Effects of Functionalization in Different Conditions and Ball Milling on the Dispersion and Thermal and Electrical Conductivity of MWCNTs in Aqueous Solution
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Instruments and Characterization
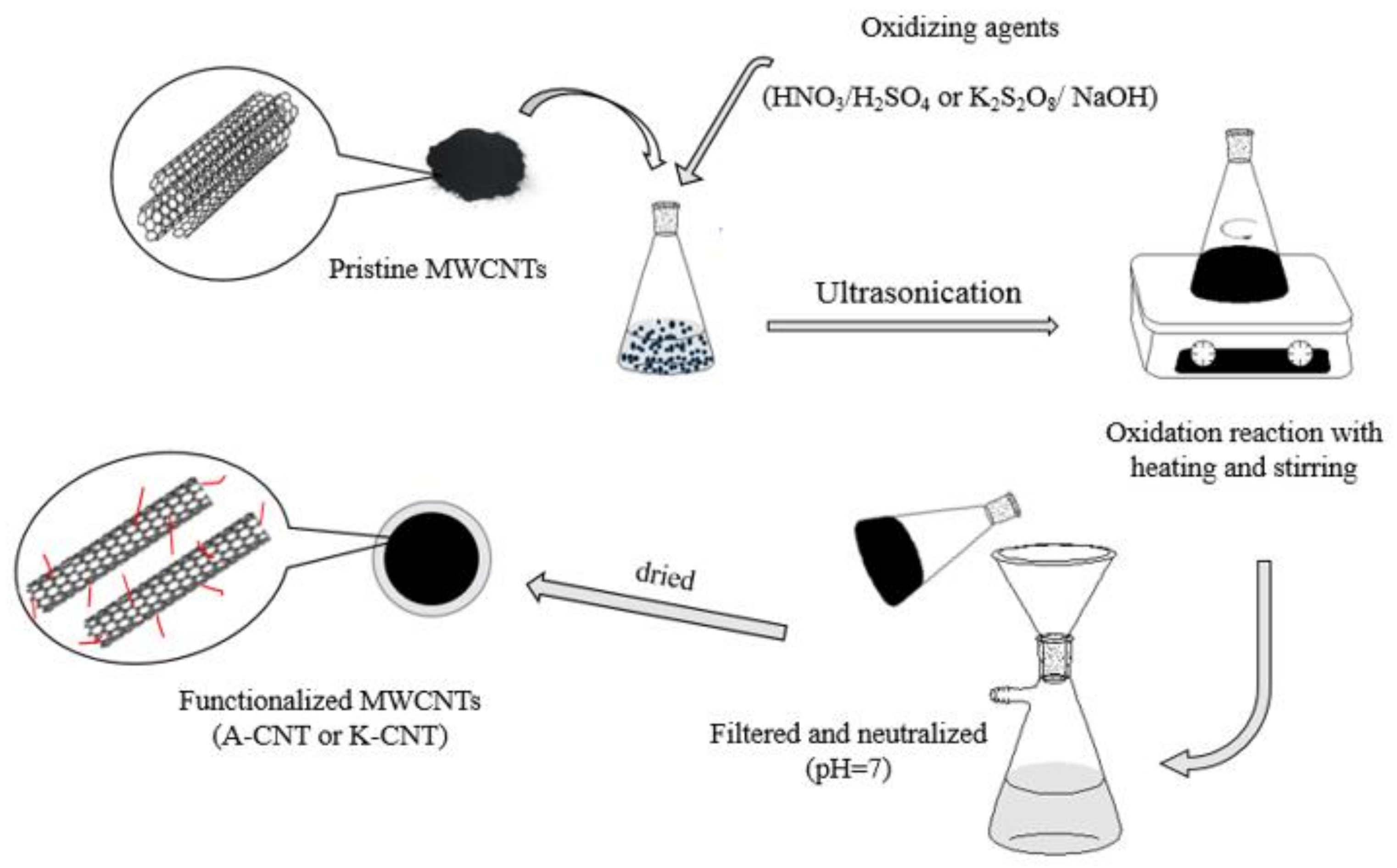
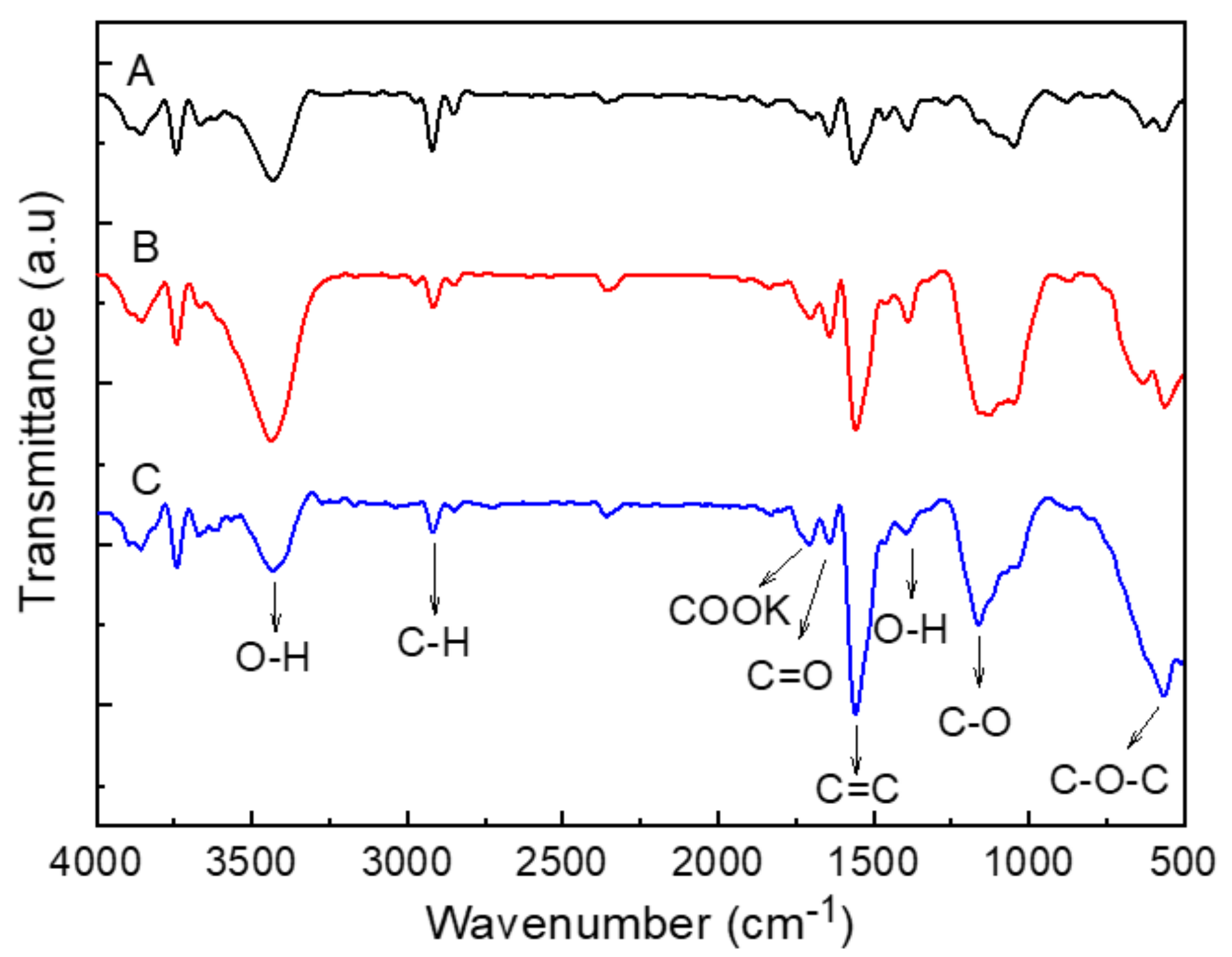
2.3. Functionalization of MWCNTs
2.4. Milling of MWCNTs
2.5. Preparation of MWCNT Nanofluids
3. Result and Discussion
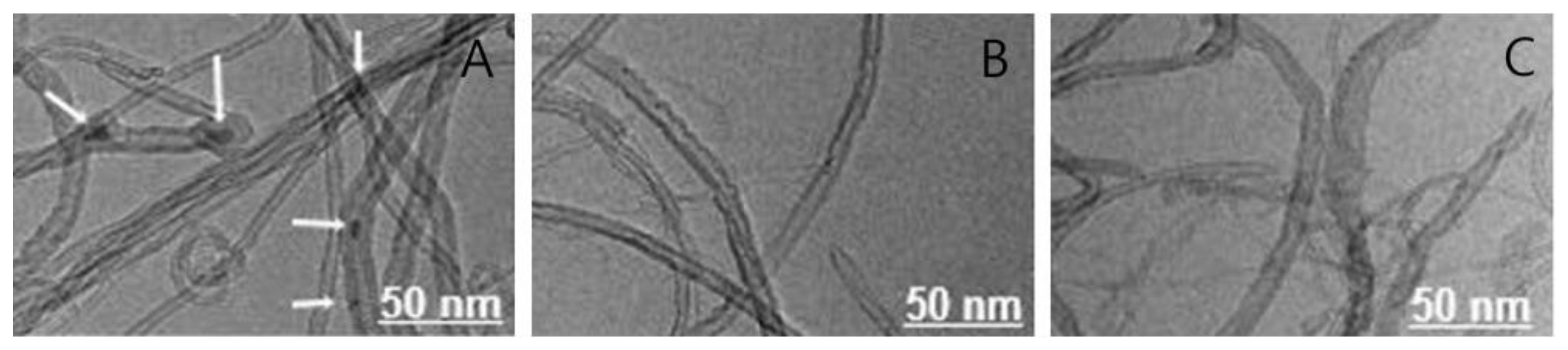
3.1. Morphological Surface Analysis of MWCNTs
3.2. Dispersion Characteristics of MWCNTs
3.3. Thermal Conductivity of MWCNTs
3.4. Electrical Conductivity of MWCNTs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moore, V.C.; Strano, M.S.; Haroz, E.H.; Hauge, R.H.; Smalley, R.E.; Schmidt, J.; Talmon, Y. Individually suspended single-walled carbon nanotubes in various surfactants. Nano Lett. 2003, 3, 1379–1382. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; De Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Vaccarini, L.; Goze, C.; Henrard, L.; Hernandez, E.; Bernier, P.; Rubio, A. Mechanical and electronic properties of carbon and boron–nitride nanotube. Carbon 2000, 38, 1681–1690. [Google Scholar] [CrossRef]
- Casas, C.; Li, W. A review of application of carbon nanotubes for lithium ion battery anode material. J. Power Sources 2012, 208, 74–85. [Google Scholar] [CrossRef]
- Chahine, N.O.; Collette, N.M.; Thomas, C.B.; Genetos, D.C.; Loots, G.G. Nanocomposite scaffold for chondrocyte growth and cartilage tissue engineering: Effects of carbon nanotube surface functionalization. Tissue Eng. Part A 2014, 20, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Apul, O.G.; Wang, Q.; Zhou, Y.; Karanfil, T. Adsorption of aromatic organic contaminants by graphene nanosheets: Comparison with carbon nanotubes and activated carbon. Water Res. 2013, 47, 1648–1654. [Google Scholar] [CrossRef]
- Sahooa, N.G.; Ranab, S.; Cho, J.W.; Li, L.; Chana, S. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Postma, H.W.C.; Teepen, T.; Yao, Z.; Grifoni, M.; Dekker, C. Carbon nanotube single-electron transistors at room temperature. Science 2001, 293, 76–79. [Google Scholar] [CrossRef]
