Effect of UV-Irradiation and ZnO Nanoparticles on Nonlinear Optical Response of Specific Photochromic Polymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodology
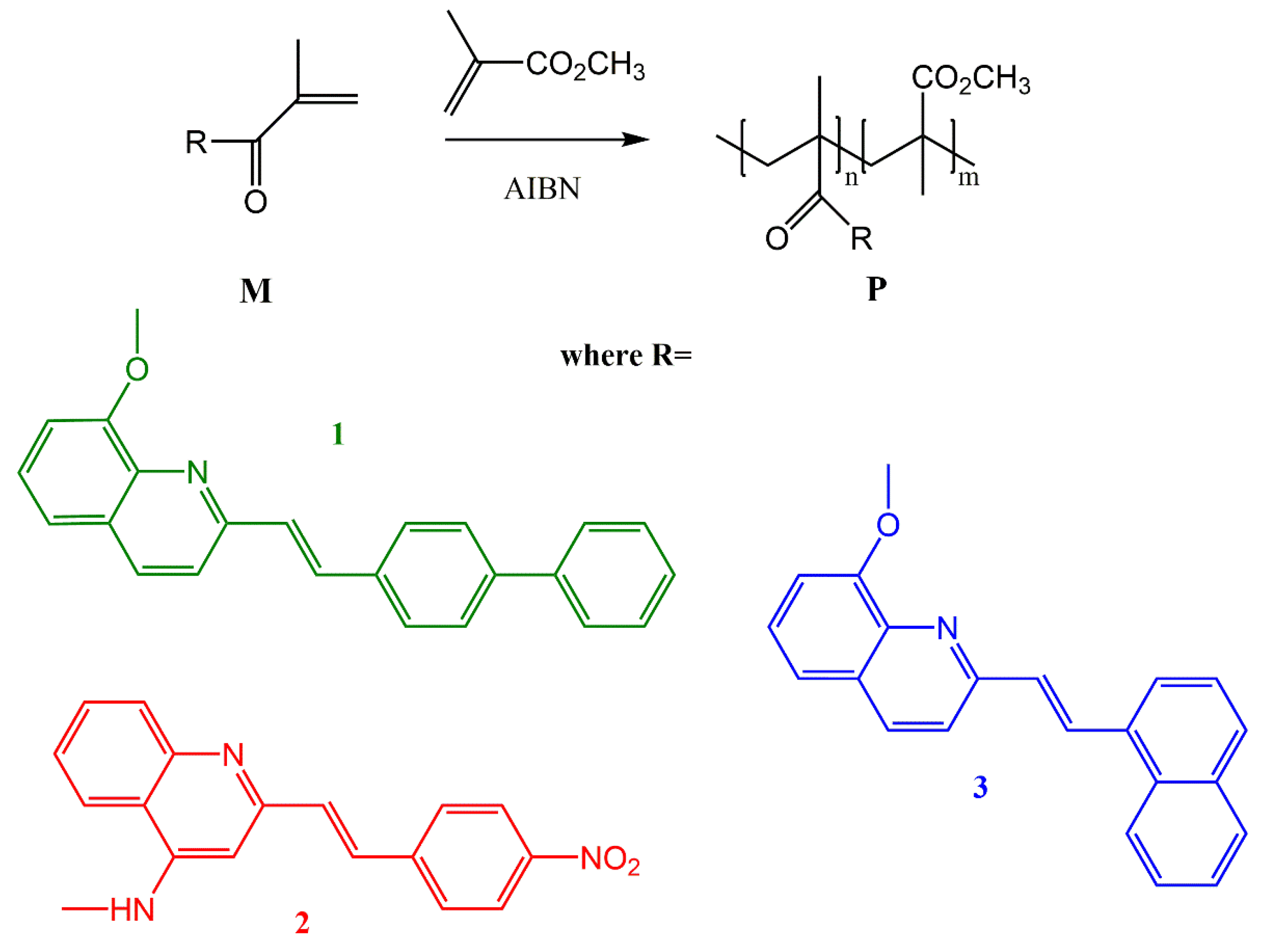
2.2. Synthesis and Thin Films Preparation
2.2.1. 2-(2-Biphenyl-4-ethenyl)quinolin-8-yl propionate (1p)
2.2.2. 2-(2-Biphenyl-4-ethenyl)quinolin-8-ol (1 h)
2.2.3. 2-(2-Naphtyl)ethenyl)quinolin-8-yl-propionate (2p)
2.2.4. 2-(2-Naphtyl)ethenyl)quinolin-8-ol (2h)
2.2.5. 2-[2-(4-Nitrophenyl)ethenyl]quinolin-4-yl-propanamide (3p)
2.2.6. 2-[2-(4-Nitrophenyl)ethenyl]quinolin-4-amine (3h)
2.2.7. 2-(2-Biphenyl-4-ethenyl)quinolin-8-yl-2-methylpropil-2-enoate (M1)
2.2.8. 2-[2-(4-Nitrophenyl)ethenyl]quinolin-4-yl-2-methyl-2-propenamide (M2)
2.2.9. 2-(2-Naphtyl)ethenyl)quinolin-8-yl-2-methylpropil-2-enoate (M3)
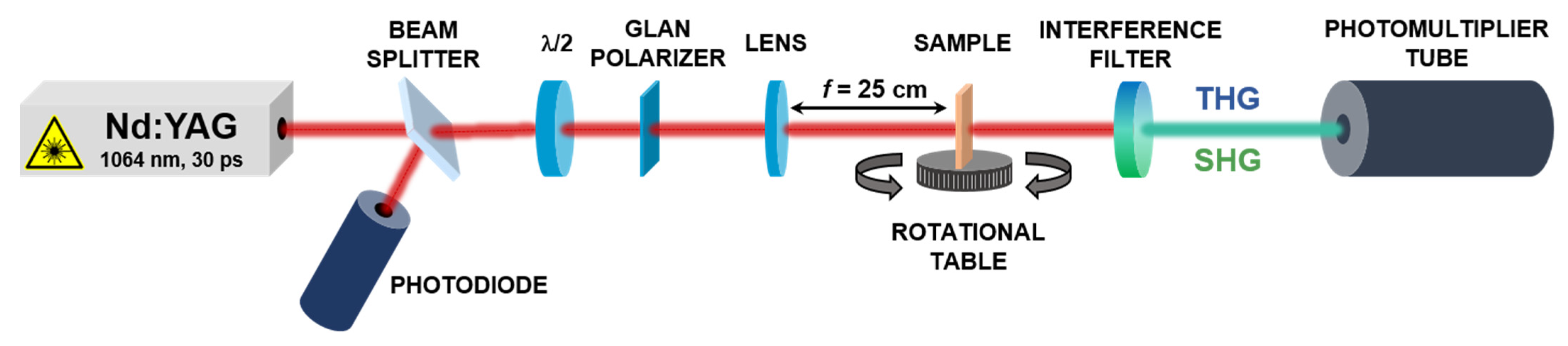
2.3. Second and Third Harmonic Generation Experiment
3. Results and Discussion
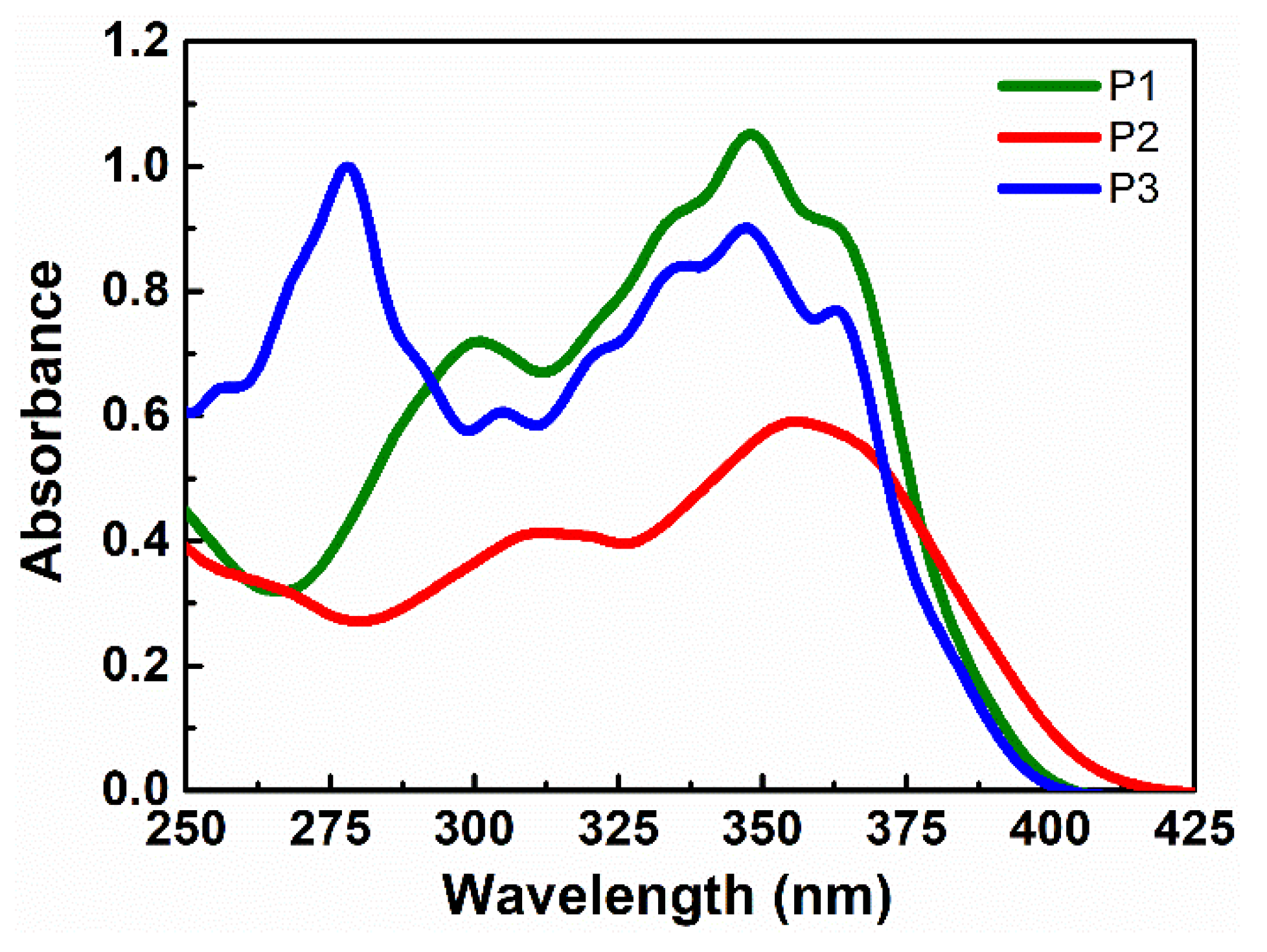
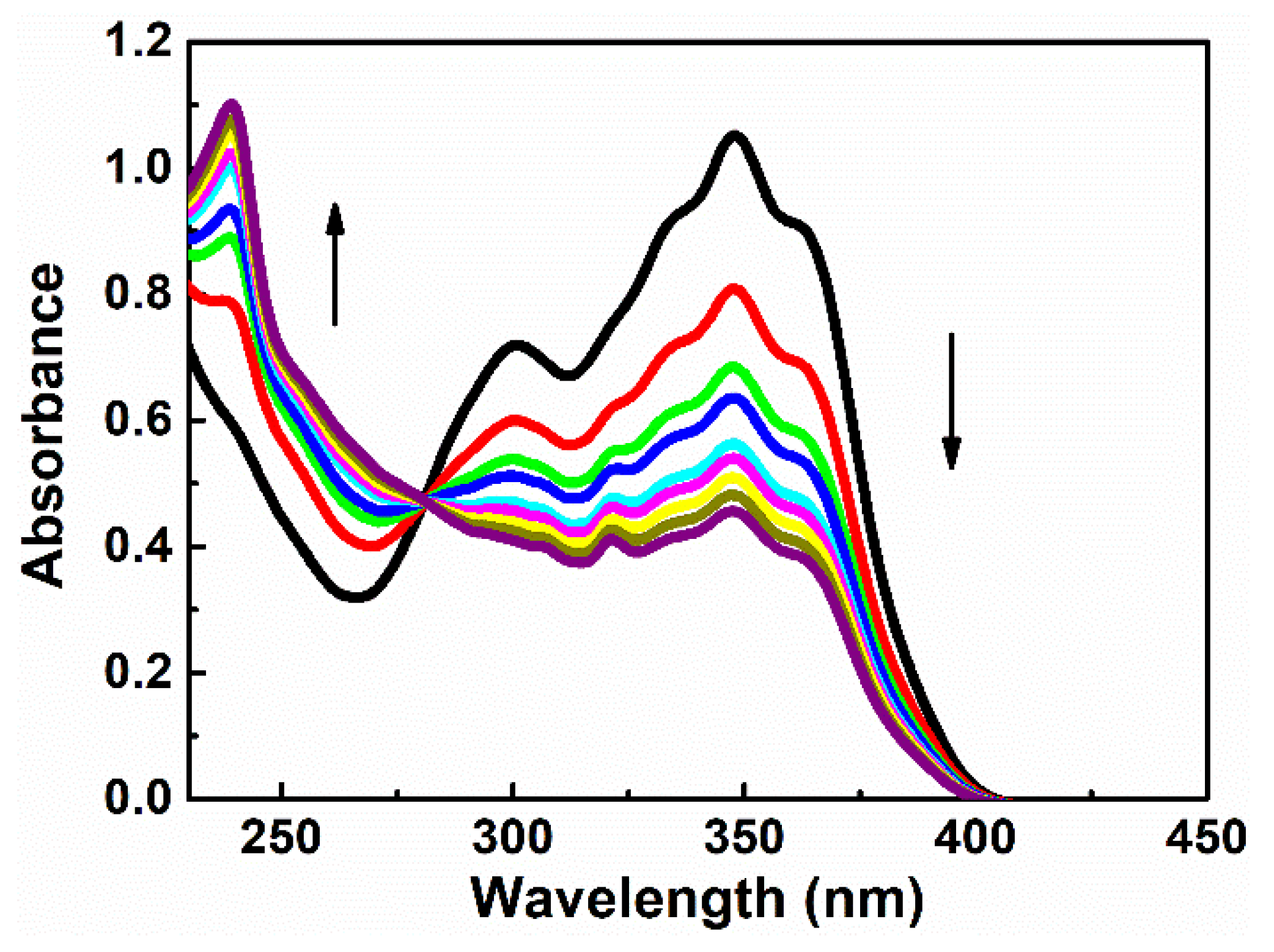
