Dimethylglyoxime Clathrate as Ligand Derived Nitrogen-Doped Carbon-Supported Nano-Metal Particles as Catalysts for Oxygen Reduction Reaction
Abstract
1. Introduction
2. Experimental Section
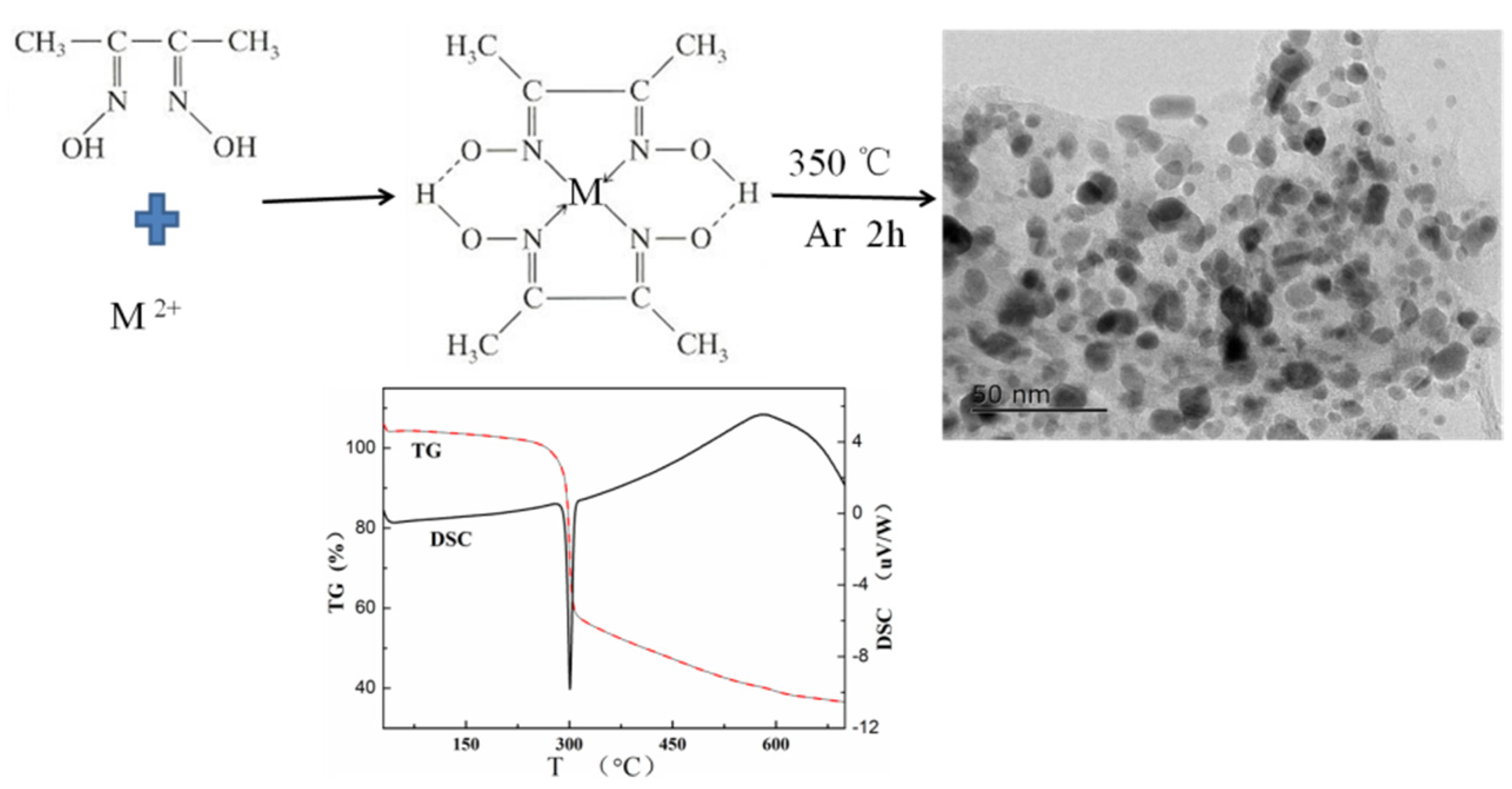
2.1. Materials Synthesis
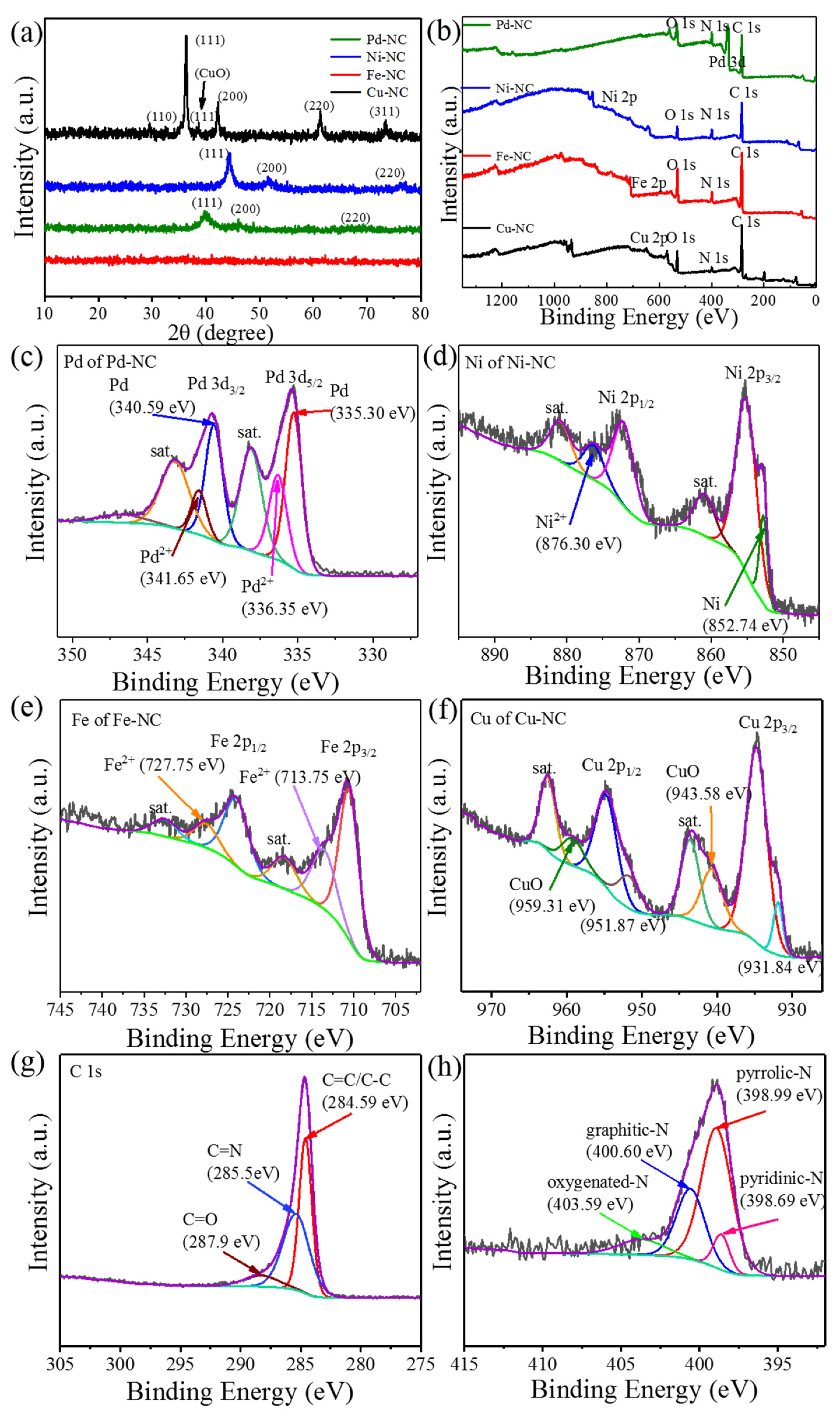
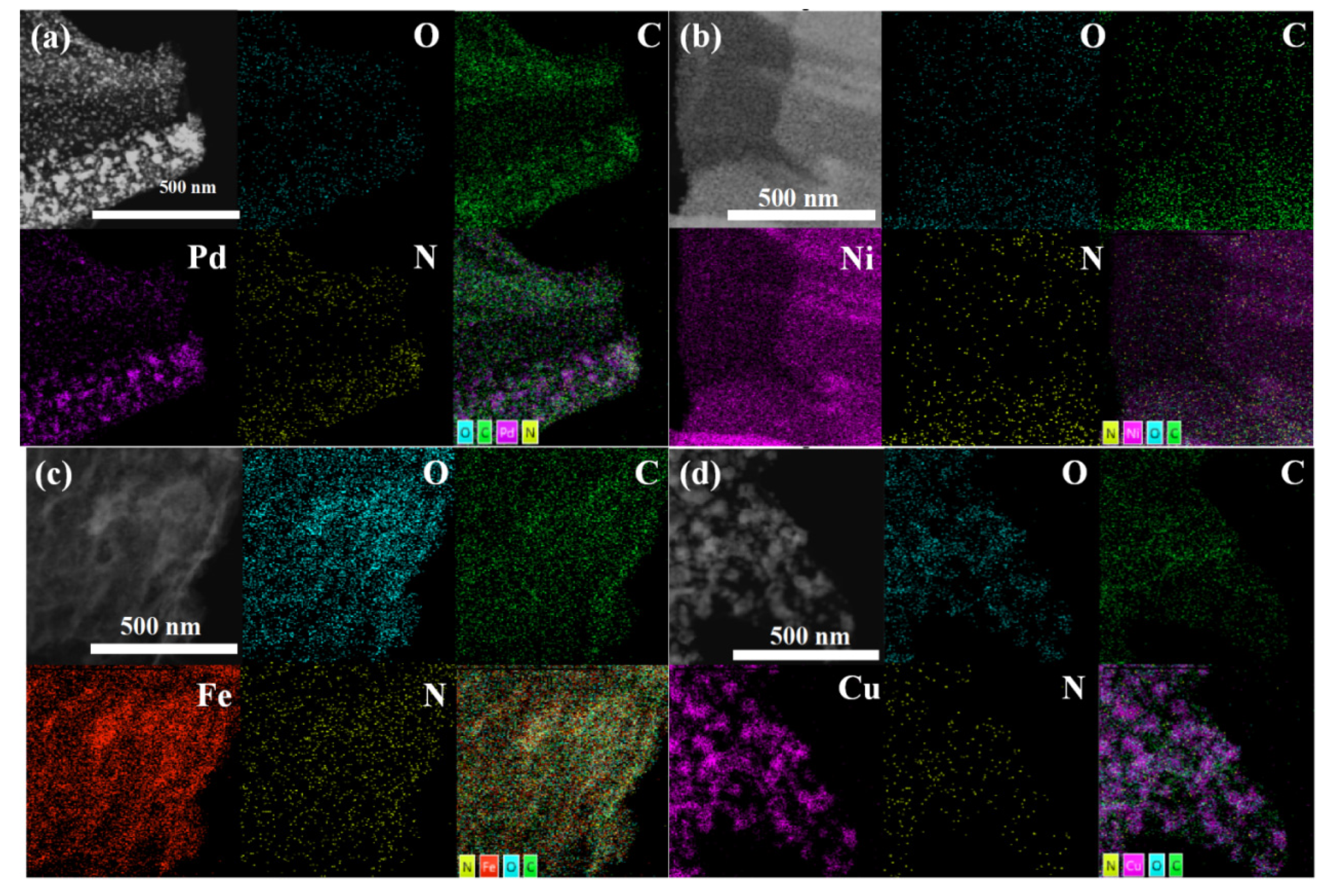
2.2. Structure Characterization
2.3. Electrochemical Measurements
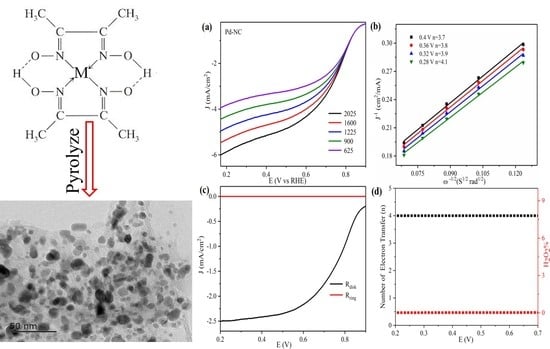
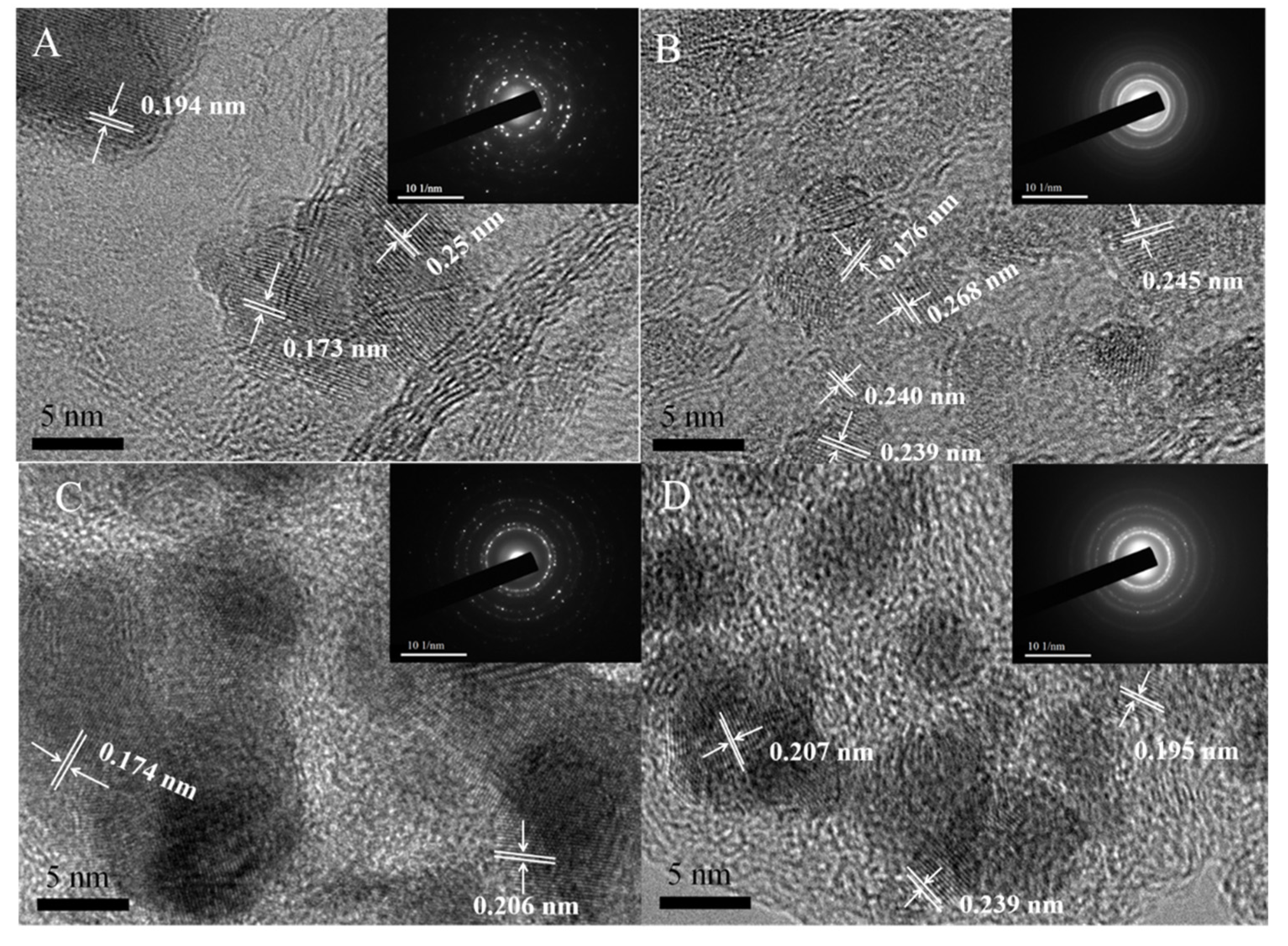
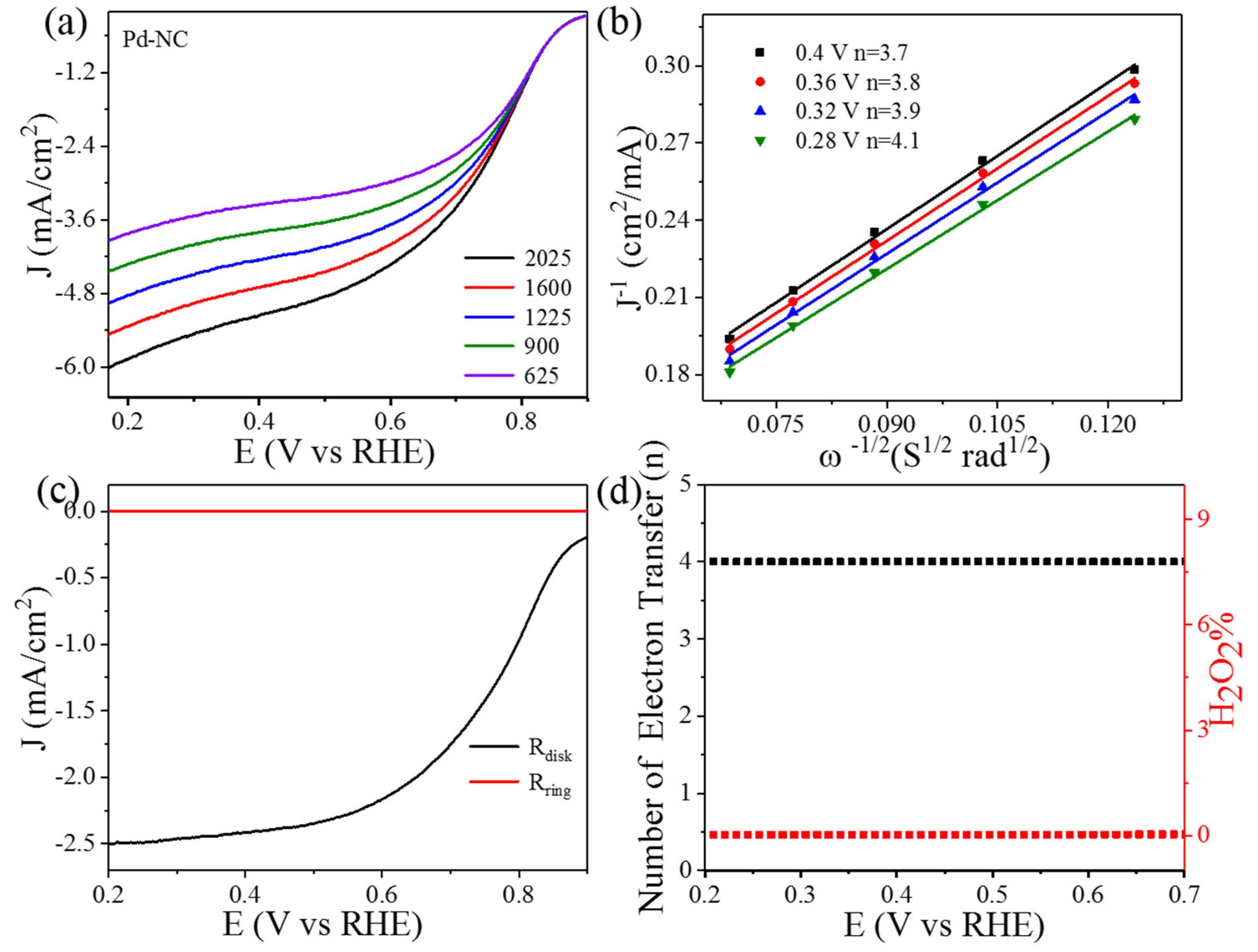
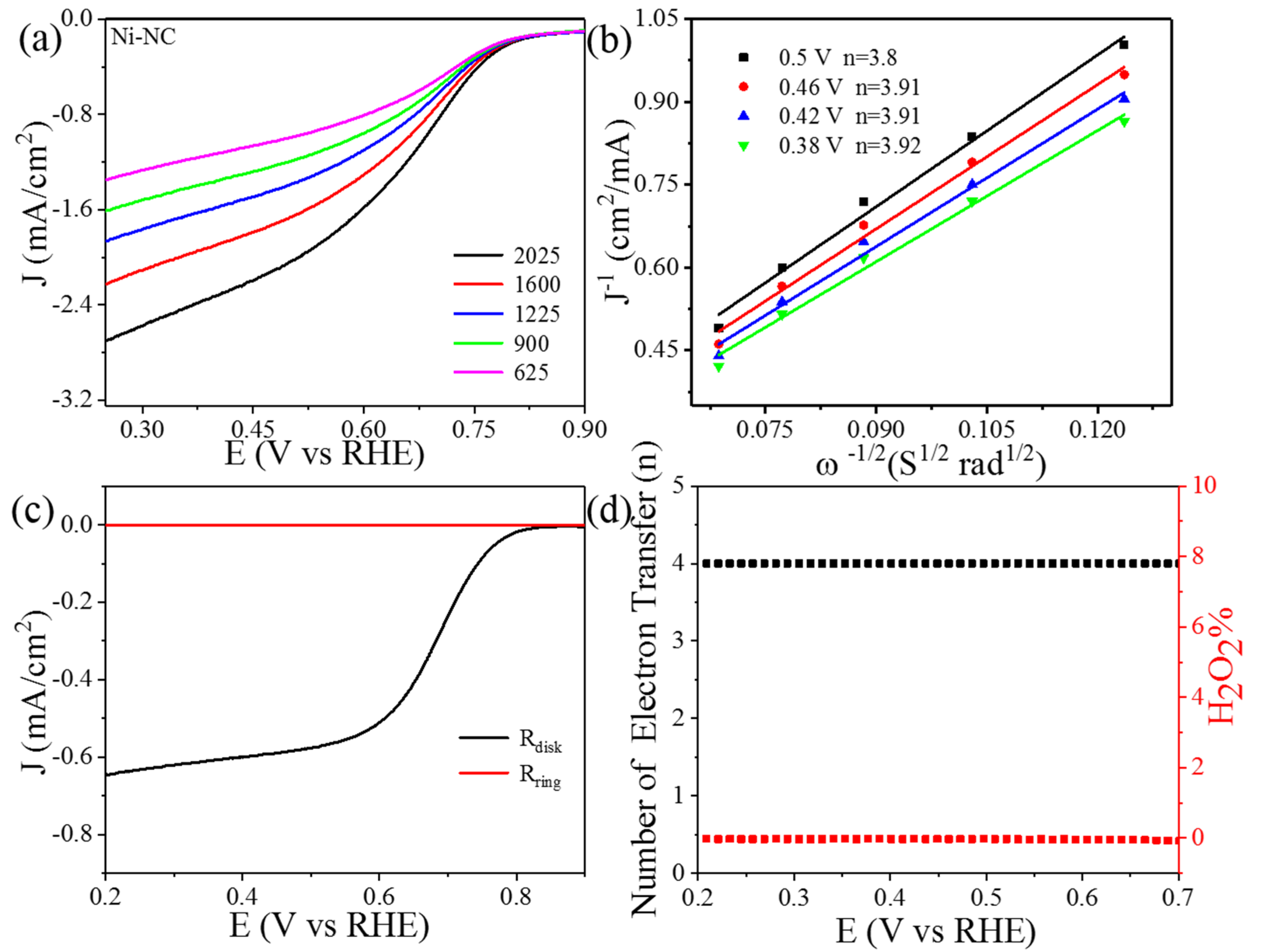
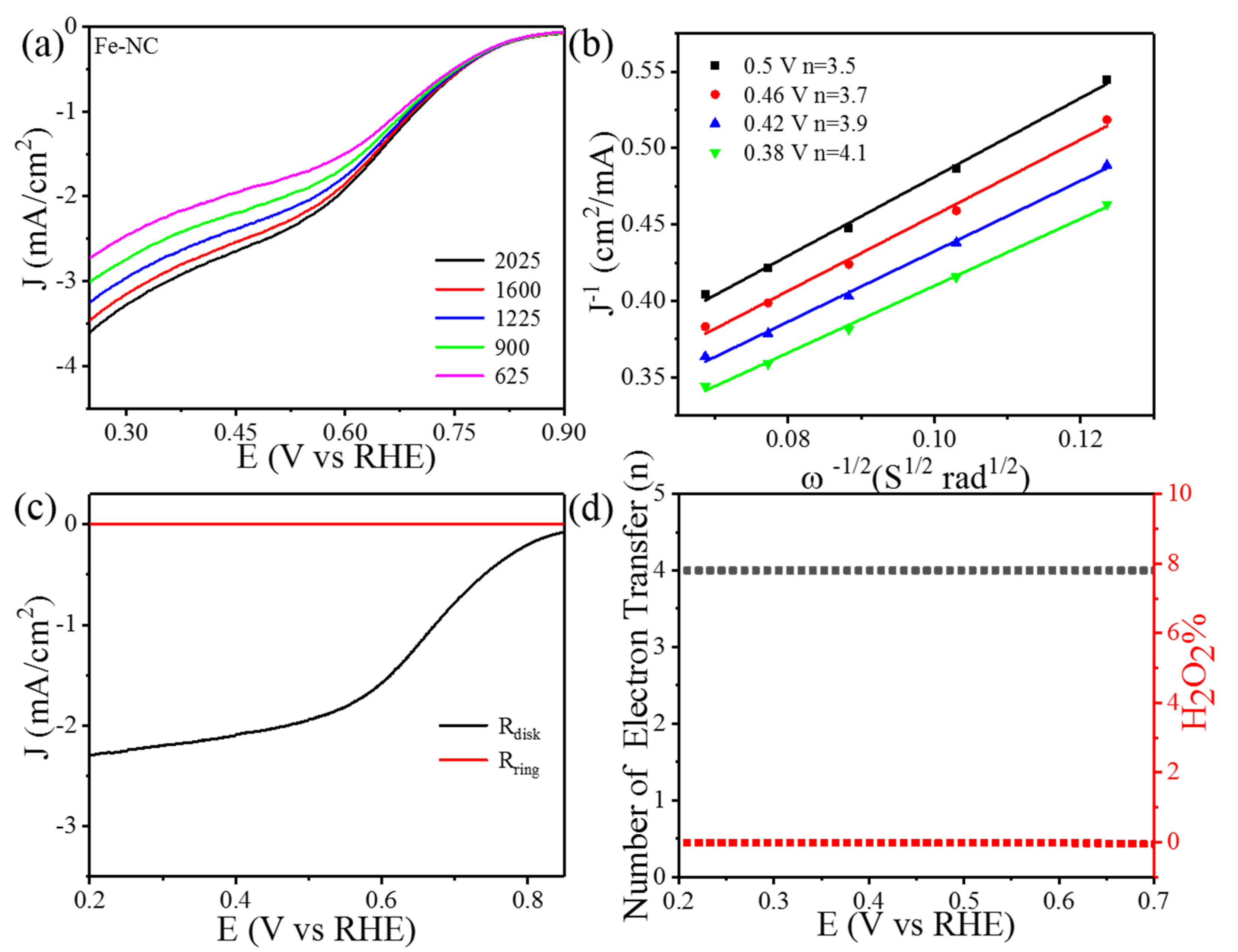
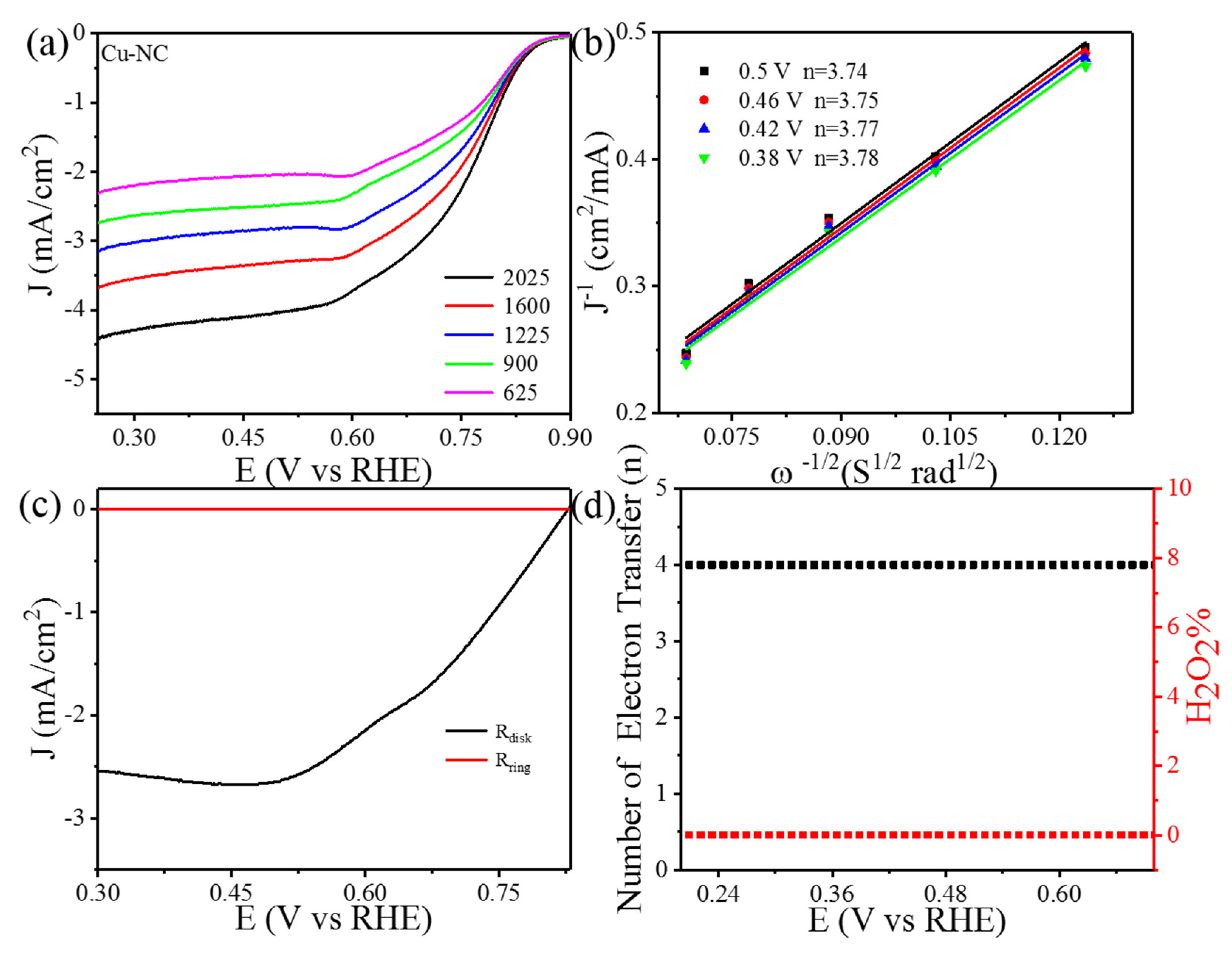
3. Result and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Gülzow, E. Alkaline fuel cells. Fuel Cells 2004, 4, 251–255. [Google Scholar] [CrossRef]
- Fang, H.; Huang, T.; Mao, J.; Yao, S.; Dinesh, M.M.; Sun, Y.; Liang, N.; Qi, L.; Yu, J.; Jiang, Z. Investigation on the catalytic performance of reduced-graphene-oxide-interpolated FeS2 and FeS for oxygen reduction reaction. ChemistrySelect 2018, 3, 10418–10427. [Google Scholar] [CrossRef]
- Pan, J.; Lu, S.; Li, Y.; Huang, A.; Zhuang, L.; Lu, J. High-Performance Alkaline Polymer Electrolyte for Fuel Cell Applications. Adv. Fun. Mater. 2010, 20, 312–319. [Google Scholar] [CrossRef]
- Banham, D.; Feng, F.; Pei, K.; Ye, S.; Birss, V. Effect of carbon support nanostructure on the oxygen reduction activity of Pt/C catalysts. J. Mater. Chem. A 2013, 1, 2812–2820. [Google Scholar] [CrossRef]
- Rivera-Cárcamo, C.; Serp, P. Single atom catalysts on carbon-based materials. ChemCatChem 2018, 10, 5058–5091. [Google Scholar] [CrossRef]