- Cherukuri, P.; Bachilo, S.M.; Litovsky, S.H.; Weisman, R.B. Near-infrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cells. J. Am. Chem. Soc. 2004, 126, 15638–15639. [Google Scholar] [CrossRef]
- Guldi, D.M.; Rahman, G.M.A.; Prato, M.; Jux, N.; Qin, S.; Ford, W. Single-wall carbon nanotubes as integrative building blocks for solar-energy conversion. Angew. Chem. 2005, 117, 2051–2054. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nat. Cell Biol. 1997, 386, 377–379. [Google Scholar] [CrossRef]
- Jiang, W.; Ding, G.; Peng, H. Measurement and model on thermal conductivities of carbon nanotube nanorefrigerants. Int. J. Therm. Sci. 2009, 48, 1108–1115. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Ahn, Y.C.; Shin, H.S.; Lee, C.G.; Kim, G.T.; Park, H.S.; Lee, J.K. Investigation on characteristics of thermal conductivity enhancement of nanofluids. Cur. Appl. Phys. 2006, 6, 1068–1071. [Google Scholar] [CrossRef]
- Munkhbayar, B.; Hwang, S.; Kim, J.; Bae, K.; Ji, M.; Chung, H.; Jeong, H. Photovaltiac performance of dye-sensitized solar cells with various MWCNT counter electrode structures produced by different coating methods. Electochimica Acta 2012, 80, 100–107. [Google Scholar] [CrossRef]
- Garg, P.; Alvarado, J.L.; Marsh, C.; Carlson, T.A.; Kessler, D.A.; Annamalai, K. An experimental study on the effect of ultrasonication viscosity and heat transfer performance of multi-wall carbon nanotube-based aqueous nanofluids. Int. J. Heat Mass Transf. 2009, 52, 5090–5101. [Google Scholar] [CrossRef]
- Krause, B.; Villmow, T.; Boldt, R.; Mende, M.; Petzold, G.; Pötschke, P. Influence of dry grinding in a ball mill on the length of multiwalled carbon nanotubes and their dispersion and percolation behaviour in melt mixed polycarbonate composites. Compos. Sci. Technol. 2011, 71, 1145–1153. [Google Scholar] [CrossRef]
- Wen, D.S.; Ding, Y.L. Effective thermal conductivity of aqueous suspension of carbon nanotubes. J. Thermophys. Heat Transf. 2004, 18, 481–485. [Google Scholar] [CrossRef]