3.1. Spectroscopic Studies
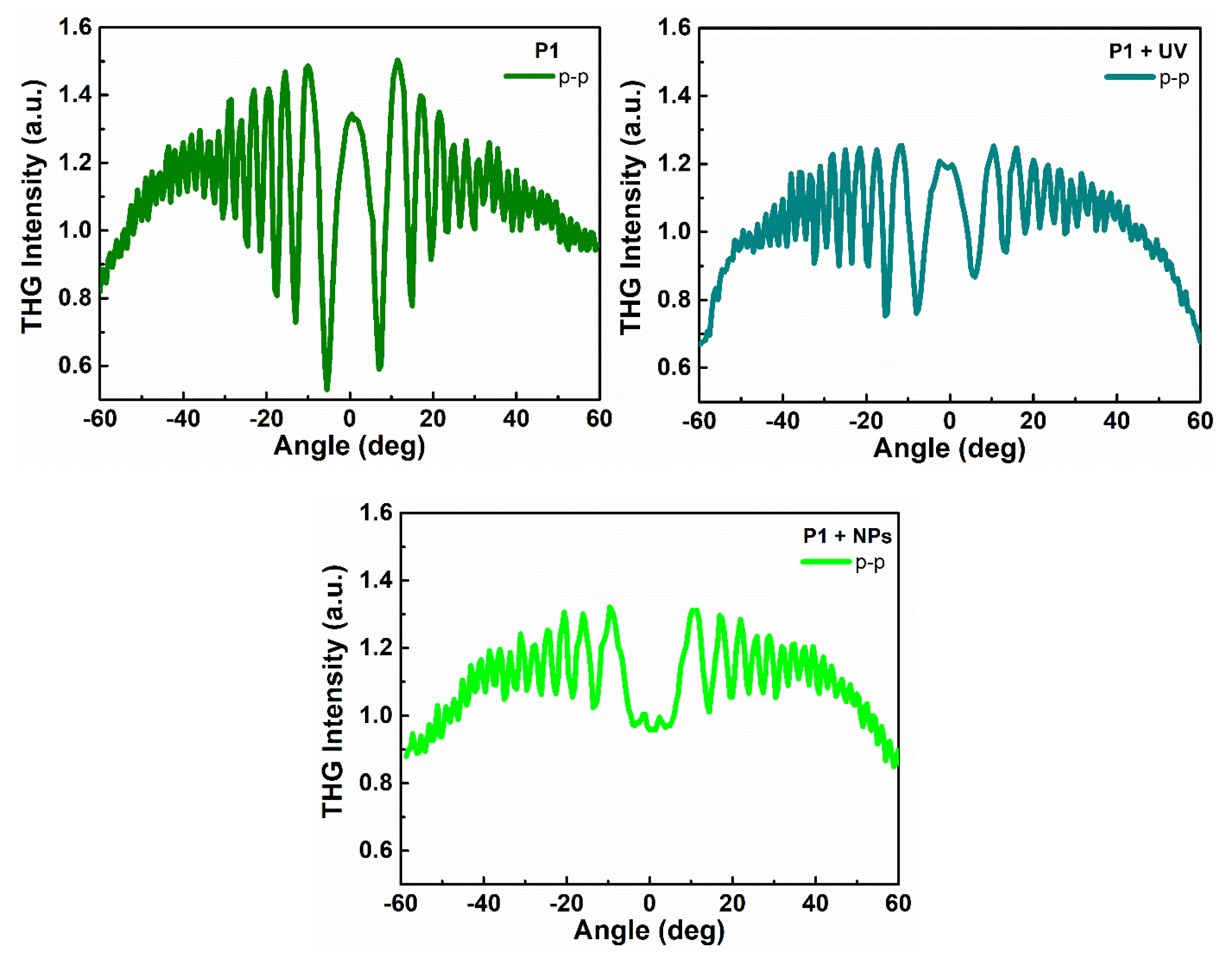
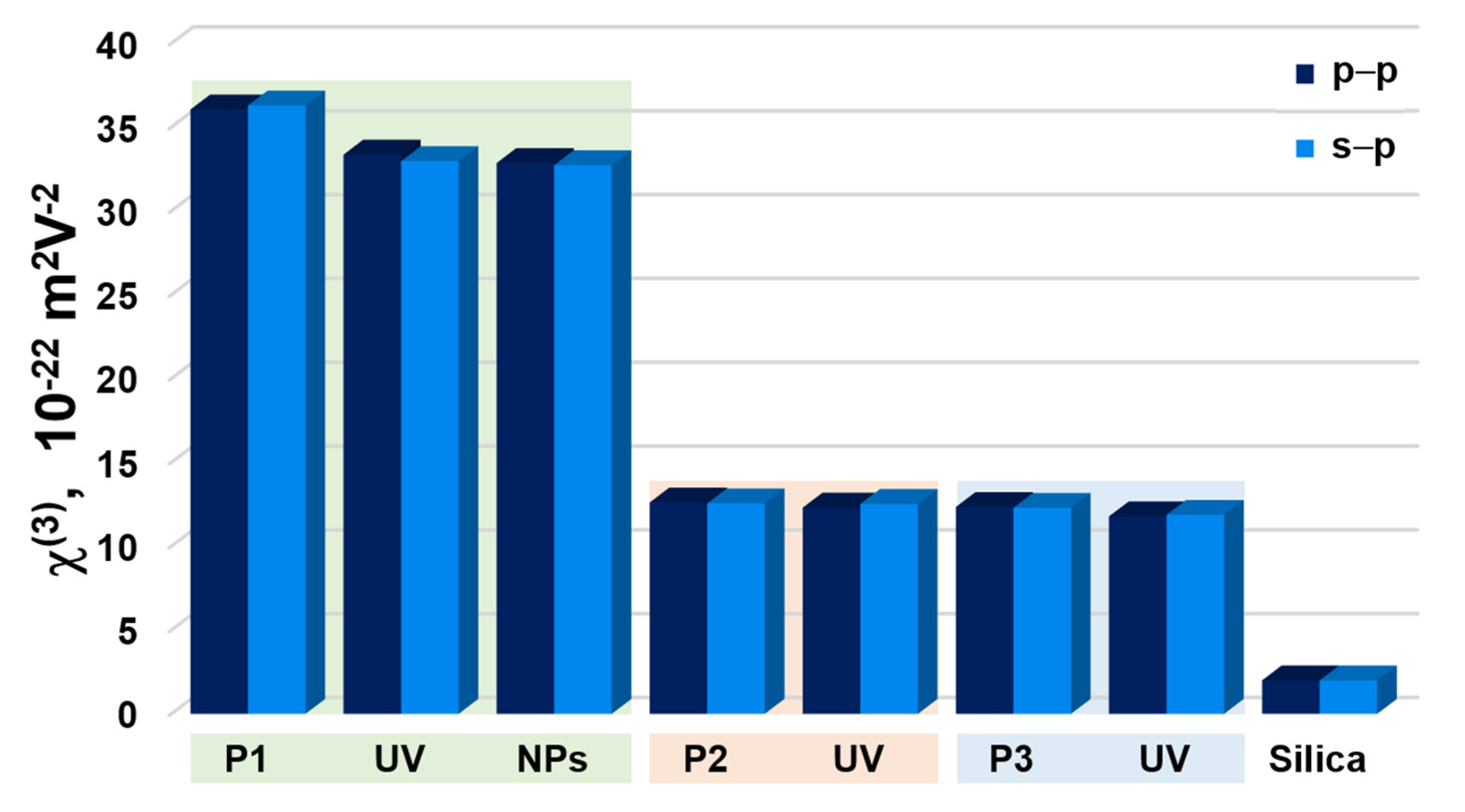
3.2. NLO Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koos, C.; Jacome, L.; Poulton, C.; Leuthold, J.; Freude, W. Nonlinear silicon-on-insulator waveguides for all-optical signal processing. Opt. Express 2007, 15, 5976–5990. [Google Scholar] [CrossRef]
- Kamanina, N.V.; Serov, S.V.; Shurpo, N.A.; Likhomanova, S.V.; Timonin, D.N.; Kuzhakov, P.V.; Rozhkova, N.N.; Kityk, I.V.; Plucinski, K.J.; Uskokovic, D.P. Polyimide-fullerene nanostructured materials for nonlinear optics and solar energy applications. J. Mater. Sci. Mater. Electron. 2012, 23, 1538–1542. [Google Scholar] [CrossRef]
- Smolyaninov, I.I. Nonlinear optics of photonic hyper-crystals: Optical limiting and hyper-computing. J. Opt. Soc. Am. B 2019, 36, 1629–1636. [Google Scholar] [CrossRef]
