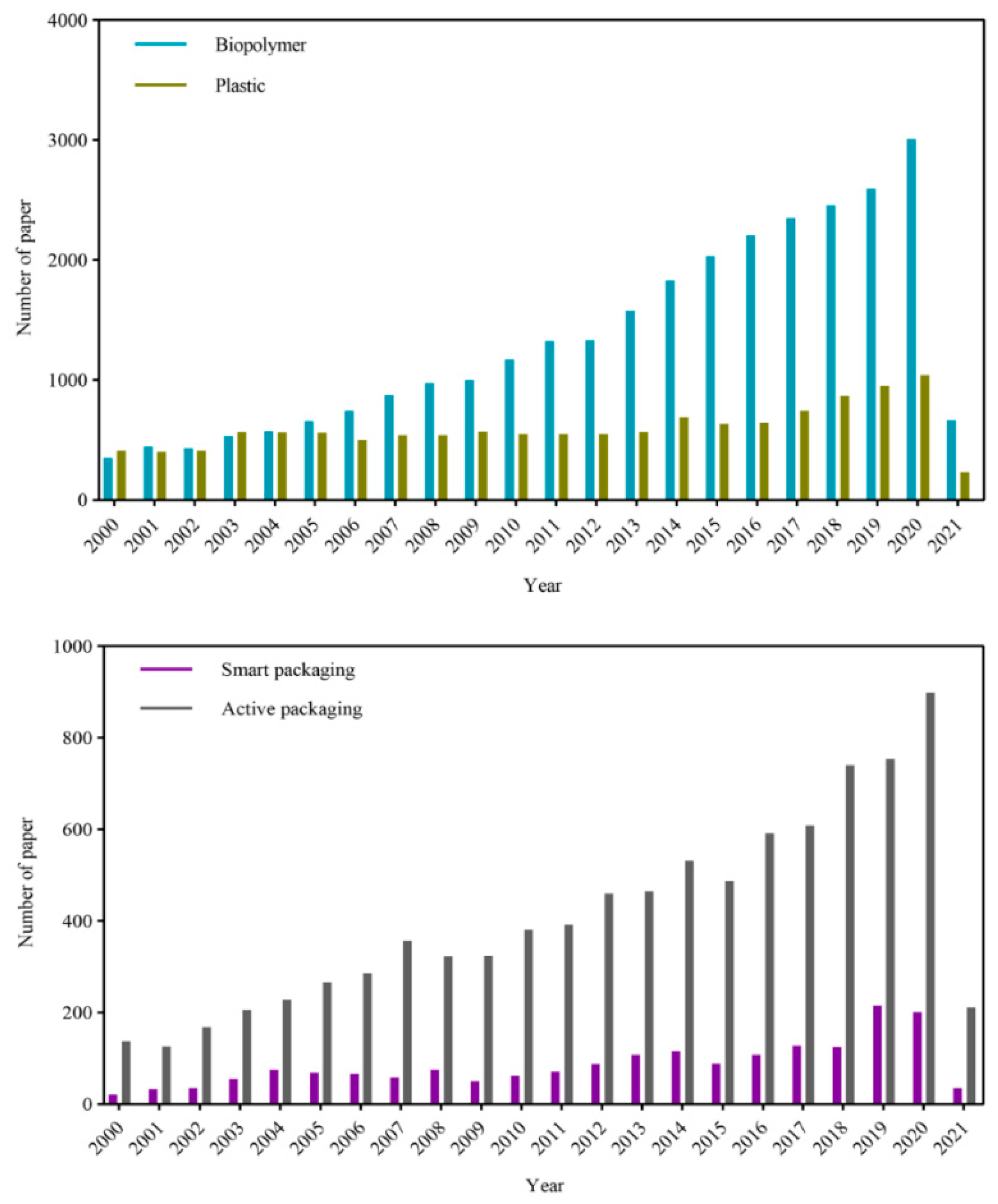
1. Introduction
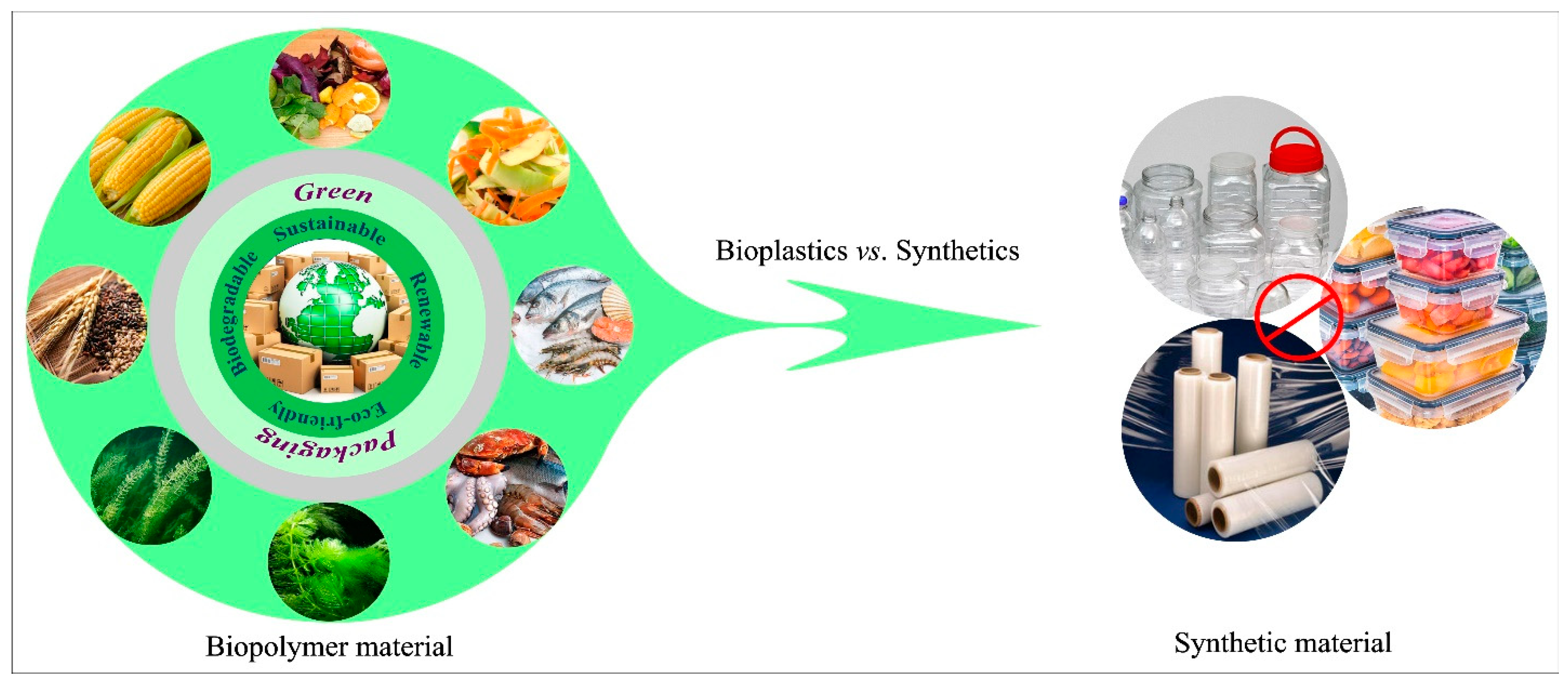
2. Overview of Biodegradable Packaging Materials
2.1. Biodegradable Materials
2.1.1. Proteins
Dairy Proteins
Meat Proteins
Plant Proteins
2.1.2. Polysaccharides
Starch
Cellulose
Chitin and Chitosan
Hydrocolloid Gums
2.1.3. Lipids
3. Fabrication of Packaging Materials
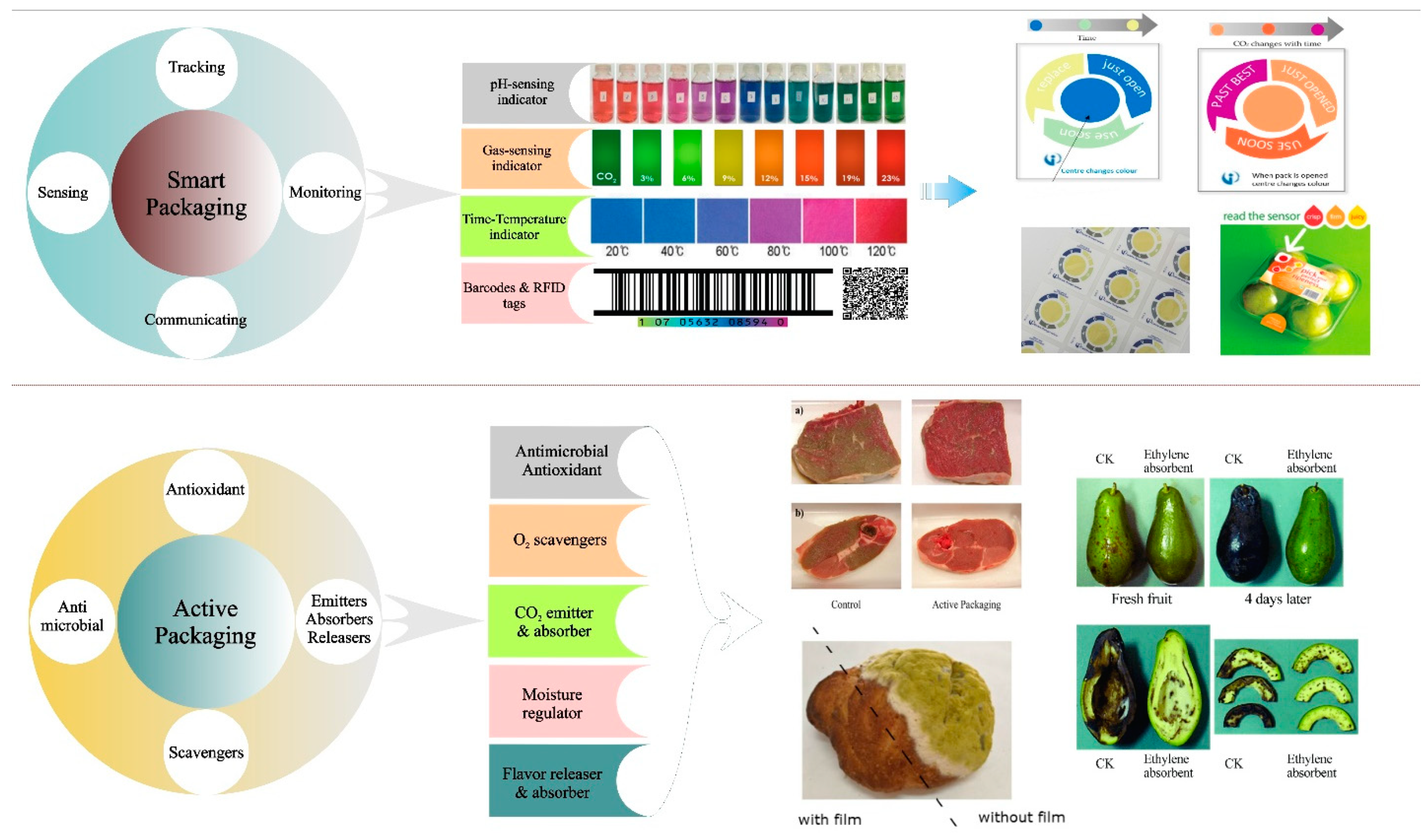
4. Active Packaging Materials
4.1. Antioxidants
4.2. Antimicrobials
4.3. Gas Controllers
5. Smart Packaging Materials
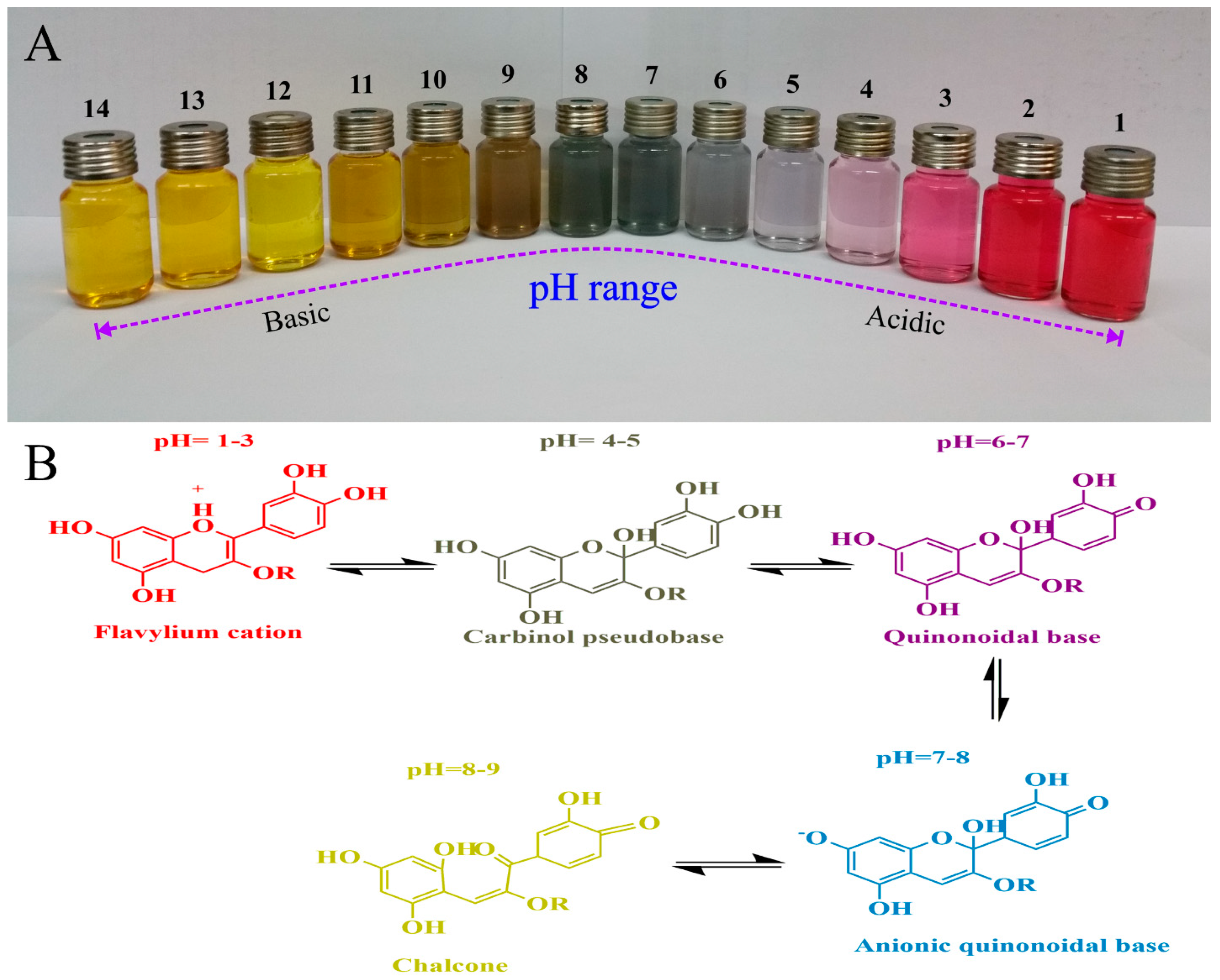
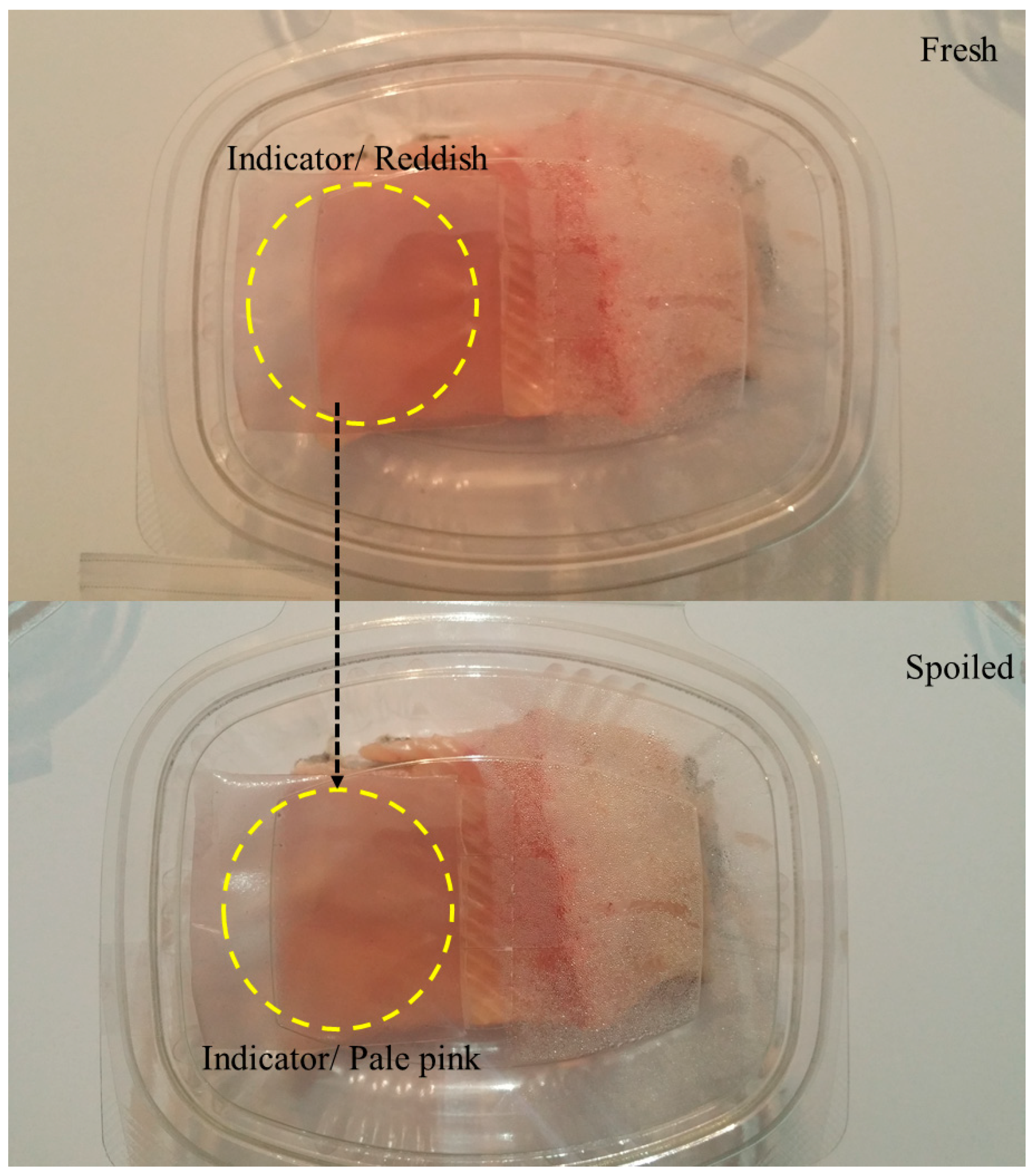
5.1. pH Indicators
5.2. Gas Indicators
5.3. Time-Temperature Indicators
6. Applications of Biodegradable Packaging Material
6.1. Meat and Seafood
6.2. Dairy Products
6.3. Fruits and Vegetables
7. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef]
- Ivonkovic, A.; Zeljko, K.; Talic, S.; Lasic, M. Biodegradable packaging in the food industry. J. Food Saf. Food Qual. 2017, 68, 26–38. [Google Scholar]
- Din, M.I.; Ghaffar, T.; Najeeb, J.; Hussain, Z.; Khalid, R.; Zahid, H. Potential perspectives of biodegradable plastics for food packaging application-review of properties and recent developments. Food Addit. Contam. Part A 2020, 37, 665–680. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Ehsani, A.; Kia, E.M.; Khezerlou, A. Microbial gums: Introducing a novel functional component of edible coatings and packaging. Appl. Microbiol. Biotechnol. 2019, 103, 6853–6866. [Google Scholar] [CrossRef] [PubMed]
- Angellier-Coussy, H.; Chalier, P.; Gastaldi, E.; Guillard, V.; Guillaume, C.; Gontard, N.; Peyron, S. Protein-based nanocomposites for food packaging. Biopolym. Nanocompos. Process. Prop. Appl. 2013, 613–654. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Iordanskii, A. Bio-Based and Biodegradable Plastics: From Passive Barrier to Active Packaging Behavior. Polymers 2020, 12, 1537. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent Trends in the Use of Pectin from Agro-Waste Residues as a Natural-Based Biopolymer for Food Packaging Applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef]
- Kakadellis, S.; Harris, Z.M. Don’t scrap the waste: The need for broader system boundaries in bioplastic food packaging life-cycle assessment—A critical review. J. Clean. Prod. 2020, 274, 122831. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Łupina, K.; Kowalczyk, D.; Zięba, E.; Kazimierczak, W.; Mężyńska, M.; Basiura-Cembala, M.; Wiącek, A.E. Edible films made from blends of gelatin and polysaccharide-based emulsifiers-A comparative study. Food Hydrocoll. 2019, 96, 555–567. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; Rhim, J.-W.; Jafari, S.M. pH-sensitive (halochromic) smart packaging films based on natural food colorants for the monitoring of food quality. Trends Food Sci. Technol. 2020, 105, 93–144. [Google Scholar] [CrossRef]
- Mohammadian, E.; Alizadeh-Sani, M.; Jafari, S.M. Smart monitoring of gas/temperature changes within food packaging based on natural colorants. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2885–2931. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Kurek, M.; Garofulić, I.E.; Bakić, M.T.; Ščetar, M.; Uzelac, V.D.; Galić, K. Development and evaluation of a novel antioxidant and pH indicator film based on chitosan and food waste sources of antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Luchese, C.L.; Sperotto, N.; Spada, J.C.; Tessaro, I.C. Effect of blueberry agro-industrial waste addition to corn starch-based films for the production of a pH-indicator film. Int. J. Biol. Macromol. 2017, 104, 11–18. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Lane, J.L.; Grant, T.; Pratt, S.; Lant, P.A.; Laycock, B. Environmental impact of biodegradable food packaging when considering food waste. J. Clean. Prod. 2018, 180, 325–334. [Google Scholar] [CrossRef]
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Chitosan/montmorillonite bionanocomposites incorporated with rosemary and ginger essential oil as packaging for fresh poultry meat. Food Packag. Shelf Life 2018, 17, 142–149. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Alizadeh-Sani, M.; Divband, B.; Ehsani, A.; McClements, D.J. Nanocomposite films consisting of functional nanoparticles (TiO2 and ZnO) embedded in 4A-Zeolite and mixed polymer matrices (gelatin and polyvinyl alcohol). Food Res. Int. 2020, 137, 109716. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Kia, E.M.; Ghasempour, Z.; Ehsani, A. Preparation of active nanocomposite film consisting of sodium caseinate, ZnO nanoparticles and rosemary essential oil for food packaging applications. J. Polym. Environ. 2021, 29, 588–598. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind. Crops Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Tavassoli, M.; Hamishehkar, H.; McClements, D.J. Carbohydrate-based films containing pH-sensitive red barberry anthocyanins: Application as biodegradable smart food packaging materials. Carbohydr. Polym. 2021, 255, 117488. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.V.; Shagolsem, L.S. Shagolsem, Biopolymer Based Nano-Structured Materials and Their Applications, in Nanostructured Materials and Their Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 337–366. [Google Scholar]
- Havstad, M.R. Biodegradable plastics. In Plastic Waste and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; pp. 97–129. [Google Scholar]
- Ahmadi, A.; Ahmadi, P.; Sani, M.A.; Ehsani, A.; Ghanbarzadeh, B. Functional biocompatible nanocomposite films consisting of selenium and zinc oxide nanoparticles embedded in gelatin/cellulose nanofiber matrices. Int. J. Biol. Macromol. 2021, 175, 87–97. [Google Scholar] [CrossRef]
- Narasagoudr, S.S.; Hegde, V.G.; Chougale, R.B.; Masti, S.P.; Dixit, S. Influence of boswellic acid on multifunctional properties of chitosan/poly (vinyl alcohol) films for active food packaging. Int. J. Biol. Macromol. 2020, 154, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.-W.; Bagheri, R. Gelatin-based functional films integrated with grapefruit seed extract and TiO2 for active food packaging applications. Food Hydrocoll. 2021, 112, 106314. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, A.K.; Duffy, B.; Jaiswal, S. Ferulic acid incorporated active films based on poly(lactide)/poly(butylene adipate-co-terephthalate) blend for food packaging. Food Packag. Shelf Life 2020, 24, 100491. [Google Scholar] [CrossRef]
- Suwanamornlert, P.; Kerddonfag, N.; Sane, A.; Chinsirikul, W.; Zhou, W.; Chonhenchob, V. Poly(lactic acid)/poly(butylene-succinate-co-adipate) (PLA/PBSA) blend films containing thymol as alternative to synthetic preservatives for active packaging of bread. Food Packag. Shelf Life 2020, 25, 100515. [Google Scholar] [CrossRef]
- Ceballos, R.L.; Ochoa-Yepes, O.; Goyanes, S.; Bernal, C.; Famá, L. Effect of yerba mate extract on the performance of starch films obtained by extrusion and compression molding as active and smart packaging. Carbohydr. Polym. 2020, 244, 116495. [Google Scholar] [CrossRef] [PubMed]
- Chenwei, C.; Zhipeng, T.; Yarui, M.; Weiqiang, Q.; Fuxin, Y.; Jun, M.; Jing, X. Physicochemical, microstructural, antioxidant and antimicrobial properties of active packaging films based on poly(vinyl alcohol)/clay nanocomposite incorporated with tea polyphenols. Prog. Org. Coat. 2018, 123, 176–184. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan based ZnO nanoparticles loaded gallic-acid films for active food packaging. Food Chem. 2021, 334, 127605. [Google Scholar] [CrossRef] [PubMed]
- Jha, P. Effect of grapefruit seed extract ratios on functional properties of corn starch-chitosan bionanocomposite films for active packaging. Int. J. Biol. Macromol. 2020, 163, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Nur Amila Najwa, I.S.; Mat Yusoff, M.; Nur Hanani, Z.A. Potential of Silver-Kaolin in Gelatin Composite Films as Active Food Packaging Materials. Food Packag. Shelf Life 2020, 26, 100564. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Rhim, J.-W.; Azizi-Lalabadi, M.; Hemmati-Dinarvand, M.; Ehsani, A. Preparation and characterization of functional sodium caseinate/guar gum/TiO2/cumin essential oil composite film. Int. J. Biol. Macromol. 2020, 145, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Tavassoli, M.; McClements, D.J.; Hamishehkar, H. Multifunctional halochromic packaging materials: Saffron petal anthocyanin loaded-chitosan nanofiber/methyl cellulose matrices. Food Hydrocoll. 2021, 111, 106237. [Google Scholar] [CrossRef]