- Huxtable, S.T.; Cahill, D.G.; Shenogin, S.; Xue, L.; Ozisik, R.; Barone, P.; Usrey, M.; Strano, M.S.; Siddons, G.; Shim, M.; et al. Interfacial heat flow in carbon nanotube suspensions. Nat. Mater. 2003, 2, 731–734. [Google Scholar] [CrossRef]
- Katkova, M.A.; Zabrodina, G.S.; Kremlev, K.V.; Gusev, S.A.; Obiedkov, A.M. Effect of Ce(III)-Cu(II) 15-metallacrown-5 compounds on the dispersion of multi-walled carbon nanotubes in aqueous solutions: Toward surfactant-free applications. Thin Solid Film. 2017, 628, 112–116. [Google Scholar] [CrossRef]
- Kim, S.; Tserengombo, B.; Choi, S.H.; Noh, J.; Huh, S.; Choi, B.; Chung, H.; Kim, J.; Jeong, H. Experimental investigation of dispersion characteristics and thermal conductivity of various surfactants on carbon based material. Int. Comm. Heat Mass Trans. 2018, 91, 95–102. [Google Scholar] [CrossRef]
- Riggs, J.E.; Guo, Z.; Carrol, D.L.; Sun, Y.P. Dispersion of carbon nanotubes and their polymer composites. J. Am. Chem. Soc. 2000, 104, 7071–7076. [Google Scholar]
- Wei, J.; Lv, R.; Guo, N.; Wang, H.; Bai, X.; Mathkar, A.; Kang, F.; Zhu, H.; Wang, K.; Wu, D.; et al. Preparation of highly oxidized nitrogen-doped carbon nanotubes. Nanotechnology 2012, 23, 155601–155606. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Sigmund, W.M. Functionalized multiwall carbon nanotube/gold nanoparticle composites. Langmuir 2004, 20, 8239–8242. [Google Scholar] [CrossRef]
- Cuentas-Gallegos, A.K.; Martínez-Rosales, R.; Rincón, M.E.; Hirata, G.A.; Orozco, G. Design of hybrid materials based on carbon nanotubes and polyoxometalates. Opt. Mater. 2006, 29, 126–133. [Google Scholar] [CrossRef]
- Datye, A.; Wu, K.-H.; Gomes, G.; Monroy, V.; Lin, H.-T.; Vleugels, J.; Vanmeensel, K. Synthesis, microstructure and mechanical properties of Yttria Stabilized Zirconia (3YTZP)—Multi-Walled Nanotube (MWNTs) nanocomposite by direct in-situ growth of MWNTs on Zirconia particles. Compos. Sci. Technol. 2010, 70, 2086–2092. [Google Scholar] [CrossRef]