- Khan, R.U.A.; Kwon, O.-P.; Tapponnier, A.; Rashid, A.N.; Günter, P. Supramolecular Ordered Organic Thin Films for Nonlinear Optical and Optoelectronic Applications. Adv. Funct. Mater. 2006, 16, 180–188. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Q.; Qiu, J. Emerging Low-Dimensional Materials for Nonlinear Optics and Ultrafast Photonics. Adv. Mater. 2017, 29, 1605886. [Google Scholar] [CrossRef] [PubMed]
- Raza, N.; Afzal, U.; Butt, A.R.; Rezazadeh, H. Optical solitons in nematic liquid crystals with Kerr and parabolic law nonlinearities. Opt. Quantum Electron. 2019, 51, 107. [Google Scholar] [CrossRef]
- Flytzanis, C.; Hache, F.; Klein, M.C.; Ricard, D.; Roussignol, P. V Nonlinear Optics in Composite Materials: 1. Semiconductor and Metal Crystallites in Dielectrics: 1. Semiconductor and Metal Crystallites in Dielectrics. Prog. Opt. 1991, 29, 321–411. [Google Scholar] [CrossRef]
- Płóciennik, P.; Guichaoua, D.; Korcala, A.; Zawadzka, A. Studies of aluminum oxide thin films deposited by laser ablation technique. Opt. Mater. 2016, 56, 49–57. [Google Scholar] [CrossRef]