- Andretta, R.; Luchese, C.L.; Tessaro, I.C.; Spada, J.C. Development and characterization of pH-indicator films based on cassava starch and blueberry residue by thermocompression. Food Hydrocoll. 2019, 93, 317–324. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart gelatin films prepared using red cabbage (Brassica oleracea L.) extracts as solvent. Food Hydrocoll. 2019, 89, 674–681. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Huang, S.; Xiong, Y.; Zou, Y.; Dong, Q.; Ding, F.; Liu, X.; Li, H. A novel colorimetric indicator based on agar incorporated with Arnebia euchroma root extracts for monitoring fish freshness. Food Hydrocoll. 2019, 90, 198–205. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart edible films based on gelatin and curcumin. Food Hydrocoll. 2017, 66, 8–15. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, P.; Guo, M.; Jiang, S.; Li, X.; Jiang, S. Films based on κ-carrageenan incorporated with curcumin for freshness monitoring. Food Hydrocoll. 2018, 83, 134–142. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. pH-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications. Food Hydrocoll. 2020, 102, 105629. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.; Mahanti, N.K. Application of biodegradable polymers in food packaging industry: A comprehensive review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Shendurse, A.; Gopikrishna, G.; Patel, A.; Pandya, A. Milk protein based edible films and coatings–preparation, properties and food applications. J. Nutr. Health Food Eng. 2018, 8, 219–226. [Google Scholar] [CrossRef]
- Qiu, Y.-T.; Wang, B.-J.; Weng, Y.-M. Preparation and characterization of genipin cross-linked and lysozyme incorporated antimicrobial sodium caseinate edible films. Food Packag. Shelf Life 2020, 26, 100601. [Google Scholar] [CrossRef]
- Azevedo, V.M.; Dias, M.V.; Borges, S.V.; Fernandes, R.V.d.B.; Silva, E.K.; Medeiros, É.A.; Ferreira Soares, N.d.F. Optical and structural properties of biodegradable whey protein isolate nanocomposite films for active packaging. Int. J. Food Prop. 2017, 20, 1869–1878. [Google Scholar] [CrossRef]
- Schmid, M.; Proels, S.; Kainz, D.M.; Hammann, F. Effect of thermally induced denaturation on molecular interaction-response relationships of whey protein isolate based films and coatings. Prog. Org. Coat. 2017, 104, 161–172. [Google Scholar] [CrossRef]
- Akhtar, M.-J.; Aïder, M. Study of the Barrier and Mechanical Properties of Packaging Edible Films Fabricated with Hydroxypropyl Methylcellulose (HPMC) Combined with Electro-Activated Whey. J. Packag. Technol. Res. 2018, 2, 169–180. [Google Scholar] [CrossRef]
- Chalermthai, B.; Chan, W.Y.; Bastidas-Oyanedel, J.-R.; Taher, H.; Olsen, B.D.; Schmidt, J.E. Preparation and characterization of whey protein-based polymers produced from residual dairy streams. Polymers 2019, 11, 722. [Google Scholar] [CrossRef]
- Brady, J.W. Introductory Food Chemistry; Cornell University Press: Ithaca, NY, USA, 2013. [Google Scholar]
- Rakhmanova, A.; Khan, Z.; Sharif, R.; Lv, X. Meeting the requirements of halal gelatin: A mini review. MOJ Food Proc. Technol. 2018, 6, 477–482. [Google Scholar] [CrossRef]
- Karim, A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Gornall, J.L.; Terentjev, E.M. Helix–coil transition of gelatin: Helical morphology and stability. Soft Matter 2008, 4, 544–549. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Sakhawy, M.; Nashy, E.-S.H.; Othman, A.M. Novel natural composite films as packaging materials with enhanced properties. Int. J. Biol. Macromol. 2019, 136, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Thermoplastic films from plant proteins. J. Appl. Polym. Sci. 2013, 130, 729–738. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ünalan, İ.U.; Korel, F.; Yemenicioğlu, A. Active packaging of ground beef patties by edible zein films incorporated with partially purified lysozyme and Na2EDTA. Int. J. Food Sci. Technol. 2011, 46, 1289–1295. [Google Scholar] [CrossRef]
- Rakotonirainy, A.; Wang, Q.; Padua, G.W. Evaluation of zein films as modified atmosphere packaging for fresh broccoli. J. Food Sci. 2001, 66, 1108–1111. [Google Scholar] [CrossRef]
- Visakh, P. Soy Protein: State-of-the-Art, New Challenges and Opportunities. SOY Protein Based Blends Compos. Nanocompos. 2017, 1–21. [Google Scholar]
- Dos Santos Paglione, I.; Galindo, M.V.; de Souza, K.C.; Yamashita, F.; Grosso, C.R.F.; Sakanaka, L.S.; Shirai, M.A. Optimization of the conditions for producing soy protein isolate films. Emir. J. Food Agric. 2019, 297–303. [Google Scholar] [CrossRef]
- Ortiz, C.M.; de Moraes, J.O.; Vicente, A.R.; Laurindo, J.B.; Mauri, A.N. Scale-up of the production of soy (Glycine max L.) protein films using tape casting: Formulation of film-forming suspension and drying conditions. Food Hydrocoll. 2017, 66, 110–117. [Google Scholar] [CrossRef]
- Lodha, P.; Netravali, A.N. Thermal and mechanical properties of environment-friendly ‘green’plastics from stearic acid modified-soy protein isolate. Ind. Crops Prod. 2005, 21, 49–64. [Google Scholar] [CrossRef]
- Mohareb, E.; Mittal, G.S. Formulation and process conditions for biodegradable/edible soy-based packaging trays. Packag. Technol. Sci. Int. J. 2007, 20, 1–15. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Jin, Z.; Jiao, A. Research progress of starch-based biodegradable materials: A review. J. Mater. Sci. 2021, 1–22. [Google Scholar]
- Shirazani, M.T.; Bakhshi, H.; Rashidi, A.; Taghizadeh, M. Starch-based activated carbon micro-spheres for adsorption of methane with superior performance in ANG technology. J. Environ. Chem. Eng. 2020, 8, 103910. [Google Scholar] [CrossRef]
- Ilyas, R.; Sapuan, S.; Ishak, M.; Zainudin, E. Sugar palm nanocrystalline cellulose reinforced sugar palm starch composite: Degradation and water-barrier properties. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK; Chicago, IL, USA, 2018. [Google Scholar]
- Palma-Rodríguez, H.M.; Aguirre-Álvarez, G.; Chavarría-Hernández, N.; Rodríguez-Hernández, A.I.; Bello-Pérez, L.A.; Vargas-Torres, A. Oxidized banana starch–polyvinyl alcohol film: Partial characterization. Starch Stärke 2012, 64, 882–889. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Moghimi, R.; Aliahmadi, A.; Rafati, H. Antibacterial hydroxypropyl methyl cellulose edible films containing nanoemulsions of Thymus daenensis essential oil for food packaging. Carbohydr. Polym. 2017, 175, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.-W. pH-responsive color indicator films based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness. Int. J. Biol. Macromol. 2020, 166, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Dharmalingam, K.; Nishizu, T.; Katsuno, N.; Anandalakshmi, R. Effect of Amylose–Amylopectin Ratios on Physical, Mechanical, and Thermal Properties of Starch-Based Bionanocomposite Films Incorporated with CMC and Nanoclay. Starch-Stärke 2020, 72, 1900121. [Google Scholar] [CrossRef]
- Tabari, M. Investigation of carboxymethyl cellulose (CMC) on mechanical properties of cold water fish gelatin biodegradable edible films. Foods 2017, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Lekjing, S. A chitosan-based edible film with clove essential oil and nisin for improving the quality and shelf life of pork patties in cold storage. RSC Adv. 2020, 10, 17777–17786. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Tran, T.X.; Rachtanapun, P. Characteristics and antimicrobial properties of active edible films based on pectin and nanochitosan. Int. J. Mol. Sci. 2020, 21, 2224. [Google Scholar] [CrossRef]
- Rai, S.K.; Chaturvedi, K.; Yadav, S.K. Evaluation of structural integrity and functionality of commercial pectin based edible films incorporated with corn flour, beetroot, orange peel, muesli and rice flour. Food Hydrocoll. 2019, 91, 127–135. [Google Scholar]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Vioque, B.; Rubio-Senent, F.; Fernández-Bolaños, J. Physical and functional properties of pectin-fish gelatin films containing the olive phenols hydroxytyrosol and 3, 4-dihydroxyphenylglycol. Carbohydr. Polym. 2017, 178, 368–377. [Google Scholar] [CrossRef]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Natural pectin polysaccharides as edible coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef]