- Eder, D. Carbon nanotube-inorganic hybrids. Chem. Rev. 2010, 110, 1348–1352. [Google Scholar] [CrossRef]
- Flahaut, E.; Peigney, A.; Laurent, C.; MarlieRe, C.; Chastel, F.; Rousset, A. Carbon nanotube–metal–oxide nanocomposites: Microstructure, electrical conductivity and mechanical properties. Act. Mater. 2000, 48, 3803–3812. [Google Scholar] [CrossRef]
- Goering, J.; Kadossov, E.; Burghaus, U. Adsorption Kinetics of Alcohols on Single-Wall Carbon Nanotubes: An ultrahigh vacuum surface chemistry study. J. Phys. Chem. C 2008, 112, 10114–10124. [Google Scholar] [CrossRef]
- Gong, J.-L.; Wang, B.; Zeng, G.-M.; Yang, C.-P.; Niu, C.-G.; Niu, Q.-Y.; Zhou, W.-J.; Liang, Y. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J. Hazard. Mater. 2009, 164, 1517–1522. [Google Scholar] [CrossRef]
- Avilés, F.; Cauich-Rodríguez, J.; Moo-Tah, L.; May-Pat, A.; Vargas-Coronado, R. Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon 2009, 47, 2970–2975. [Google Scholar] [CrossRef]
- Rosca, I.D.; Watari, F.; Uo, M.; Akasaka, T. Oxidation of multiwalled carbon nanotubes by nitric acid. Carbon 2005, 43, 3124–3131. [Google Scholar] [CrossRef]
- Nasiri, A.; Shariaty-Niasar, M.; Rashidi, A.; Khodafarin, R. Effect of CNT structures on thermal conductivity and stability of nanofluid. Int. J. Heat Mass Transf. 2012, 55, 1529–1535. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Shariaty-Niasar, M.; Rashidi, A.M. Preparation of nanofluids from functionalized Graphene by new alkaline method and study on the thermal conductivity and stability. Int. Commun. Heat Mass Transf. 2013, 42, 89–94. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Tokmakci, T.; Ozturk, A.; Park, J. Boron and zirconium co-doped TiO2 powders prepared through mechanical ball milling. Ceram. Int. 2013, 39, 5893–5899. [Google Scholar] [CrossRef]
- Tiwary, C.; Verma, A.; Biswas, K.; Mondal, A.K.; Chattopadhyay, K. Preparation of ultrafine CsCl crystallites by combined cryogenic and room temperature ball milling. Ceram. Int. 2011, 37, 3677–3686. [Google Scholar] [CrossRef]