- Taboukhat, S.; Kichou, N.; Fillaut, J.-L.; Alévêque, O.; Waszkowska, K.; Zawadzka, A.; El-Ghayoury, A.; Migalska-Zalas, A.; Sahraoui, B. Transition metals induce control of enhanced NLO properties of functionalized organometallic complexes under laser modulations. Sci. Rep. 2020, 10, 15292. [Google Scholar] [CrossRef]
- Cho, M.J.; Choi, D.H.; Sullivan, P.A.; Akelaitis, A.J.P.; Dalton, L.R. Recent progress in second-order nonlinear optical polymers and dendrimers. Prog. Polym. Sci. 2008, 33, 1013–1058. [Google Scholar] [CrossRef]
- Prasad, P.N. Third-Order Nonlinear Optical Effects in Molecular and Polymeric Materials. In Materials for Nonlinear Optics; ACS Symposium Series; Marder, S.R., Sohn, J.E., Stucky, G.D., Eds.; American Chemical Society: Washington, DC, USA, 1991; Chapter 3; Volume 455, pp. 50–66. [Google Scholar] [CrossRef]
- Yesodha, S.K.; Sadashiva Pillai, C.K.; Tsutsumi, N. Stable polymeric materials for nonlinear optics: A review based on azobenzene systems. Prog. Polym. Sci. 2004, 29, 45–74. [Google Scholar] [CrossRef]