- Stephen, A.J.; Phillips, G.O.; Williams, P.A. Food Polysaccharides and Their Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Williams, P.A.; Phillips, G.O. Handbook of Hydrocolloids, 3rd ed.; Woodhead Publishing: Kidlington, UK, 2021. [Google Scholar]
- Akoh, C.C. Food Lipids: Chemistry, Nutrition and Biotechnology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Cemistry; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Chow, C.K. Fatty Acids in Foods and Their Health Implications; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Cunha, A.P.; Brito, E.S.; Azeredo, H.M.; Gallão, M.I. Mesquite seed gum and palm fruit oil emulsion edible films: Influence of oil content and sonication. Food Hydrocoll. 2016, 56, 227–235. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A. Application of chitosan-sunflower oil edible films to pork meat hamburgers. Procedia Food Sci. 2011, 1, 39–43. [Google Scholar] [CrossRef]
- Randazzo, W.; Jiménez-Belenguer, A.; Settanni, L.; Perdones, A.; Moschetti, M.; Palazzolo, E.; Guarrasi, V.; Vargas, M.; Germanà, M.A.; Moschetti, G. Antilisterial effect of citrus essential oils and their performance in edible film formulations. Food Control 2016, 59, 750–758. [Google Scholar] [CrossRef]
- Syafiq, R.; Sapuan, S.; Zuhri, M.; Ilyas, R.; Nazrin, A.; Sherwani, S.; Khalina, A. Antimicrobial activities of starch-based biopolymers and biocomposites incorporated with plant essential oils: A review. Polymers 2020, 12, 2403. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2020, In press, 1–29. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun functional materials toward food packaging applications: A review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.; Saba, N.; Asiri, A.M.; Jawaid, M.; Indarti, E.; Wanrosli, W. Preparation and characterization of nanocomposite films from oil palm pulp nanocellulose/poly (Vinyl alcohol) by casting method. Carbohydr. Polym. 2018, 191, 103–111. [Google Scholar] [CrossRef]
- Khan, W.S.; Asmatulu, R.; Ceylan, M.; Jabbarnia, A. Recent progress on conventional and non-conventional electrospinning processes. Fibers Polym. 2013, 14, 1235–1247. [Google Scholar] [CrossRef]
- Park, S.; Park, K.; Yoon, H.; Son, J.; Min, T.; Kim, G. Apparatus for preparing electrospun nanofibers: Designing an electrospinning process for nanofiber fabrication. Polym. Int. 2007, 56, 1361–1366. [Google Scholar] [CrossRef]
- Zhan, S.; Chen, D.; Jiao, X.; Tao, C. Long TiO2 hollow fibers with mesoporous walls: Sol− gel combined electrospun fabrication and photocatalytic properties. J. Phys. Chem. B 2006, 110, 11199–11204. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, M.; Jabeen, R.; Kärki, T. The Modelling of Extrusion Processes for Polymers—A Review. Polymers 2020, 12, 1306. [Google Scholar] [CrossRef]
- Krepker, M.; Zhang, C.; Nitzan, N.; Prinz-Setter, O.; Massad-Ivanir, N.; Olah, A.; Baer, E.; Segal, E. Antimicrobial LDPE/EVOH layered films containing carvacrol fabricated by multiplication extrusion. Polymers 2018, 10, 864. [Google Scholar] [CrossRef]
- Guerrero, P.; Muxika, A.; Zarandona, I.; De La Caba, K. Crosslinking of chitosan films processed by compression molding. Carbohydr. Polym. 2019, 206, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Maisanaba, S.; Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Puerto, M.; Prieto, A.I.; Jos, A.; Cameán, A.M. New advances in active packaging incorporated with essential oils or their main components for food preservation. Food Rev. Int. 2017, 33, 447–515. [Google Scholar] [CrossRef]
- Ozdemir, M.; Floros, J.D. Active Food Packaging Technologies. Crit. Rev. Food Sci. Nutr. 2004, 44, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Moreirinha, C.; Vilela, C.; Silva, N.H.C.S.; Pinto, R.J.B.; Almeida, A.; Rocha, M.A.M.; Coelho, E.; Coimbra, M.A.; Silvestre, A.J.D.; Freire, C.S.R. Antioxidant and antimicrobial films based on brewers spent grain arabinoxylans, nanocellulose and feruloylated compounds for active packaging. Food Hydrocoll. 2020, 108, 105836. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Ghanbarzadeh, B.; Divband, B. Polyvinyl alcohol/gelatin nanocomposite containing ZnO, TiO2 or ZnO/TiO2 nanoparticles doped on 4A zeolite: Microbial and sensory qualities of packaged white shrimp during refrigeration. Int. J. Food Microbiol. 2020, 312, 108375. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Liu, J.; Meng, C.-G.; Liu, S.; Kan, J.; Jin, C.-H. Preparation and characterization of protocatechuic acid grafted chitosan films with antioxidant activity. Food Hydrocoll. 2017, 63, 457–466. [Google Scholar] [CrossRef]
- Khajeh, B.; Dashti-Khavidaki, S.; Nasiri-Toosi, M.; Mohammadi, K.; Jafari, A. Effects of pre-transplant L-carnitine supplementation on primary graft dysfunction in liver transplant recipients: A pilot, randomized, placebo-controlled clinical trial. Res. Pharm. Sci. 2019, 14, 504–514. [Google Scholar] [CrossRef]
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time–Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Aday, M.S.; Caner, C. The Applications of ‘active packaging and chlorine dioxide’ for extended shelf life of fresh strawberries. Packag. Technol. Sci. 2011, 24, 123–136. [Google Scholar] [CrossRef]
- Shruthy, R.; Jancy, S.; Preetha, R. Cellulose nanoparticles synthesised from potato peel for the development of active packaging film for enhancement of shelf life of raw prawns (Penaeus monodon) during frozen storage. Int. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Antioxidative activity and properties of fish skin gelatin films incorporated with BHT and α-tocopherol. Food Hydrocoll. 2008, 22, 449–458. [Google Scholar] [CrossRef]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Vulić, I. Green tea preparation and its influence on the content of bioactive compounds. Food Res. Int. 2010, 43, 167–176. [Google Scholar] [CrossRef]
- Li, J.-H.; Miao, J.; Wu, J.-L.; Chen, S.-F.; Zhang, Q.-Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Kamkar, A.; Molaee-Aghaee, E.; Khanjari, A.; Akhondzadeh-Basti, A.; Noudoost, B.; Shariatifar, N.; Sani, M.A.; Soleimani, M. Nanocomposite active packaging based on chitosan biopolymer loaded with nano-liposomal essential oil: Its characterizations and effects on microbial, and chemical properties of refrigerated chicken breast fillet. Int. J. Food Microbiol. 2021, 342, 109071. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Melo, N.R.d.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Djenane, D. Chemical Profile, Antibacterial and Antioxidant Activity of Algerian Citrus Essential Oils and Their Application in Sardina pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef] [PubMed]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Sanfuentes, E.A. The synergistic antimicrobial effect of carvacrol and thymol in clay/polymer nanocomposite films over strawberry gray mold. LWT Food Sci. Technol. 2015, 64, 390–396. [Google Scholar] [CrossRef]
- Wiburanawong, S.; Petchwattana, N.; Covavisaruch, S. Carvacrol as an Antimicrobial Agent for Poly(butylene succinate): Tensile Properties and Antimicrobial Activity Observations. Adv. Mater. Res. 2014, 931–932, 111–115. [Google Scholar] [CrossRef]
- Zaman, H.U.; Hun, P.D.; Khan, R.A.; Yoon, K.-B. Morphology, mechanical, and crystallization behaviors of micro- and nano-ZnO filled polypropylene composites. J. Reinf. Plast. Compos. 2012, 31, 323–329. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2020, 1–29. [Google Scholar] [CrossRef]
- Liang, T.; Sun, G.; Cao, L.; Li, J.; Wang, L. A pH and NH3 sensing intelligent film based on Artemisia sphaerocephala Krasch. gum and red cabbage anthocyanins anchored by carboxymethyl cellulose sodium added as a host complex. Food Hydrocoll. 2019, 87, 858–868. [Google Scholar] [CrossRef]
- Tavassoli, M.; Sani, M.A.; Khezerlou, A.; Ehsani, A.; McClements, D.J. Multifunctional nanocomposite active packaging materials: Immobilization of quercetin, lactoferrin, and chitosan nanofiber particles in gelatin films. Food Hydrocoll. 2021, 118, 106747. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Fabrication of eugenol loaded gelatin nanofibers by electrospinning technique as active packaging material. LWT 2021, 139, 110800. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.-E.; Mitrea, L.; Călinoiu, L.-F.; Martău, G.-A.; Simon, E.; Varvara, R.-A.; Vodnar, D.C. Active Packaging—Poly(Vinyl Alcohol) Films Enriched with Tomato By-Products Extract. Coatings 2020, 10. [Google Scholar] [CrossRef]