- Gao, M.R.; Jiang, J.; Yu, S.H. Solution-based synthesis and design of late transition metal chalcogenide materials for oxygen reduction reaction (ORR). Small 2012, 8, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-S.; Li, S.-L.; Tang, Y.-J.; Han, M.; Dai, Z.-H.; Bao, J.-C.; Lan, Y.-Q. Nitrogen-doped Fe/Fe3C@graphitic layer/carbon nanotube hybrids derived from MOFs: Efficient bifunctional electrocatalysts for ORR and OER. Chem. Commun. 2015, 51, 2710–2713. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhu, J.; Li, G.; Li, N.; Li, S.; Cano, Z.P.; Ma, L.; Cui, P.; Xu, P.; Jiang, G.; et al. A single-atom iridium heterogeneous catalyst in oxygen reduction reaction. Angew. Chem. Int. Ed. 2019, 58, 9640–9645. [Google Scholar] [CrossRef]
- Lei, C.; Wang, Y.; Hou, Y.; Liu, P.; Yang, J.; Zhang, T.; Zhuang, X.; Chen, M.; Yang, B.; Lei, L.; et al. Efficient alkaline hydrogen evolution on atomically dispersed Ni–Nx Species anchored porous carbon with embedded Ni nanoparticles by accelerating water dissociation kinetics. Energy Environ. Sci. 2019, 12, 149–156. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef]
- Liu, X.; Liu, W.; Ko, M.; Park, M.; Kim, M.G.; Oh, P.; Chae, S.; Park, S.; Casimir, A.; Wu, G.; et al. Metal (Ni, Co)-metal oxides/graphene nanocomposites as multifunctional electrocatalysts. Adv. Fun. Mater. 2015, 25, 5799–5808. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Zhu, Y.; Yang, X.; Li, C. Transition metals (Fe, Co, and Ni) encapsulated in nitrogen-doped carbon nanotubes as bi-functional catalysts for oxygen electrode reactions. J. Mater. Chem. A 2016, 4, 1694–1701. [Google Scholar] [CrossRef]