- Fedus, K.; Smokal, V.; Krupka, O.; Boudebs, G. Synthesis and non-resonant nonlinear optical properties of push-pull side-chain azobenzene polymers. J. Nonlinear Opt. Phys. Mater. 2011, 20, 1–13. [Google Scholar] [CrossRef]
- Trefon-Radziejewska, D.; Hamaoui, G.; Chirtoc, M.; Horny, N.; Smokal, V.; Biitseva, A.; Krupka, O.; Derkowska-Zielinska, B. Thermophysical properties of methacrylic polymer films with guest-host and side-chain azobenzene. Mater. Chem. Phys. 2019, 223, 700–707. [Google Scholar] [CrossRef]
- Szukalski, A.; Korbut, A.; Ortyl, E. Structural and light driven molecular engineering in photochromic polymers. Polymer 2020, 192, 122311. [Google Scholar] [CrossRef]
- Gindre, D.; Iliopoulos, K.; Krupka, O.; Evrard, M.; Champigny, E.; Sallé, M. Coumarin-Containing Polymers for High Density Non-Linear Optical Data Storage. Molecules 2016, 21, 147. [Google Scholar] [CrossRef] [PubMed]
- Guichaoua, D.; Kulyk, B.; Smokal, V.; Migalska-Zalas, A.; Kharchenko, O.; Krupka, O.; Kolendo, O.; Sahraoui, B. UV irradiation induce NLO modulation in photochromic styrylquinoline-based polymers: Computational and experimental studies. Org. Electron. 2019, 66, 175–182. [Google Scholar] [CrossRef]
- Derkowska-Zielinska, B.; Matczyszyn, K.; Dudek, M.; Samoc, M.; Czaplicki, R.; Kaczmarek-Kedziera, A.; Smokal, V.; Biitseva, A.; Krupka, O. All-Optical Poling and Two-Photon Absorption in Heterocyclic Azo Dyes with Different Side Groups. J. Phys. Chem. C 2019, 123, 725–734. [Google Scholar] [CrossRef]
- Zawadzka, A.; Płóciennik, P.; El Kouari, Y.; Bougharraf, H.; Sahraoui, B. Linear and nonlinear optical properties of ZnO thin films deposited by pulsed laser deposition. J. Lumin. 2016, 169, 483–491. [Google Scholar] [CrossRef]
- Kulyk, B.; Essaidi, Z.; Luc, J.; Sofiani, Z.; Boudebs, G.; Sahraoui, B. Second and third order nonlinear optical properties of microrod ZnO films deposited on sapphire substrates by thermal oxidation of metallic zinc. J. Appl. Phys. 2007, 102, 113113. [Google Scholar] [CrossRef]