- Randazzo, W.; Fabra, M.J.; Falcó, I.; López-Rubio, A.; Sánchez, G. Polymers and Biopolymers with Antiviral Activity: Potential Applications for Improving Food Safety. Compr. Rev. Food Sci. Food Saf. 2018, 17, 754–768. [Google Scholar] [CrossRef]
- Amankwaah, C. Incorporation of Selected Plant Extracts into Edible Chitosan Films and the Effect on the Antiviral, Antibacterial and Mechanical Properties of the Material. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2013. [Google Scholar]
- Martínez-Abad, A.; Ocio, M.J.; Lagarón, J.M.; Sánchez, G. Evaluation of silver-infused polylactide films for inactivation of Salmonella and feline calicivirus in vitro and on fresh-cut vegetables. Int. J. Food Microbiol. 2013, 162, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, M.P.; Lopez-Carballo, G.; Catala, R.; Gavara, R.; Hernandez-Munoz, P. Antifungal properties of gliadin films incorporating cinnamaldehyde and application in active food packaging of bread and cheese spread foodstuffs. Int. J. Food Microbiol. 2013, 166, 369–377. [Google Scholar] [CrossRef]
- Dey, A.; Neogi, S. Oxygen scavengers for food packaging applications: A review. Trends Food Sci. Technol. 2019, 90, 26–34. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Polyphenol and flavonoid profiles and radical scavenging activity in leafy vegetable Amaranthus gangeticus. BMC Plant Biol. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Pant, A.F.; Sängerlaub, S.; Müller, K. Gallic Acid as an Oxygen Scavenger in Bio-Based Multilayer Packaging Films. Materials 2017, 10, 489. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Negi, Y.S. Ethylene scavengers for active packaging of fresh food produce. Environ. Chem. Lett. 2020, 18, 269–284. [Google Scholar] [CrossRef]
- Romani, V.P.; Martins, V.G.; Goddard, J.M. Radical scavenging polyethylene films as antioxidant active packaging materials. Food Control 2020, 109, 106946. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Emerging trends in food packaging. Nutr. Food Sci. 2018, 48, 764–779. [Google Scholar] [CrossRef]
- Kadam, A.A.; Singh, S.; Gaikwad, K.K. Chitosan based antioxidant films incorporated with pine needles (Cedrus deodara) extract for active food packaging applications. Food Control 2021, 107877. [Google Scholar] [CrossRef]
- Zhang, N.; Bi, F.; Xu, F.; Yong, H.; Bao, Y.; Jin, C.; Liu, J. Structure and functional properties of active packaging films prepared by incorporating different flavonols into chitosan based matrix. Int. J. Biol. Macromol. 2020, 165, 625–634. [Google Scholar] [CrossRef]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Mohamad, N.; Mazlan, M.M.; Tawakkal, I.S.M.A.; Talib, R.A.; Kian, L.K.; Fouad, H.; Jawaid, M. Development of active agents filled polylactic acid films for food packaging application. Int. J. Biol. Macromol. 2020, 163, 1451–1457. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Koutsoumanis, K.P.; Biliaderis, C.G. Physical and thermo-mechanical properties of whey protein isolate films containing antimicrobials, and their effect against spoilage flora of fresh beef. Food Hydrocoll. 2010, 24, 49–59. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Dufresne, A. Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr. Polym. 2016, 151, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Higueras, L.; López-Carballo, G.; Hernández-Muñoz, P.; Gavara, R.; Rollini, M. Development of a novel antimicrobial film based on chitosan with LAE (ethyl-Nα-dodecanoyl-l-arginate) and its application to fresh chicken. Int. J. Food Microbiol. 2013, 165, 339–345. [Google Scholar] [CrossRef]
- Aydemir Sezer, U.; Sanko, V.; Yuksekdag, Z.N.; Uzundağ, D.; Sezer, S. Use of oxidized regenerated cellulose as bactericidal filler for food packaging applications. Cellulose 2016, 23, 3209–3219. [Google Scholar] [CrossRef]
- Li, L.; Zhao, C.; Zhang, Y.; Yao, J.; Yang, W.; Hu, Q.; Wang, C.; Cao, C. Effect of stable antimicrobial nano-silver packaging on inhibiting mildew and in storage of rice. Food Chem. 2017, 215, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Azlin-Hasim, S.; Cruz-Romero, M.C.; Morris, M.A.; Padmanabhan, S.C.; Cummins, E.; Kerry, J.P. The Potential Application of Antimicrobial Silver Polyvinyl Chloride Nanocomposite Films to Extend the Shelf-Life of Chicken Breast Fillets. Food Bioprocess Technol. 2016, 9, 1661–1673. [Google Scholar] [CrossRef]
- Akbar, A.; Anal, A.K. Zinc oxide nanoparticles loaded active packaging, a challenge study against Salmonella typhimurium and Staphylococcus aureus in ready-to-eat poultry meat. Food Control 2014, 38, 88–95. [Google Scholar] [CrossRef]
- Min, S.; Harris, L.J.; Krochta, J.M. Antimicrobial Effects of Lactoferrin, Lysozyme, and the Lactoperoxidase System and Edible Whey Protein Films Incorporating the Lactoperoxidase System Against Salmonella enterica and Escherichia coli O157:H7. J. Food Sci. 2005, 70, m332–m338. [Google Scholar] [CrossRef]
- Biji, K.; Ravishankar, C.; Mohan, C.; Gopal, T.S. Smart packaging systems for food applications: A review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef]
- Kuswandi, B.; Wicaksono, Y.; Abdullah, A.; Heng, L.Y.; Ahmad, M. Smart packaging: Sensors for monitoring of food quality and safety. Sens. Instrum. Food Qual. Saf. 2011, 5, 137–146. [Google Scholar] [CrossRef]
- Vo, T.-V.; Dang, T.-H.; Chen, B.-H. Synthesis of Intelligent pH Indicative Films from Chitosan/Poly(vinyl alcohol)/Anthocyanin Extracted from Red Cabbage. Polymers 2019, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Chi, W.; Zhang, C.; Xu, S.; Li, J.; Wang, L. Developing a green film with pH-sensitivity and antioxidant activity based on к-carrageenan and hydroxypropyl methylcellulose incorporating Prunus maackii juice. Food Hydrocoll. 2019, 94, 345–353. [Google Scholar] [CrossRef]
- Wardana, A.A.; Widyaningsih, T.D. Development of edible films from tapioca starch and agar, enriched with red cabbage (Brassica oleracea) as a sausage deterioration bio-indicator. IOP Conf. Ser. Earth Environ. Sci. 2017, 109, 012031. [Google Scholar] [CrossRef]
- Luchese, C.L.; Abdalla, V.F.; Spada, J.C.; Tessaro, I.C. Evaluation of blueberry residue incorporated cassava starch film as pH indicator in different simulants and foodstuffs. Food Hydrocoll. 2018, 82, 209–218. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Zandraa, O.; Pummerová, M.; Sáha, P. Essential Oil Based PVP-CMC-BC-GG Functional Hydrogel Sachet for ‘Cheese’: Its Shelf Life Confirmed with Anthocyanin (Isolated from Red Cabbage) Bio Stickers. Foods 2020, 9, 307. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Zhang, J.; Shi, J.; Zou, X.; Huang, X.; Zhang, D.; Sun, Y.; Yang, Z.; Holmes, M.; et al. Natural Biomaterial-Based Edible and pH-Sensitive Films Combined with Electrochemical Writing for Intelligent Food Packaging. J. Agric. Food Chem. 2018, 66, 12836–12846. [Google Scholar] [CrossRef]
- Othman, M.; Yusup, A.A.; Zakaria, N.; Khalid, K. Bio-polymer chitosan and corn starch with extract of hibiscus rosa-sinensis (hibiscus) as PH indicator for visually-smart food packaging. AIP Conf. Proc. 2018, 1985, 050004. [Google Scholar] [CrossRef]
- Aghaei, Z.; Emadzadeh, B.; Ghorani, B.; Kadkhodaee, R. Cellulose Acetate Nanofibres Containing Alizarin as a Halochromic Sensor for the Qualitative Assessment of Rainbow Trout Fish Spoilage. Food Bioprocess Technol. 2018, 11, 1087–1095. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Almasi, H.; Forough, M.; Ezati, P. A novel pH-sensing indicator based on bacterial cellulose nanofibers and black carrot anthocyanins for monitoring fish freshness. Carbohydr. Polym. 2019, 222, 115030. [Google Scholar] [CrossRef]
- Apriliyanti, M.W.; Wahyono, A.; Fatoni, M.; Poerwanto, B.; Suryaningsih, W. The Potency of betacyanins extract from a peel of dragon fruits as a source of colourimetric indicator to develop intelligent packaging for fish freshness monitoring. IOP Conf. Ser. Earth Environ. Sci. 2018, 207, 012038. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. The application of natural food colorants as indicator substances in intelligent biodegradable packaging materials. Food Chem. Toxicol. 2020, 135, 110975. [Google Scholar] [CrossRef]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Huang, X.; Zhang, W.; Holmes, M. Novel colorimetric films based on starch/polyvinyl alcohol incorporated with roselle anthocyanins for fish freshness monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef]