- Shin, H.; Lee, S.; Jung, H.S.; Kim, J.-B. Effect of ball size and powder loading on the milling efficiency of a laboratory-scale wet ball mill. Ceram. Int. 2013, 39, 8963–8968. [Google Scholar] [CrossRef]
- Mio, H.; Kano, J.; Saito, F.; Kaneko, K. Optimum revolution and rotational directions and their speeds in planetary ball milling. Int. J. Miner. Process. 2004, 74, S85–S92. [Google Scholar] [CrossRef]
- Liu, S.-S.; Sun, L.-X.; Zhang, Y.; Xu, F.; Zhang, J.; Chu, H.-L.; Fan, M.-Q.; Zhang, T.; Song, X.-Y.; Grolier, J.P. Effect of ball milling time on the hydrogen storage properties of TiF3-doped LiAlH4. Int. J. Hydrog. Energy 2009, 34, 8079–8085. [Google Scholar] [CrossRef]
- Munkhbayar, B.; Nine, M.J.; Jeoung, J.; Munkhjargal, B.; Chung, H.; Jeong, H. Influence of dry and wet ball milling on dispersion characteristics of the multi-walled carbon nanotubes in aqueous solution with and without surfactant. Powder Technol. 2013, 234, 132–140. [Google Scholar] [CrossRef]
- Yudianti, R.; Onggo, H.; Sudirman; Saito, Y.; Iwata, T.; Azuma, J. Analysis of functional group sited on multi-wall carbon nanotube surface. Open Mater. Sci. J. 2011, 5, 242–247. [Google Scholar] [CrossRef]
- Shim, J.-W.; Park, S.-J.; Ryu, S.-K. Effect of modification with HNO3 and NaOH on metal adsorption by pitch-based activated carbon fibers. Carbon 2001, 39, 1635–1642. [Google Scholar] [CrossRef]
- Yadav, S.K.; Mahapatra, S.S.; Yoo, H.J.; Cho, J.W. Synthesis of multi-walled carbon nanotube/polyhedral oligomeric silsesquioxane nanohybrid by utilizing click chemistry. Nanoscale Res. Lett. 2011, 6, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Saleh, T.A. Synthesis of carbon nanotube-metal oxides composites; adsorption and photo-degradation. Carbon Nanotub. Res. Appl. 2011, 295–312. [Google Scholar]
- Castaldo, R.; Lama, G.C.; Aprea, P.; Gentile, G.; Lavorgna, M.; Ambrogi, V.; Cerruti, P. Effect of the oxidation degree on self-assembly, adsorption and barrier properties of nano-graphene. Microporous Mesoporous Mater. 2018, 260, 102–115. [Google Scholar] [CrossRef]