- Yan, X.Y.; Tong, X.L.; Zhang, Y.F.; Han, X.D.; Wang, Y.Y.; Jin, G.Q.; Qin, Y.; Guo, X.Y. Cuprous oxide nanoparticles dispersed on reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction. Chem. Commun. 2012, 48, 1892–1894. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Liu, W.; Chang, C.; Tang, H.; Li, Z.; Chen, W.; Jia, C.; Yao, T.; Wei, S.; et al. Design of N-coordinated dual-metal sites: A stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 2017, 139, 17281–17284. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, S.; Sun, S. Tuning Nanoparticle Catalysis for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 8526–8544. [Google Scholar] [CrossRef]
- Ma, Z.; Dou, S.; Shen, A.; Tao, L.; Dai, L.; Wang, S. Sulfur-doped graphene derived from cycled lithium-sulfur batteries as a metal-free electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. Engl. 2015, 54, 1888–1892. [Google Scholar] [CrossRef]
- Li, T.; Liu, J.; Song, Y.; Wang, F. Photochemical solid-phase synthesis of platinum single atoms on nitrogen-doped carbon with high loading as bifunctional catalysts for hydrogen evolution and oxygen reduction reactions. ACS Catal. 2018, 8, 8450–8458. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, Z.; Xin, S.; Ma, L.; Xiao, C.; Ding, S.; Li, F.; Gao, G. Ultrafine Co-doped ZnO nanoparticles on reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction. Electrochim. Acta 2017, 224, 561–570. [Google Scholar] [CrossRef]