- Maciel, V.B.; Franco, T.T.; Yoshida, C.M. Alternative intelligent material for packaging using chitosan films as colorimetric temperature indicators. Polímeros 2012, 22, 318–324. [Google Scholar] [CrossRef]
- Listyarini, A.; Sholihah, W.; Imawan, C. A paper-based colorimetric indicator label using natural dye for monitoring shrimp spoilage. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK; Chicago, IL, USA, 2018. [Google Scholar]
- Ma, Q.; Du, L.; Wang, L. Tara gum/polyvinyl alcohol-based colorimetric NH3 indicator films incorporating curcumin for intelligent packaging. Sens. Actuators B Chem. 2017, 244, 759–766. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef]
- Padhi, B. Pollution due to synthetic dyes toxicity & carcinogenicity studies and remediation. Int. J. Environ. Sci. 2012, 3, 940. [Google Scholar]
- Suslick, K.S.; Rakow, N.A.; Sen, A. Colorimetric sensor arrays for molecular recognition. Tetrahedron 2004, 60, 11133–11138. [Google Scholar] [CrossRef]
- Wells, N.; Yusufu, D.; Mills, A. Colourimetric plastic film indicator for the detection of the volatile basic nitrogen compounds associated with fish spoilage. Talanta 2019, 194, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Lu, X.; Wang, W.; Hubbe, M.A.; Liu, Y.; Mu, J.; Wang, J.; Sun, J.; Rojas, O.J. Recent developments in colorimetric and optical indicators stimulated by volatile base nitrogen to monitor seafood freshness. Food Packag. Shelf Life 2021, 28, 100634. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, X.; Qin, Y.-R.; Zhang, Y.-H.; Wang, X.-P.; Wang, J.-Y.; Ning, Z.-X.; Ruan, Q.-J.; Zhang, Y.-S. Preparation and characterization of a novel colorimetric indicator film based on gelatin/polyvinyl alcohol incorporating mulberry anthocyanin extracts for monitoring fish freshness. Food Res. Int. 2019, 126, 108604. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an intelligent film based on chitosan/oxidized chitin nanocrystals incorporating black rice bran anthocyanins for seafood spoilage monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef]
- Peralta, J.; Bitencourt-Cervi, C.M.; Maciel, V.B.; Yoshida, C.M.; Carvalho, R.A. Aqueous hibiscus extract as a potential natural pH indicator incorporated in natural polymeric films. Food Packag. Shelf Life 2019, 19, 47–55. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yuan, L.; Yong, H.; Liu, J. Preparation and characterization of antioxidant, antimicrobial and pH-sensitive films based on chitosan, silver nanoparticles and purple corn extract. Food Hydrocoll. 2019, 96, 102–111. [Google Scholar] [CrossRef]
- Liang, T.; Sun, G.; Cao, L.; Li, J.; Wang, L. Rheological behavior of film-forming solutions and film properties from Artemisia sphaerocephala Krasch. gum and purple onion peel extract. Food Hydrocoll. 2018, 82, 124–134. [Google Scholar] [CrossRef]
- Huang, J.; Chen, M.; Zhou, Y.; Li, Y.; Hu, Y. Functional characteristics improvement by structural modification of hydroxypropyl methylcellulose modified polyvinyl alcohol films incorporating roselle anthocyanins for shrimp freshness monitoring. Int. J. Biol. Macromol. 2020, 162, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Wei, Y.-C.; Cheng, C.-H.; Ho, Y.-C.; Tsai, M.-L.; Mi, F.-L. Active gellan gum/purple sweet potato composite films capable of monitoring pH variations. Food Hydrocoll. 2017, 69, 491–502. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Sun, J.; Lu, Y.; Tong, C.; Wang, L.; Yan, Z.; Pang, J. Novel konjac glucomannan films with oxidized chitin nanocrystals immobilized red cabbage anthocyanins for intelligent food packaging. Food Hydrocoll. 2020, 98, 105245. [Google Scholar] [CrossRef]
- Stoll, L.; Rech, R.; Flôres, S.H.; Nachtigall, S.M.B.; de Oliveira Rios, A. Carotenoids extracts as natural colorants in poly (lactic acid) films. J. Appl. Polym. Sci. 2018, 135, 46585. [Google Scholar] [CrossRef]
- Stoll, L.; Rech, R.; Flôres, S.H.; Nachtigall, S.M.B.; de Oliveira Rios, A. Poly (acid lactic) films with carotenoids extracts: Release study and effect on sunflower oil preservation. Food Chem. 2019, 281, 213–221. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Zhang, X.; Liu, J. Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocoll. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Hu, H.; Yao, X.; Qin, Y.; Yong, H.; Liu, J. Development of multifunctional food packaging by incorporating betalains from vegetable amaranth (Amaranthus tricolor L.) into quaternary ammonium chitosan/fish gelatin blend films. Int. J. Biol. Macromol. 2020, 159, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Chavoshizadeh, S.; Pirsa, S.; Mohtarami, F. Conducting/smart color film based on wheat gluten/chlorophyll/polypyrrole nanocomposite. Food Packag. Shelf Life 2020, 24, 100501. [Google Scholar] [CrossRef]
- Castaneda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Torskangerpoll, K.; Andersen, Ø.M. Colour stability of anthocyanins in aqueous solutions at various pH values. Food Chem. 2005, 89, 427–440. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J.; Qin, Y.; Bai, R.; Zhang, X.; Liu, J. Antioxidant and pH-sensitive films developed by incorporating purple and black rice extracts into chitosan matrix. Int. J. Biol. Macromol. 2019, 137, 307–316. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Shi, J.; Huang, X.; Sun, Z.; Zhang, D.; Zou, X.; Sun, Y.; Zhang, J.; Holmes, M. A colorimetric hydrogen sulfide sensor based on gellan gum-silver nanoparticles bionanocomposite for monitoring of meat spoilage in intelligent packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef]
- Tirtashi, F.E.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Cellulose/chitosan pH-responsive indicator incorporated with carrot anthocyanins for intelligent food packaging. Int. J. Biol. Macromol. 2019, 136, 920–926. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, T.; Cao, L.; Wang, L. Intelligent poly (vinyl alcohol)-chitosan nanoparticles-mulberry extracts films capable of monitoring pH variations. Int. J. Biol. Macromol. 2018, 108, 576–584. [Google Scholar] [CrossRef]
- Huang, X.-W.; Zou, X.-B.; Shi, J.-Y.; Guo, Y.; Zhao, J.-W.; Zhang, J.; Hao, L. Determination of pork spoilage by colorimetric gas sensor array based on natural pigments. Food Chem. 2014, 145, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Kulawik, P.; Guzik, P.; Duda, I. The verification of intelligent properties of furcellaran films with plant extracts on the stored fresh Atlantic mackerel during storage at 2 °C. Food Hydrocoll. 2019, 97, 105211. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, D.-W.; Zhu, Z. Recent developments in intelligent packaging for enhancing food quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef] [PubMed]
- Saliu, F.; Della Pergola, R. Carbon dioxide colorimetric indicators for food packaging application: Applicability of anthocyanin and poly-lysine mixtures. Sens. Actuators B Chem. 2018, 258, 1117–1124. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, L.-T. Colorimetric array indicator for NH3 and CO2 detection. Sens. Actuators B Chem. 2018, 255, 3216–3226. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation—structural and chromatic aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Gordon, H.T.; Bauernfeind, J.C.; Furia, T.E. Carotenoids as food colorants. CRC Crit. Rev. Food Sci. Nutr. 1983, 18, 59–97. [Google Scholar] [CrossRef]
- Won, K.; Jang, N.Y.; Jeon, J. A natural component-based oxygen indicator with in-pack activation for intelligent food packaging. J. Agric. Food Chem. 2016, 64, 9675–9679. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Jung, J.; Ko, S. Carbon dioxide sensors for intelligent food packaging applications. Food Control 2012, 25, 328–333. [Google Scholar] [CrossRef]
- Koskela, J.; Sarfraz, J.; Ihalainen, P.; Määttänen, A.; Pulkkinen, P.; Tenhu, H.; Nieminen, T.; Kilpelä, A.; Peltonen, J. Monitoring the quality of raw poultry by detecting hydrogen sulfide with printed sensors. Sens. Actuators B Chem. 2015, 218, 89–96. [Google Scholar] [CrossRef]
- Huang, X.; Zou, X.; Shi, J.; Li, Z.; Zhao, J. Colorimetric sensor arrays based on chemo-responsive dyes, orfood, odor visualization. Trends Food Sci. Technol. 2018, 81, 90–107. [Google Scholar] [CrossRef]
- Chen, H.-z.; Zhang, M.; Bhandari, B.; Guo, Z. Applicability of a colorimetric indicator label for monitoring freshness of fresh-cut green bell pepper. Postharvest Biol. Technol. 2018, 140, 85–92. [Google Scholar] [CrossRef]