- Yan, H.; Wu, H.; Li, K.; Wang, Y.; Tao, X.; Yang, H.; Li, A.; Cheng, R. Influence of the surface structure of graphene oxide on the adsorption of aromatic organic compounds from water. ACS Appl. Mater. Interfaces 2015, 7, 6690–6697. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Aksay, I.A.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, T.; Choi, J.; Park, J.; Kim, Y.S.; Chang, M.S.; Jung, H.; Park, K.T.; Yang, S.J.; Park, C.R. Hidden second oxidation step of hummers method. Chem. Mater. 2016, 28, 756–764. [Google Scholar] [CrossRef]
- Qiu, Y.; Collin, F.; Hurt, R.H.; Külaots, I. Thermochemistry and kinetics of graphite oxide exothermic decomposition for safety in large-scale storage and processing. Carbon 2016, 96, 20–28. [Google Scholar] [CrossRef]
- Bai, H.; Jiang, W.; Kotchey, G.P.; Saidi, W.A.; Bythell, B.J.; Jarvis, J.M.; Marshall, A.G.; Robinson, R.A.S.; Star, A. Insight into the mechanism of graphene oxide degradation via the photo-fenton reaction. J. Phys. Chem. C 2014, 118, 10519–10529. [Google Scholar] [CrossRef]
- Pham, V.H.; Pham, H.D.; Dang, T.T.; Hur, S.H.; Kim, E.J.; Kong, B.S.; Kim, S.; Chung, J.S. Chemical reduction of an aqueous suspension of graphene oxide by nascent hydrogen. J. Mater. Chem. 2012, 22, 10530–10536. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, T.; Kim, Y.S.; Choi, H.S.; Lim, H.J.; Yang, S.J.; Park, C.R. Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon 2012, 50, 3–33. [Google Scholar] [CrossRef]
- O’Brien, R.W.; Midmore, B.R.; Lamb, A.; Hunter, R.J. Electroacoustic studies of moderately concentrated colloidal suspensions. Faraday Discuss. Chem. Soc. 1990, 19, 301–312. [Google Scholar] [CrossRef]
- Lee, J.H.; Rhee, K.Y.; Park, S.J. Effects of cryomilling on the structures and hydrogen storage characteristics of multi-walled carbon nanotubes. Int. J. Hydrogen Energy 2010, 35, 7850–7857. [Google Scholar] [CrossRef]
- Greenwood, R.; Kendall, K. Electroacoustic studies of moderately concentrated colloidal suspensions. J. Eur. Ceram. Soc. 1999, 19, 479–488. [Google Scholar] [CrossRef]