- Li, Q.K.; Li, X.F.; Zhang, G.; Jiang, J. Cooperative spin transition of monodispersed FeN3 sites within graphene induced by CO adsorption. J. Am. Chem. Soc. 2018, 140, 15149–15152. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Nandan, R.; Pandey, P.; Gautam, A.; Bisen, O.Y.; Chattopadhyay, K.; Titirici, M.-M.; Nanda, K.K. Atomic Arrangement Modulation in CoFe Nanoparticles Encapsulated in N-Doped Carbon Nanostructures for Efficient Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2021, 13, 3771–3781. [Google Scholar] [CrossRef]
- Meganathan, M.D.; Mao, S.; Huang, T.; Sun, G. Reduced graphene oxide intercalated Co2C or Co4N nanoparticles as an efficient and durable fuel cell catalyst for oxygen reduction. J. Mater. Chem. A 2017, 5, 2972–2980. [Google Scholar] [CrossRef]
- Bayati, M.; Scott, K. Synthesis and Activity of A Single Active Site N-doped Electro-catalyst for Oxygen Reduction. Electrochim. Acta 2016, 213, 927–932. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; McCue, A.J.; Miao, C.; Feng, J.; Li, D.; Anderson, J.A. Palladium phosphide nanoparticles as highly selective catalysts for the selective hydrogenation of acetylene. J. Catal. 2018, 364, 406–414. [Google Scholar] [CrossRef]
- Hickman, A.J.; Sanford, M.S. High-valent organometallic copper and palladium in catalysis. Nature 2012, 484, 177–185. [Google Scholar] [CrossRef]
- He, D.; Zhang, L.; He, D.; Zhou, G.; Lin, Y.; Deng, Z.; Hong, X.; Wu, Y.; Chen, C.; Li, Y. Amorphous nickel boride membrane on a platinum–nickel alloy surface for enhanced oxygen reduction reaction. Nat. Commun. 2015, 7, 12362–12369. [Google Scholar]