- Heising, J.K.; Dekker, M.; Bartels, P.V.; Van Boekel, M. Monitoring the quality of perishable foods: Opportunities for intelligent packaging. Crit. Rev. Food Sci. Nutr. 2014, 54, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Su, H.-M.; Imani, S.M.; Alkhaldi, K..; M. Filipe, C.D.; Didar, T.F. Intelligent food packaging: A review of smart sensing technologies for monitoring food quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, A.; Cerdán-Cartagena, F.; Suardíaz-Muro, J.; Boluda-Aguilar, M.; Hernández-Hernández, M.E.; López-Serrano, M.A.; López-Coronado, J. Radiofrequency identification and surface acoustic wave technologies for developing the food intelligent packaging concept. Food Eng. Rev. 2015, 7, 11–32. [Google Scholar] [CrossRef]
- Mijanur Rahman, A.; Kim, D.; Jang, H.; Yang, J.; Lee, S. Preliminary study on biosensor-type time-temperature integrator for intelligent food packaging. Sensors 2018, 18, 1949. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent packaging in the food sector: A brief overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Yang, M.; Zhang, Y.; Xiang, K.; Tang, R. Review of time temperature indicators as quality monitors in food packaging. Packag. Technol. Sci. 2015, 28, 839–867. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, G.; Xiao, X.; Liu, Y.; Zheng, X. Application of microbial TTIs as smart label for food quality: Response mechanism, application and research trends. Trends Food Sci. Technol. 2016, 51, 12–23. [Google Scholar] [CrossRef]
- Taoukis, P.; Labuza, T.P. Applicability of time-temperature indicators as shelf life monitors of food products. J. Food Sci. 1989, 54, 783–788. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stability of anthocyanin-based aqueous extracts of Andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants. Food Chem. 2004, 86, 69–77. [Google Scholar] [CrossRef]
- Shaked-Sachray, L.; Weiss, D.; Reuveni, M.; Nissim-Levi, A.; Oren-Shamir, M. Increased anthocyanin accumulation in aster flowers at elevated temperatures due to magnesium treatment. Physiol. Plant. 2002, 114, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Alighourchi, H.; Barzegar, M. Some physicochemical characteristics and degradation kinetic of anthocyanin of reconstituted pomegranate juice during storage. J. Food Eng. 2009, 90, 179–185. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; Da Pieve, S.; Butler, F. Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purées. Innov. Food Sci. Emerg. Technol. 2009, 10, 308–313. [Google Scholar] [CrossRef]
- Maciel, V.B.; Yoshida, C.M.; Franco, T.T. Development of a prototype of a colourimetric temperature indicator for monitoring food quality. J. Food Eng. 2012, 111, 21–27. [Google Scholar] [CrossRef]
- Park, Y.W.; Kim, S.M.; Lee, J.Y.; Jang, W. Application of biosensors in smart packaging. Mol. Cell. Toxicol. 2015, 11, 277–285. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; Wagner, J.R. Use of edible films and coatings for functional foods developments: A review. Funct. Foods Sources Health Eff. Future Perspect. 2017, 1–26. [Google Scholar]
- Ejaz, M.; Arfat, Y.A.; Mulla, M.; Ahmed, J. Zinc oxide nanorods/clove essential oil incorporated Type B gelatin composite films and its applicability for shrimp packaging. Food Packag. Shelf Life 2018, 15, 113–121. [Google Scholar] [CrossRef]
- Mohammadi, H.; Kamkar, A.; Misaghi, A.; Zunabovic-Pichler, M.; Fatehi, S. Nanocomposite films with CMC, okra mucilage, and ZnO nanoparticles: Extending the shelf-life of chicken breast meat. Food Packag. Shelf Life 2019, 21, 100330. [Google Scholar] [CrossRef]
- Park, H.-Y.; Kim, S.-J.; Kim, K.M.; You, Y.-S.; Kim, S.Y.; Han, J. Development of Antioxidant Packaging Material by Applying Corn-Zein to LLDPE Film in Combination with Phenolic Compounds. J. Food Sci. 2012, 77, E273–E279. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Lee, J.-H.; Won, M.; Song, K.B. Antioxidant activities of distiller dried grains with solubles as protein films containing tea extracts and their application in the packaging of pork meat. Food Chem. 2016, 196, 174–179. [Google Scholar] [CrossRef]
- Sani, M.A.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Pereira de Abreu, D.A.; Paseiro Losada, P.; Maroto, J.; Cruz, J.M. Natural antioxidant active packaging film and its effect on lipid damage in frozen blue shark (Prionace glauca). Innov. Food Sci. Emerg. Technol. 2011, 12, 50–55. [Google Scholar] [CrossRef]
- Souza, C.O.; Silva, L.T.; Silva, J.R.; López, J.A.; Veiga-Santos, P.; Druzian, J.I. Mango and Acerola Pulps as Antioxidant Additives in Cassava Starch Bio-based Film. J. Agric. Food Chem. 2011, 59, 2248–2254. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Rodríguez, G.M.; Sibaja, J.C.; Espitia, P.J.; Otoni, C.G. Antioxidant active packaging based on papaya edible films incorporated with Moringa oleifera and ascorbic acid for food preservation. Food Hydrocoll. 2020, 103, 105630. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Dao, U.T.T.; Bui, Q.P.T.; Bach, G.L.; Thuc, C.H.; Thuc, H.H. Enhanced antimicrobial activities and physiochemical properties of edible film based on chitosan incorporated with Sonneratia caseolaris (L.) Engl. leaf extract. Prog. Org. Coat. 2020, 140, 105487. [Google Scholar] [CrossRef]
- de Oliveira, T.M.; de Fátima Ferreira Soares, N.; Pereira, R.M.; de Freitas Fraga, K. Development and evaluation of antimicrobial natamycin-incorporated film in gorgonzola cheese conservation. Packag. Technol. Sci. Int. J. 2007, 20, 147–153. [Google Scholar] [CrossRef]
- Kurnianto, M.; Poerwanto, B.; Wahyono, A.; Apriliyanti, M.; Lestari, I. Monitoring of banana deteriorations using intelligent-packaging containing brazilien extract (Caesalpina sappan L.). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK; Chicago, IL, USA, 2020. [Google Scholar]
- Liu, B.; Xu, H.; Zhao, H.; Liu, W.; Zhao, L.; Li, Y. Preparation and characterization of intelligent starch/PVA films for simultaneous colorimetric indication and antimicrobial activity for food packaging applications. Carbohydr. Polym. 2017, 157, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Tiekstra, S.; Dopico-Parada, A.; Koivula, H.; Lahti, J.; Buntinx, M. Holistic Approach to a Successful Market Implementation of Active and Intelligent Food Packaging. Foods 2021, 10, 465. [Google Scholar] [CrossRef]
- Ribeiro Sanches, M.A.; Camelo-Silva, C.; da Silva Carvalho, C.; Rafael de Mello, J.; Barroso, N.G.; Lopes da Silva Barros, E.; Silva, P.P.; Pertuzatti, P.B. Active packaging with starch, red cabbage extract and sweet whey: Characterization and application in meat. LWT 2021, 135, 110275. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Guo, M.; Li, L.; Chen, M.; Jiang, S.; Li, X.; Jiang, S. Extract from Lycium ruthenicum Murr. Incorporating κ-carrageenan colorimetric film with a wide pH–sensing range for food freshness monitoring. Food Hydrocoll. 2019, 94, 1–10. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, M.; Lee, Y.S. Temperature sensitive smart packaging for monitoring the shelf life of fresh beef. J. Food Eng. 2018, 234, 41–49. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Iqbal, H.M. Development and characterization of essential oils incorporated chitosan-based cues with antibacterial and antifungal potentialities. J. Radiat. Res. Appl. Sci. 2020, 13, 174–179. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Guo, X.; Ge, S.; Zhang, Q. Physicochemical properties, antimicrobial activity and oil release of fish gelatin films incorporated with cinnamon essential oil. Aquac. Fish. 2017, 2, 185–192. [Google Scholar] [CrossRef]
- Karaman, A.D.; Özer, B.; Pascall, M.A.; Alvarez, V. Recent Advances in Dairy Packaging. Food Rev. Int. 2015, 31, 295–318. [Google Scholar] [CrossRef]
- Conte, A.; Scrocco, C.; Sinigaglia, M.; Del Nobile, M.A. Innovative Active Packaging Systems to Prolong the Shelf Life of Mozzarella Cheese. J. Dairy Sci. 2007, 90, 2126–2131. [Google Scholar] [CrossRef] [PubMed]
- Kuorwel, K.K.; Bigger, S.W.; Cran, M.J.; Sonneveld, K.; Miltz, J. The antimicrobial activity of carvacrol and linalool against S. aureus for the packaging of Cheddar cheese. In Proceedings of the 17th IAPRI World Conference of Packaging, Tianjin, China, 12–15 October 2010; Binglin, L.U., Ed.; Scientific Research Publishing: Irvine, CA, USA, 2010. [Google Scholar]