- Mandzy, N.; Grulke, E.; Druffel, T. Breakage of TiO2 agglomerates in electrostatically stabilized aqueous dispersions. Powder Technol. 2005, 160, 121–126. [Google Scholar] [CrossRef]
- Zhang, L.; Ni, Q.-Q.; Fu, Y.; Natsuki, T. One-step preparation of water-soluble single-walled carbon nanotubes. Appl. Surf. Sci. 2009, 255, 7095–7099. [Google Scholar] [CrossRef]
- Incropera, F.P.; DeWitt, D.P. Fundamentals of Heat and Mass Transfer, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Jang, S.P.; Choi, S.U.S. Role of Brownian motion in the enhanced thermal conductivity of nanofliuds. Appl. Phys. Lett. 2004, 84, 4316–4318. [Google Scholar] [CrossRef]
- Gao, J.; Itkis, M.E.; Yu, A.; Bekyarova, E.; Zhao, B.; Haddon, R.C. Continuous spinning of a single-walled carbon nanotube−nylon composite fiber. J. Am. Chem. Soc. 2005, 127, 3847–3854. [Google Scholar] [CrossRef]
- Xie, H.; Chen, L. Adjustable thermal conductivity in carbon nanotube nanofluids. Phys. Lett. A 2009, 373, 1861–1864. [Google Scholar] [CrossRef]
- Bordi, F.; Cametti, C.; Codastefano, P.; Tartaglia, P. Electrical conductivity of colloidal systems during irreversible aggregation. Phys. A Stat. Mech. Appl. 1990, 164, 663–672. [Google Scholar] [CrossRef]
- Sarojini, K.K.; Manoj, S.V.; Singh, P.K.; Pradeep, T.; Das, S.K. Electrical conductivity of ceramic and metallic nanofluids. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 417, 39–46. [Google Scholar] [CrossRef]
- Srinivasan, B.; Le Tonquesse, S.; Gellé, A.; Bourgès, C.; Monier, L.; Ohkubo, I.; Halet, J.-F.; Berthebaud, D.; Mori, T. Screening of transition (Y, Zr, Hf, V, Nb, Mo, and Ru) and rare-earth (La and Pr) elements as potential effective dopants for thermoelectric GeTe–an experimental and theoretical appraisal. J. Mater. Chem. A 2020, 8, 19805–19821. [Google Scholar] [CrossRef]
- Bourgès, C.; Srinivasan, B.; Fontaine, B.; Sauerschnig, P.; Minard, A.; Halet, J.F.; Halet, J.-F.; Miyazaki, Y.; Berthebaud, D.; Mori, T. Tailoring the thermoelectric and structural properties of Cu–Sn based thiospinel compounds [CuM 1+ x Sn 1− x S 4 (M= Ti, V, Cr, Co)]. J. Mater. Chem. C 2020, 8, 16368–16383. [Google Scholar] [CrossRef]