- Ci, S.; Huang, T.; Wen, Z.; Cui, S.; Mao, S.; Steeber, D.A.; Chen, J. Nickel oxide hollow microsphere for non-enzyme glucose detection. Biosens. Bioelectron. 2014, 54, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Yu, F.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Ammon, C.; Bayer, A.; Held, G.; Richter, B.; Schmidt, T.; Steinruck, H.P. Dissociation and oxidation of methanol on Cu(110). Surf. Sci. 2002, 507, 845–850. [Google Scholar] [CrossRef]
- Amin, R.S.; Hameed, R.M.A.; El-Khatib, K.M.; El-Abd, H.; Souaya, E.R. Effect of preparation conditions on the performance of nano Pt-CuO/C electrocatalysts for methanol electro-oxidation. Int. J. Hydrogen Energy 2012, 37, 18870–18881. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Mu, X.; Du, J.; Wang, H.; Huang, B.; Zhou, J.; Pan, X.; Xie, E. The carbonization temperature effect on the electrochemical performance of nitrogen-doped carbon monoliths. Electrochim. Acta 2017, 242, 100–106. [Google Scholar] [CrossRef]
- Hu, B.-C.; Wu, Z.-Y.; Chu, S.-Q.; Zhu, H.-W.; Liang, H.-W.; Zhang, J.; Yu, S.-H. SiO2-protected shell mediated templating synthesis of Fe–N-doped carbon nanofibers and their enhanced oxygen reduction reaction performance. Energy Environ. Sci. 2018, 11, 2208–2215. [Google Scholar] [CrossRef]
- Jena, H.S.; Krishnaraj, C.; Parwaiz, S.; Lecoeuvre, F.; Schmidt, J.; Pradhan, D.; Van Der Voort, P. Illustrating the role of quaternary-N of BINOL covalent triazine-based frameworks in oxygen reduction and hydrogen evolution reactions. ACS Appl. Mater. Interfaces 2020, 12, 44689–44699. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cheng, D.; Cao, D.; Zeng, X.C. A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 2018, 1, 339–348. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.-M.; Meng, X.-Y.; Wu, X.-R.; Li, S.-N.; Chen, Y. Direct chemical synthesis of ultrathin holey iron doped cobalt oxide nanosheets on nickel foam for oxygen evolution reaction. Nano Energy 2018, 54, 238–250. [Google Scholar] [CrossRef]
- Xue, Q.; Bai, J.; Han, C.; Chen, P.; Jiang, J.-X.; Chen, Y. Au nanowires@Pd-polyethylenimine nanohybrids as highly active and methanol-tolerant electrocatalysts toward oxygen reduction reaction in alkaline media. ACS Catal. 2018, 8, 11287–11295. [Google Scholar] [CrossRef]
- Vorobyeva, E.; Fako, E.; Chen, Z.; Collins, S.M.; Johnstone, D.; Midgley, P.A.; Hauert, R.; Safonova, O.V.; Vilé, G.; López, N.; et al. Atom-by-atom resolution of structure-function relations over low-nuclearity metal catalysts. Angew. Chem. Int. Ed. Engl. 2019, 58, 8724–8729. [Google Scholar] [CrossRef]
- Chen, Y.; Gokhale, R.; Serov, A.; Artyushkova, K.; Atanassov, P. Novel highly active and selective Fe-N-C oxygen reduction electrocatalysts derived from in-situ polymerization pyrolysis. Nano Energy 2017, 38, 201–209. [Google Scholar] [CrossRef]
- Guo, H.-L.; Su, P.; Kang, X.; Ning, S.-K. Synthesis and characterization of nitrogen-doped graphene hydrogels by hydrothermal route with urea as reducing-doping agents. J. Mater. Chem. A 2013, 1, 2248–2255. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, Y.; Ma, D.; Foucher, A.C.; Xiong, L.; Zhang, J.; Stach, E.A.; Yue, Q.; Kang, Y. Atomic Fe Dispersed Hierarchical Mesoporous Fe–N–C Nanostructures for an Efficient Oxygen Reduction Reaction. ACS Catal. 2020, 11, 74–81. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Diao, P.; Chang, W.; Hong, G.; Li, Y.; Gong, M.; Xie, L.; Zhou, J.; Wang, J.; et al. Oxygen reduction electrocatalyst based on strongly coupled cobalt oxide nanocrystals and carbon nanotubes. J. Am. Chem. Soc. 2012, 134, 15849–15857. [Google Scholar] [CrossRef]
- Bezerra, C.W.B.; Zhang, L.; Lee, K.; Liu, H.; Marques, A.L.B.; Marques, E.P.; Wang, H.; Zhang, J. A review of Fe–N/C and Co–N/C catalysts for the oxygen reduction reaction. Electrochim. Acta 2008, 53, 4937–4951. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly coupled inorganic/nanocarbon hybrid materials for advanced electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Chen, X.; Liu, W.; Wang, T.; Qi, D.; Zhi, Q.; Liu, W.; Li, W.; Wang, K.; Jiang, J. Atomic Zn Sites on N and S Codoped Biomass-Derived Graphene for a High-Efficiency Oxygen Reduction Reaction in both Acidic and Alkaline Electrolytes. ACS Appl. Energy Mater. 2021, 4, 2481–2488. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Y.; Dou, M.; Zhang, Z.; Wang, F. Edge-functionalized polyphthalocyanine networks with high oxygen reduction reaction activity. J. Am. Chem. Soc. 2020, 142, 17524–17530. [Google Scholar] [CrossRef]
- Chisaka, M.; Xiang, R.; Maruyama, S.; Daiguji, H. Efficient phosphorus doping into the surface oxide layers on TiN to enhance oxygen reduction reaction activity in acidic media. ACS Appl. Energy Mater. 2020, 3, 9866–9876. [Google Scholar] [CrossRef]