- da Rosa, C.G.; Sganzerla, W.G.; Maciel, M.V.d.O.B.; de Melo, A.P.Z.; da Rosa Almeida, A.; Nunes, M.R.; Bertoldi, F.C.; Barreto, P.L.M. Development of poly (ethylene oxide) bioactive nanocomposite films functionalized with zein nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124268. [Google Scholar] [CrossRef]
- Ramesh, M.; Narendra, G.; Sasikanth, S. A review on biodegradable packaging materials in extending the shelf life and quality of fresh fruits and vegetables. In Waste Management as Economic Industry towards Circular Economy; Springer: Berlin/Heidelberg, Germany, 2020; pp. 59–65. [Google Scholar]
- Pavinatto, A.; de Almeida Mattos, A.V.; Malpass, A.C.G.; Okura, M.H.; Balogh, D.T.; Sanfelice, R.C. Coating with chitosan-based edible films for mechanical/biological protection of strawberries. Int. J. Biol. Macromol. 2020, 151, 1004–1011. [Google Scholar] [CrossRef]
- Torres-León, C.; Vicente, A.A.; Flores-López, M.L.; Rojas, R.; Serna-Cock, L.; Alvarez-Pérez, O.B.; Aguilar, C.N. Edible films and coatings based on mango (var. Ataulfo) by-products to improve gas transfer rate of peach. LWT 2018, 97, 624–631. [Google Scholar] [CrossRef]
- Taqi, A.; Mutihac, L.; Stamatin, I. Physical and Barrier Properties of Apple Pectin/Cassava Starch Composite Films Incorporating Laurus nobilis L. Oil and Oleic Acid. J. Food Process. Preserv. 2014, 38, 1982–1993. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Pereira Ribeiro, C.P.; Uliana, N.R.; Cassetari Rodrigues, M.B.; da Rosa, C.G.; Ferrareze, J.P.; Veeck, A.P.d.L.; Nunes, M.R. Bioactive and pH-sensitive films based on carboxymethyl cellulose and blackberry (Morus nigra L.) anthocyanin-rich extract: A perspective coating material to improve the shelf life of cherry tomato (Solanum lycopersicum L. var. cerasiforme). Biocatal. Agric. Biotechnol. 2021, 33, 101989. [Google Scholar] [CrossRef]
- Buendía-Moreno, L.; Soto-Jover, S.; Ros-Chumillas, M.; Antolinos, V.; Navarro-Segura, L.; Sánchez-Martínez, M.J.; Martínez-Hernández, G.B.; López-Gómez, A. Innovative cardboard active packaging with a coating including encapsulated essential oils to extend cherry tomato shelf life. LWT 2019, 116, 108584. [Google Scholar] [CrossRef]
- Da Rocha Neto, A.C.; Beaudry, R.; Maraschin, M.; Di Piero, R.M.; Almenar, E. Double-bottom antimicrobial packaging for apple shelf-life extension. Food Chem. 2019, 279, 379–388. [Google Scholar] [CrossRef]
| Polymer(s)/Biopolymer(s) | Active Material(s) | Smart/or Active Packaging | Characteristics of Packaging Films | Thermal | Ref. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical | Mechanical | Barrier | Optical | ||||||||||||
| WS | MC | WCA | Th | TS | EB | YM | WVP | OP | T600/Op | Color | |||||
| Chitosan/poly (vinyl alcohol) | Boswellic acid | Active | + | − | + | ± | + | − | + | − | − | −/+ | − | − | [32] |
| Gelatin | Grapefruit seed extract/TiO2 NPs | Active | N | N | − | + | − | + | − | + | N | −/+ | + | − | [33] |
| Poly(lactide)/poly(butylene adipate-co-terephthalate) | Ferulic acid | Active | N | N | − | + | + | − | + | N | N | −/+ | N | − | [34] |
| Poly(lactic acid)/poly(butylene-succinate-co-adipate) (PLA/PBSA) | Thymol EOs | Active | − | − | N | + | − | + | − | − | − | − | N | − | [35] |
| Starch | Yerba mate extract | Active | − | − | N | + | + | + | − | − | − | N | N | N | [36] |
| Poly(vinyl alcohol)/clay | Tea polyphenols | Active | − | − | ± | ± | + | − | N | − | − | −/+ | + | N | [37] |
| Chitosan/gallic-acid | ZnO NPs | Active | − | − | N | + | − | + | N | − | − | −/+ | N | N | [38] |
| Corn starch/chitosan | Grapefruit seed extract | Active | + | + | N | + | − | + | − | − | − | N | N | − | [39] |
| Gelatin | Silver-Kaolin NPs | Active | − | − | + | + | + | − | + | − | N | −/+ | N | N | [40] |
| Sodium caseinate/guar gum | TiO2 NPs/cumin EOs | Active | − | N | − | + | + | ± | + | ± | N | −/+ | N | − | [41] |
| Methyl cellulose/chitosan nanofibers | Saffron petal anthocyanins | Smart | − | − | N | + | + | + | − | − | N | − | + | − | [42] |
| Cassava starch | Blueberry residue | Smart | + | + | + | ± | − | + | − | ± | + | −/+ | + | − | [43] |
| Chitosan | Black soybean seed coat extract | Smart | + | − | N | + | + | + | N | − | N | + | + | − | [44] |
| Gelatin | Red cabbage (Brassica oleracea L.) extracts | Smart | + | − | N | + | + | + | − | + | N | + | − | N | [45] |
| Chitosan | Purple-fleshed sweet potato extract | Smart | + | − | N | + | − | − | N | + | N | − | − | − | [46] |
| Agar | Arnebia euchroma root extracts | Smart | − | − | + | − | + | + | + | + | N | − | + | N | [47] |
| Gelatin | Curcumin | Smart | ± | ± | N | + | − | + | − | − | N | −/+ | + | N | [48] |
| k-carrageenan | Curcumin | Smart | N | N | N | + | + | − | + | − | − | − | + | + | [49] |
| Chitosan | Blueberry and blackberry pomace extracts | Smart | ± | − | N | ± | ± | − | + | − | ± | + | ± | N | [19] |
| Chitosan | Alizarin | Smart | N | N | + | + | − | + | + | − | − | + | − | + | [50] |
| Packaging Film Matrix | Active Additives | Additive Functions | Remarks | Ref. |
|---|---|---|---|---|
| Chitosan | Pine needle extract (Cedrus deodara) | Antioxidant/physical/oxygen/water vapor permeability/color/microstructures | Films showed high antioxidant activity and protected oxygen-sensitive foods. | [148] |
| Chitosan | Flavanols (kaempferol, quercetin, myricetin) | Antimicrobial/Antioxidant/water vapor permeability/oxygen permeability/UV–vis light transmittance | Prevention of microbial growth | [149] |
| Poly(lactic acid)/ Poly(ε-caprolactone) | EOs (thymol, carvacrol) | Antioxidant | A PLA film impregnated with thymol and carvacrol had the best antioxidant activity. | [150] |
| Chitosan | Poly (vinyl alcohol) | Antimicrobial/ultraviolet blocking/morphology/mechanical properties/water solubility/hydrophilicity | Films exhibited antimicrobial activity against Escherichia coli, Staphylococcus aureus, and Candida albicans. | [32] |
| Polylactic acid | EOs (thymol, kesum, curry) | Antimicrobial/Morphology/functional chemistry/thermal stability/permeability | Films inhibited bacterial growth and extended shelf life of meats, fruits, and vegetable products | [151] |
| Sodium lactate/ whey protein isolate | ɛ-Poly lysine | Mechanical behavior/Antimicrobial | Films extended shelf-life by reduction of total flora and inhibiting lactic acid bacteria growth | [152] |
| Chitosan/ Carboxymethyl cellulose | ZnO nanoparticles | Antimicrobial/Physicochemical and physical properties | Films had good activity against gram-positive bacteria and fungi | [153] |
| Chitosan | ethyl-Nα-dodecanoyl-Larginate | Antimicrobial | Films exhibited antibacterial activity | [154] |
| Poly(ε-caprolactone) | Oxidized regenerated cellulose | Antimicrobial | Films reduced total colony-forming units on salami during storage. | [155] |
| LDPE/LLDPE | Ag/TiO2 nanoparticles | Antimicrobial | Nanoparticle addition improved antimildew and physicochemical properties of films. | [156] |
| Polyvinyl chloride | Ag nanoparticles | Antimicrobial/Antioxidant | Films inhibited bacterial growth, reduced oxidation, and extended shelf life | [157] |
| Sodium alginate | ZnO nanoparticles | Antimicrobial | Films reduced initial bacterial count | [158] |
| Whey protein isolate | Lactoferrin, Lysozyme, and the Lactoperoxidase | Antimicrobial | Films extended shelf-life by inhibiting bacterial growth | [159] |
| Packaging Film Matrix | Colorant Agent/Source | Trigger | Remarks | Ref. |
|---|---|---|---|---|
| Chitosan/ Polyvinyl alcohol (PVA) | Anthocyanin/Red cabbage | pH indicator | Additives increased tensile strength of film and provided color indication of pork spoilage during storage. | [162] |
| Chitosan/Starch/ Polyvinyl alcohol | Anthocyanin/Roselle calyx | pH indicator | Color changes in film provided indication of spoilage in pork. | [163] |
| Hydroxy propyl methylcellulose/ κ-carrageenan | Anthocyanin/Prunus maackii juice | pH indicator | Color changes in film provided indication of spoilage. | [164] |
| Agar/Tapioca starch | Anthocyanin/Red cabbage | pH indicator | Color changes in film provided indication of spoilage in sausage. | [165] |
| Cassava starch | Anthocyanin/Blueberry residue | pH indicator | Color changes in film provided indication of spoilage. | [166] |
| Methylcellulose/ Chitosan nanofiber | Anthocyanin/Barberry (BA) | pH indicator | Films underwent color changes when exposed to different pH conditions. | [79] |