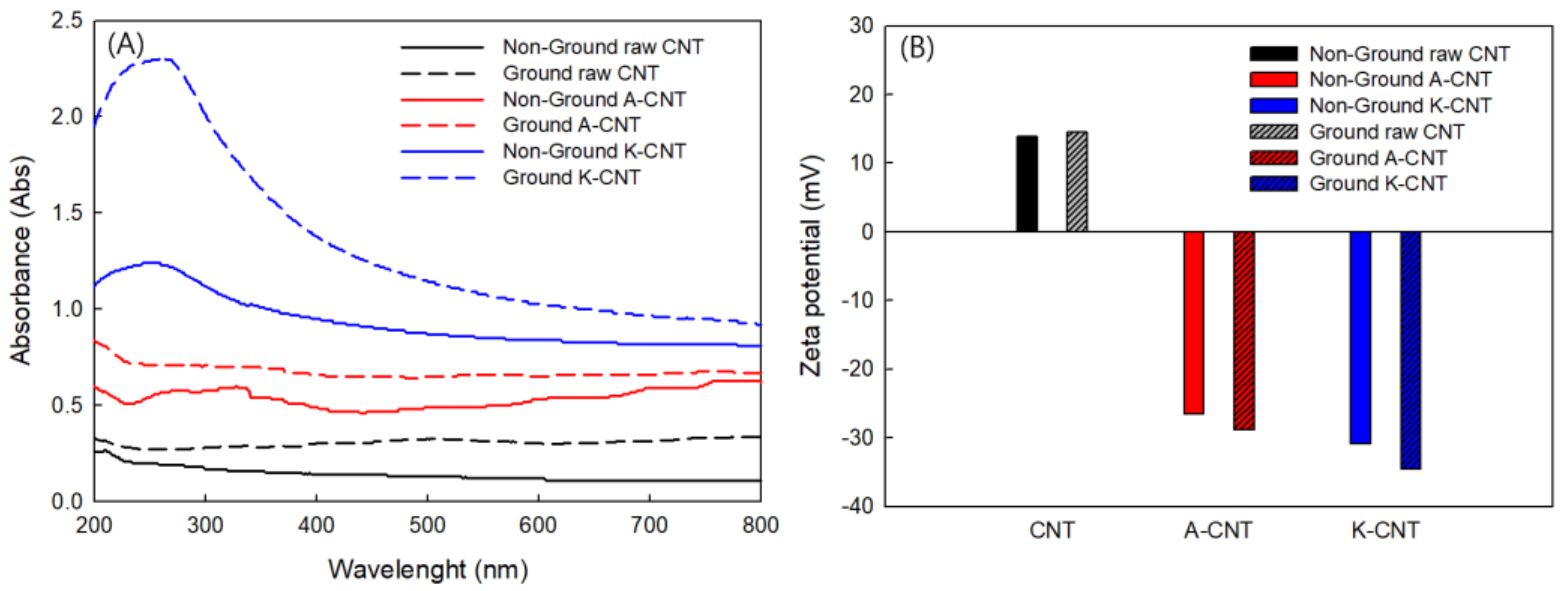
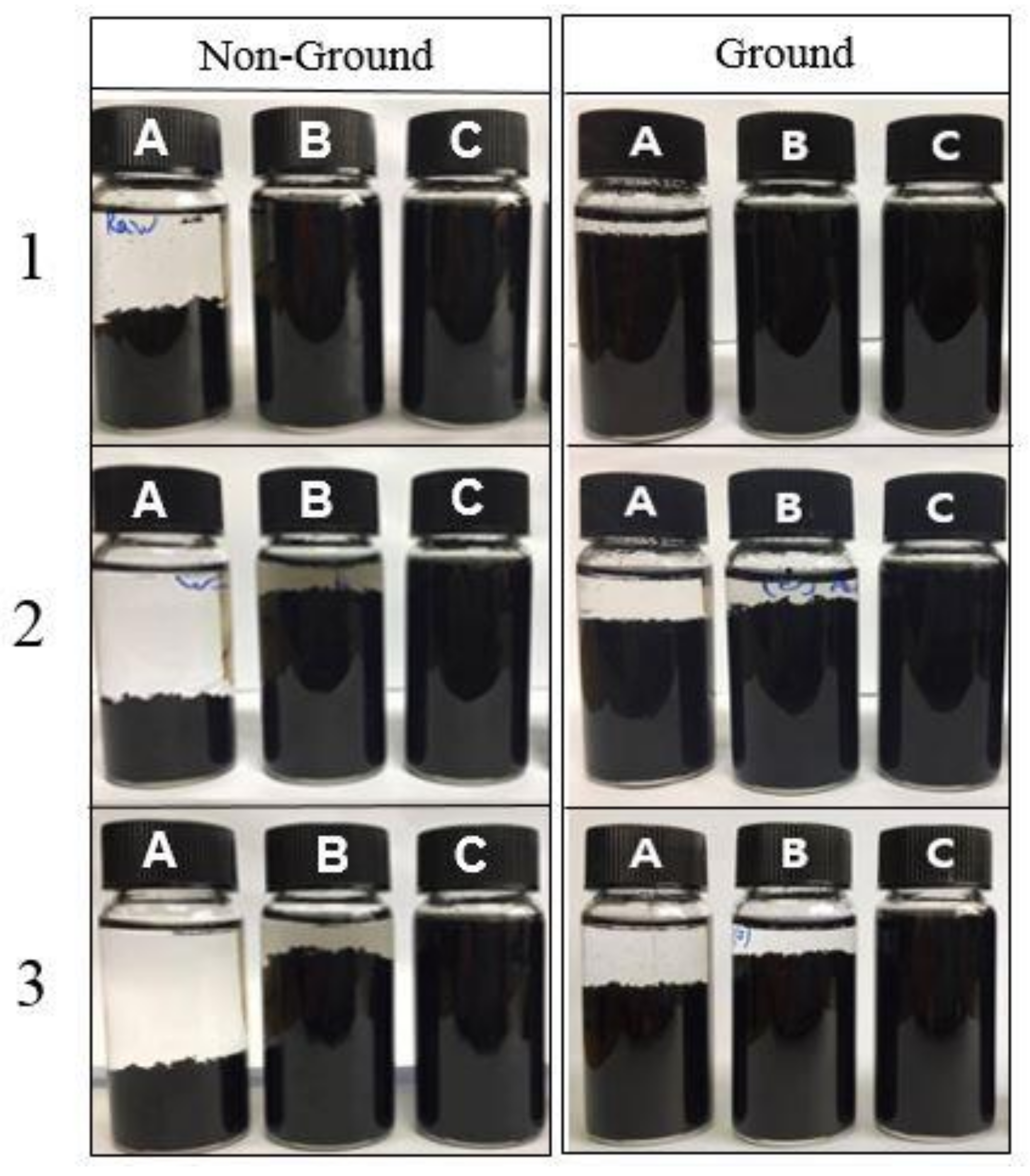
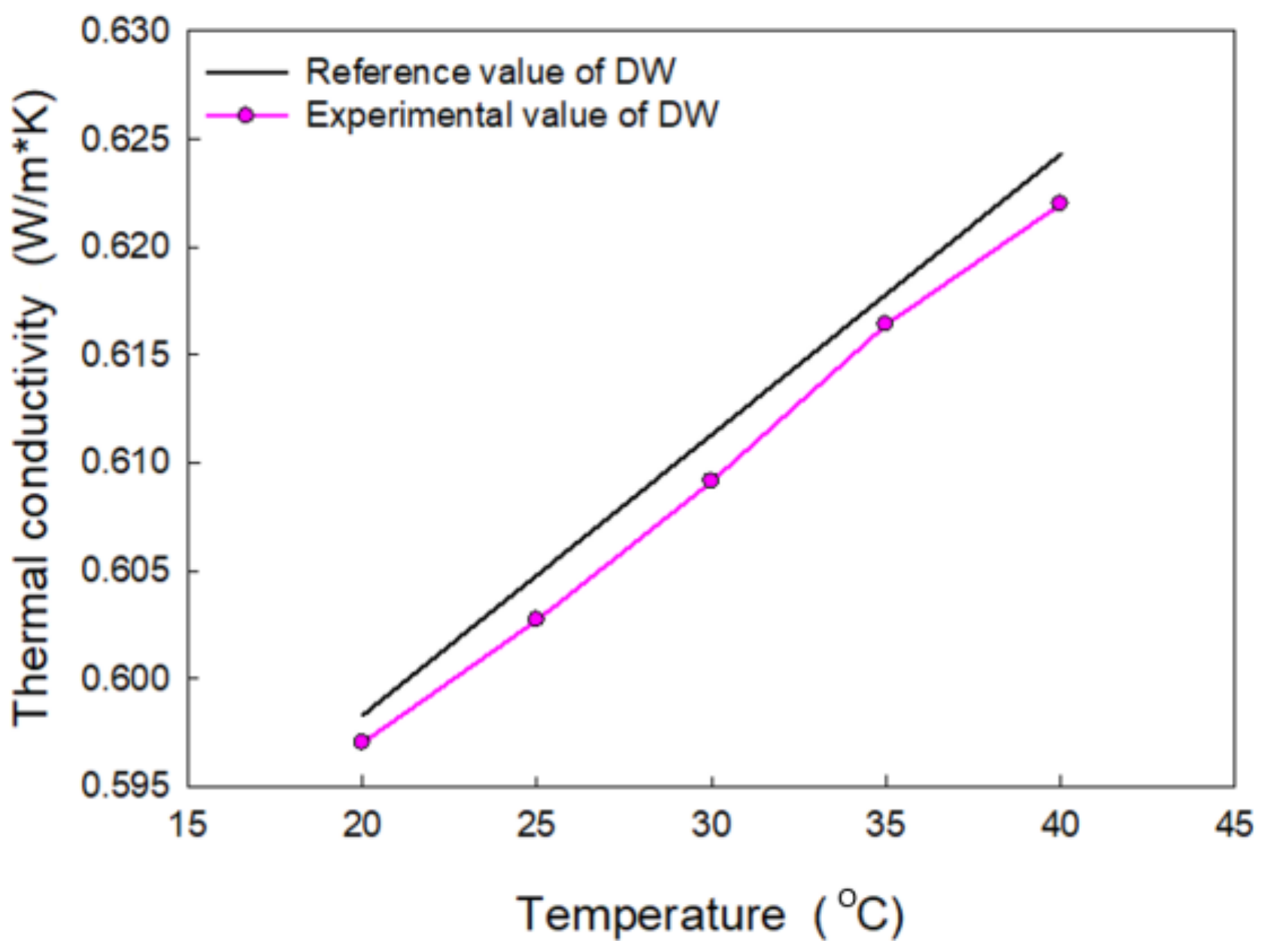
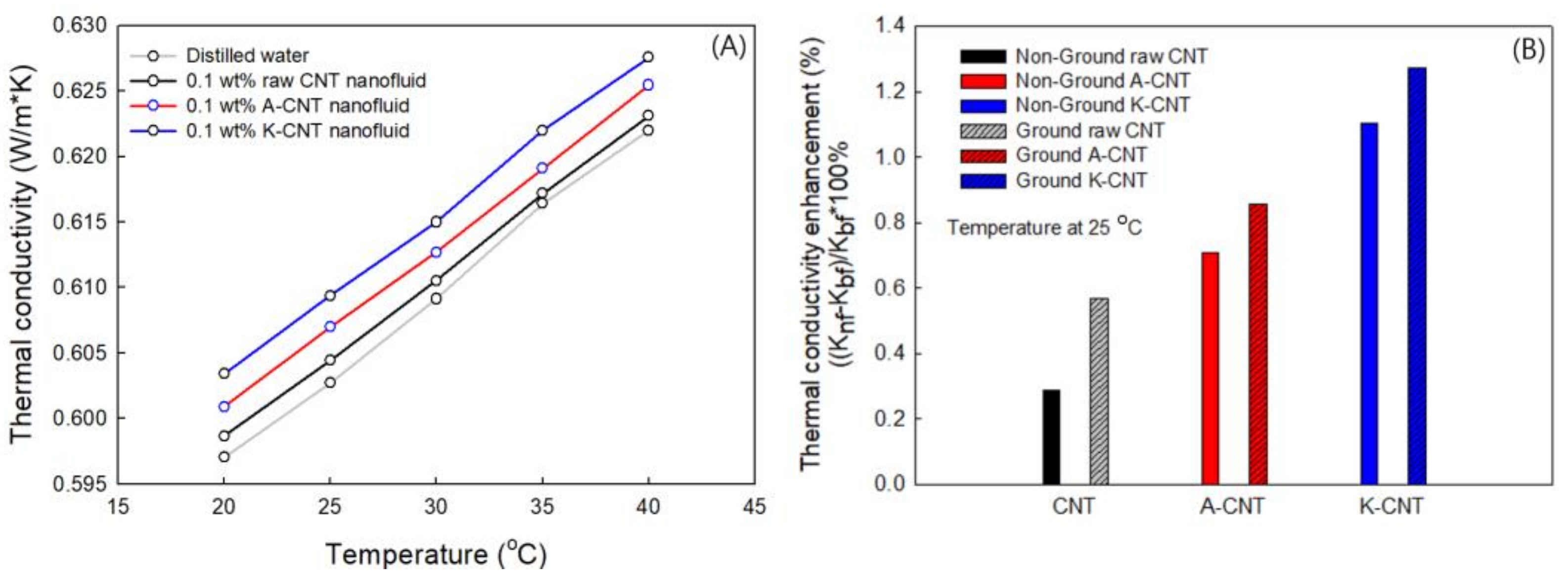
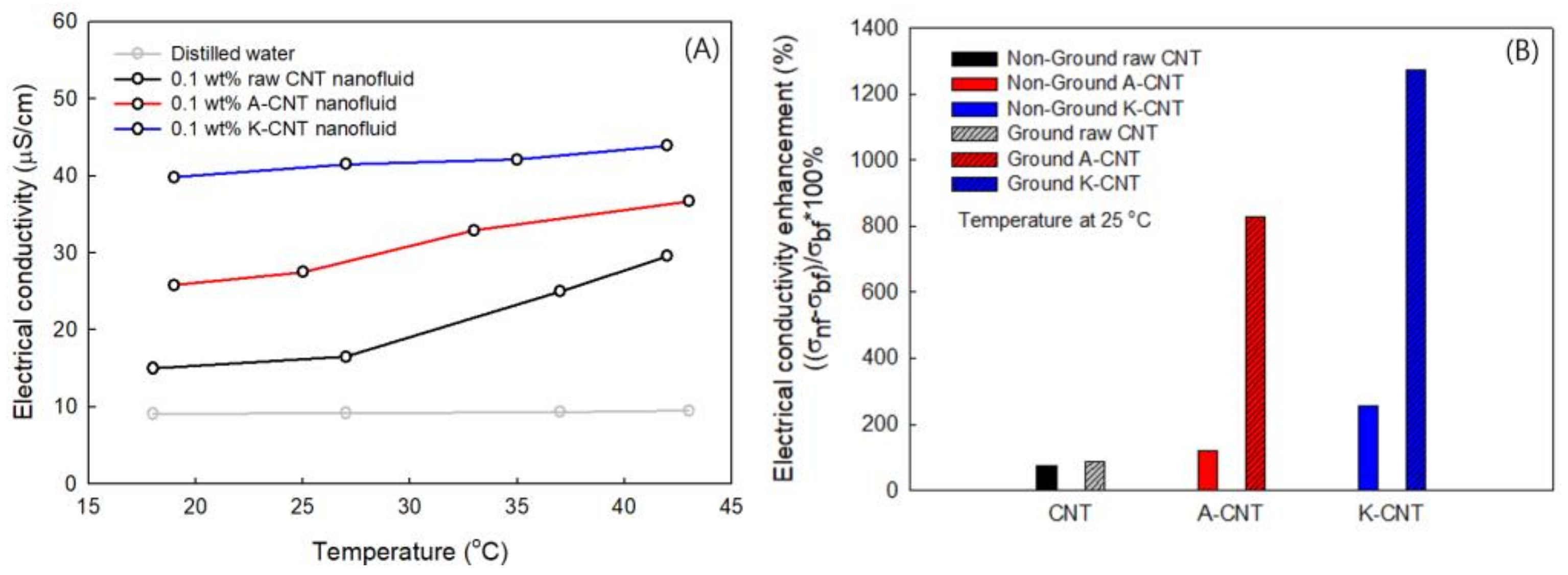
| No. | Sample | Type of Agent | Conc. (wt%) | Zeta Pot. (mV) | Thermal Cond. (W/m·K) at 25 °C | Electrical Сond. (μS/cm) at 25 °C |
|---|---|---|---|---|---|---|
| 1 | Non-ground Pristine CNT | - | 0.1 | 13.87 | 0.6035 | 16.19 |
| 2 | Ground Pristine CNT | - | 0.1 | 14.54 | 0.6061 | 17.32 |
| 3 | Non-ground A-CNT | H2SO4/HNO3 | 0.1 | −26.6 | 0.6069 | 20.2 |
| 4 | Ground A-CNT | H2SO4/HNO3 | 0.1 | −28.83 | 0.6078 | 85.1 |
| 5 | Non-ground K-CNT | K2S2O8/NaOH | 0.1 | −30.9 | 0.6094 | 32.7 |
| 6 | Ground K-CNT | K2S2O8/NaOH | 0.1 | −34.57 | 0.6104 | 125.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tserengombo, B.; Jeong, H.; Dolgor, E.; Delgado, A.; Kim, S. Effects of Functionalization in Different Conditions and Ball Milling on the Dispersion and Thermal and Electrical Conductivity of MWCNTs in Aqueous Solution. Nanomaterials 2021, 11, 1323. https://doi.org/10.3390/nano11051323
Tserengombo B, Jeong H, Dolgor E, Delgado A, Kim S. Effects of Functionalization in Different Conditions and Ball Milling on the Dispersion and Thermal and Electrical Conductivity of MWCNTs in Aqueous Solution. Nanomaterials. 2021; 11(5):1323. https://doi.org/10.3390/nano11051323
Chicago/Turabian StyleTserengombo, Baasandulam, Hyomin Jeong, Erdenechimeg Dolgor, Antonio Delgado, and Sedong Kim. 2021. "Effects of Functionalization in Different Conditions and Ball Milling on the Dispersion and Thermal and Electrical Conductivity of MWCNTs in Aqueous Solution" Nanomaterials 11, no. 5: 1323. https://doi.org/10.3390/nano11051323
APA StyleTserengombo, B., Jeong, H., Dolgor, E., Delgado, A., & Kim, S. (2021). Effects of Functionalization in Different Conditions and Ball Milling on the Dispersion and Thermal and Electrical Conductivity of MWCNTs in Aqueous Solution. Nanomaterials, 11(5), 1323. https://doi.org/10.3390/nano11051323