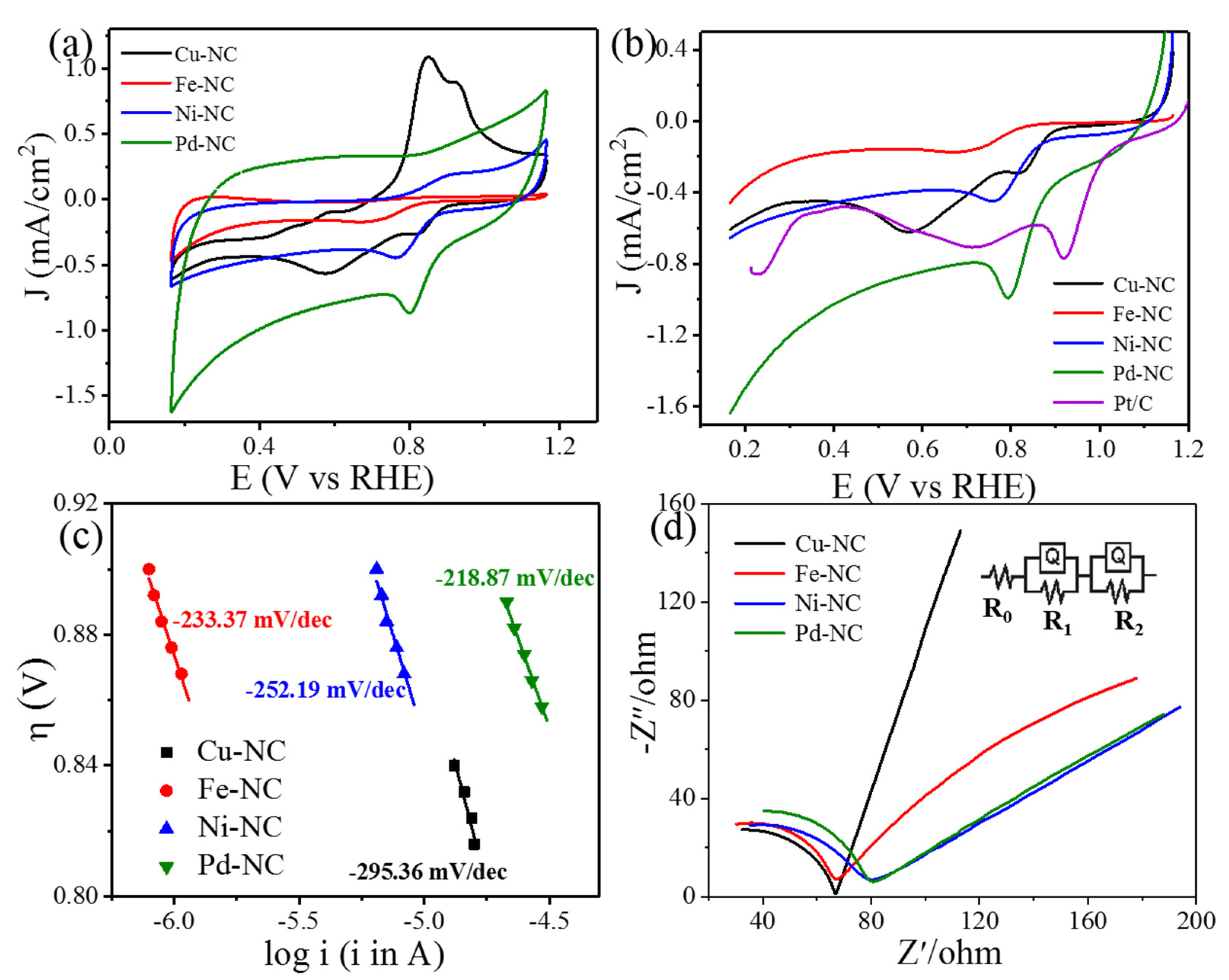
| Material | R0 (Ω) | R1 (Ω) | R2 (Ω) |
|---|---|---|---|
| Cu-NC | 10 | 75 | 1620 |
| Ni-NC | 8 | 72 | 1580 |
| Pd-NC | 7 | 68 | 1380 |
| Fe-NC | 11 | 82 | 1468 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Guo, Z.; Jiang, H.; Xu, S.; Ma, J.; Hu, M.; Yu, J.; Zhao, F.; Huang, T. Dimethylglyoxime Clathrate as Ligand Derived Nitrogen-Doped Carbon-Supported Nano-Metal Particles as Catalysts for Oxygen Reduction Reaction. Nanomaterials 2021, 11, 1329. https://doi.org/10.3390/nano11051329
Xu L, Guo Z, Jiang H, Xu S, Ma J, Hu M, Yu J, Zhao F, Huang T. Dimethylglyoxime Clathrate as Ligand Derived Nitrogen-Doped Carbon-Supported Nano-Metal Particles as Catalysts for Oxygen Reduction Reaction. Nanomaterials. 2021; 11(5):1329. https://doi.org/10.3390/nano11051329
Chicago/Turabian StyleXu, Luping, Zhongqin Guo, Hanyu Jiang, Siyu Xu, Juanli Ma, Mi Hu, Jiemei Yu, Fengqi Zhao, and Taizhong Huang. 2021. "Dimethylglyoxime Clathrate as Ligand Derived Nitrogen-Doped Carbon-Supported Nano-Metal Particles as Catalysts for Oxygen Reduction Reaction" Nanomaterials 11, no. 5: 1329. https://doi.org/10.3390/nano11051329
APA StyleXu, L., Guo, Z., Jiang, H., Xu, S., Ma, J., Hu, M., Yu, J., Zhao, F., & Huang, T. (2021). Dimethylglyoxime Clathrate as Ligand Derived Nitrogen-Doped Carbon-Supported Nano-Metal Particles as Catalysts for Oxygen Reduction Reaction. Nanomaterials, 11(5), 1329. https://doi.org/10.3390/nano11051329