| Poly vinyl pyrrolidone/CMC/Bacterial cellulose/Guar gum | Anthocyanin/Red cabbage | pH indicator | Anthocyanin addition improved physicochemical properties of films and were suitable as color sensors of pH changes. | [167] |
| Gelatin/Gellan gum | Anthocyanins/Red radish | pH indicator | Films underwent color changes when exposed to different pH conditions. | [168] |
| Chitosan/Pectin | Anthocyanin Hibiscus rosa-sinensis | pH indicator | Color changes in film provided indication of spoilage during storage. | [169] |
| Cellulose acetate nanofibers | Alizarin | pH indicator | Color changes in film provided indication of spoilage. | [170] |
| Bacterial cellulose nanofiber | Anthocyanin/Black carrot | pH indicator | Films underwent color changes when exposed to different pH conditions. | [171] |
| Glucomannan/Polyvinyl alcohol | Betacyanin | pH indicator | Films underwent color changes when exposed to different pH conditions. | [172] |
| Methylcellulose/ Chitin nanofiber | Anthocyanins/Red barberry | pH indicator | Color changes in film provided indication of spoilage in fish and meat samples during storage. | [28] |
| Artemisia sphaerocephala Krasch. gum (ASKG)/Carboxymethyl cellulose sodium | Anthocyanins/Red cabbage | pH/Gas/volatile compounds indicator (NH3) | Color changes in film in response to pH changes or NH3 production provided indication of spoilage | [132] |
| Polylactide/Poly hydroxybutyrate | β-carotene, Chlorophyll, Curcumin, Lutein | Temperature/Light | Color changes in film in response to changes in temperature or light exposure | [173] |
| Starch/Polyvinyl alcohol | Anthocyanins/Roselle | Temperature/pH indicator | Color changes in film in response to changes in pH or light exposure | [174] |
| Agar | Arnebia euchroma root | Temperature/Freshness | Film changed color when fish spoiled. | [47] |
| Chitosan/Polyvinyl alcohol | Anthocyanins/ Red cabbage | Time/Temperature | The colorimetric film on pasteurized milk shows visual color changes to consumers. | [118] |
| Chitosan | Chlorophyll | Temperature | Film changed color when exposed to elevated temperatures (>50 °C). | [175] |
| Cellulose | Anthocyanin/Ruellia Simplex flowers | Time/Temperature | Film changed color when exposed to different temperatures: pink/blue (at 13 °C); purplish/blue (at 25 °C); yellow/gray (at 40 °C) | [176] |
| Bacterial cellulose nanofibers | Anthocyanin/Black carrot | Gas/volatile ammonia compounds | Film changed color in response to gas production | [171] |
| Tara gum/Polyvinyl alcohol | Curcumin | Gas/volatile compounds (TVBN, NH3) | Film changed color in response to gas production | [177] |
| Food model | Polymers | Active materials | Smart or Active | Function | Remarks | Ref. |
|---|---|---|---|---|---|---|
| Shrimp | Bovine skin gelatin | ZnO nanoparticles/clove essential oil | Active | Antibacterial | Composite films showed antibacterial activity against Listeria monocytogenes and Salmonella Typhimurium inoculated in shrimp during refrigerated storage. | [232] |
| Chicken breast meat | Carboxymethyl cellulose | Okra mucilage/ ZnO nanoparticles | Active | Antimicrobial/Antioxidant | Incorporating okra mucilage and ZnO nanoparticles in films reduced microbial growth, oxidation, and gas production. | [233] |
| Vacuum-packed beef patties | Corn-zein-laminated linear LDPE film | Thymol, carvacrol, and eugenol essential oil | Active | Antioxidant | Incorporating essential oils in films reduced lipid oxidation and color changes in fresh ground beef patties during storage. | [234] |
| Pork meat | Distiller dried grains with soluble protein | Green tea, oolong tea, and black tea extracts | Active | Antioxidant | Incorporating tea extracts increased the antioxidant activity of films. | [235] |
| Lamb meat | Whey protein isolate/cellulose nanofibre/ | TiO2 nanoparticle/rosemary essential oil | Active | Antimicrobial/Antioxidant | Nanocomposite films reduced total viable count, Pseudomonas spp, Enterobacteriaceae, Lactic acid bacteria, Staphylococcus aureus, Listeria monocytogenes, and Escherichia coli counts. Higher inhibition observed for Gram-positive than Gram-negative bacteria | [236] |
| Frozen blue shark (Prionace glauca) | low density polyethylene (LDPE) | Barley husk extracts | Active | Antioxidant | Hydrolytic activity and lipid oxidation are sensitive to antioxidant content and storage time. | [237] |
| Palm oil | Cassava starch | Mango and acerola pulp | Active | Antioxidant | Antioxidants were effective additives for protecting the packaged product. | [238] |
| Strawberry | Clay/PE polymer | Carvacrol and thymol essential oils | Active | Antifungal | Incorporating essential oils in films increased antifungal activity against Botrytis. | [128] |
| Tomato | Chitosan | TiO2 nanoparticles | Active | Gas scavenger | Nanocomposite films delayed tomato ripening. | [239] |
| Pear | Papaya (Carica papaya L.) puree | Ascorbic acid and Moringa leaf extract | Active | Antioxidant | Films increased shelf-life and improved sensory properties of pears. | [240] |
| Banana | Chitosan | Sonneratia caseolaris (L.) Engl. leaf extract | Active | Antimicrobial | Incorporating a leaf extract into the films increase the shelf-life of bananas | [241] |
| Gorgonzola cheese | Cellulose polymeric films and laminated films | Natamycin | Active | Antifungal | Incorporating the antifungal agent into film led to increased inhibition of P. roqueforti | [242] |
| Fish | Chitin nanofiber/methylcellulose | Red barberry anthocyanins (RBAs) | Active/Smart | Antimicrobial/Antioxidant/ Colorimetric | Films exhibited good antioxidant and antimicrobial activity, as well as ability to detect quality changes. | [28] |
| Chicken | Chitosan/corn starch | Hibiscus rosa-sinensis anthocyanin | Smart | Colorimetric | Films exhibited good optical and morphological properties and are sensitive to pH changes. | [169] |
| Sausage | Agar/Tapioca starch | Red cabbage anthocyanin | Smart | Colorimetric | Anthocyanins change color in response to quality changes in sausage during storage. | [165] |
| Chicken | Cassava starch | Blueberry residue anthocyanin | Smart | Colorimetric | Anthocyanins change color in response to pH (quality) changes in chicken during storage. | [166] |
| Pork/Fish | Chitosan | Bauhinia blakeana Dunn. flower anthocyanin | Smart | Colorimetric | Anthocyanins change color in response to quality changes in pork and fish during storage. | [204] |
| Lamb meat | Chitosan nanofibers/methylcellulose | Saffron petal anthocyanins | Active/Smart | Antimicrobial/Antioxidant/Colorimetric | Chitosan provides antimicrobial activity while anthocyanins provide antioxidant activity and change color in response to changes in lamb quality during storage. | [42] |
| Red meat | Methylcellulose/chitosan nanofiber | Barberry anthocyanin | Active/Smart | Antioxidant/Colorimetric | Chitosan provides antimicrobial activity while anthocyanins change color in response to changes in meat quality during storage. | [79] |
| Banana | PVA/glucomannan | Sappan Wood extracts | Smart | Antioxidant | The wood extract changed color in response to quality changes in banana during storage. | [243] |
| Milk | Starch/Polyvinyl alcohol | Purple sweet potato anthocyanin | Smart | Antimicrobial/Colorimetric | The anthocyanins gave a color change in response to alterations in milk quality. The films also exhibited antimicrobial activity against Aspergillus niger, Bacillus subtilis, and Staphylococcus aureus. | [244] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sani, M.A.; Azizi-Lalabadi, M.; Tavassoli, M.; Mohammadi, K.; McClements, D.J. Recent Advances in the Development of Smart and Active Biodegradable Packaging Materials. Nanomaterials 2021, 11, 1331. https://doi.org/10.3390/nano11051331
Sani MA, Azizi-Lalabadi M, Tavassoli M, Mohammadi K, McClements DJ. Recent Advances in the Development of Smart and Active Biodegradable Packaging Materials. Nanomaterials. 2021; 11(5):1331. https://doi.org/10.3390/nano11051331
Chicago/Turabian StyleSani, Mahmood Alizadeh, Maryam Azizi-Lalabadi, Milad Tavassoli, Keyhan Mohammadi, and David Julian McClements. 2021. "Recent Advances in the Development of Smart and Active Biodegradable Packaging Materials" Nanomaterials 11, no. 5: 1331. https://doi.org/10.3390/nano11051331
APA StyleSani, M. A., Azizi-Lalabadi, M., Tavassoli, M., Mohammadi, K., & McClements, D. J. (2021). Recent Advances in the Development of Smart and Active Biodegradable Packaging Materials. Nanomaterials, 11(5), 1331. https://doi.org/10.3390/nano11051331