- Kulyk, B.; Kapustianyk, V.; Krupka, O.; Sahraoui, B. Optical absorption and photoluminescence properties of ZnO/PMMA nanocomposite films. J. Phys. Conf. Ser. 2011, 289, 012003. [Google Scholar] [CrossRef]
- Waszkowska, K.; Krupka, O.; Kharchenko, O.; Figà, V.; Smokal, V.; Kutsevol, N.; Sahraoui, B. Influence of ZnO nanoparticles on nonlinear optical properties. Appl. Nanosci. 2020, 10, 4977–4982. [Google Scholar] [CrossRef]
- Maker, P.D.; Terhune, R.W.; Nisenoff, M.; Savage, C.M. Effects of dispersion and focusing on the production of optical harmonics. Phys. Rev. Lett. 1962, 8. [Google Scholar] [CrossRef]
- Budyka, M.F.; Potashova, N.I.; Gavrishova, T.N.; Li, V.M. Photoisomerization of 2–Styrylquinoline in Neutral and Protonated Forms. High Energy Chem. 2008, 42, 446–453. [Google Scholar] [CrossRef]
- Budyka, M.F.; Potashova, N.I.; Gavrishova, T.N.; Lee, V.M. Reconfigurable molecular logic gate operating in polymer film. J. Mater. Chem. 2009, 19, 7721–7724. [Google Scholar] [CrossRef]
- Lee, G.J.; Cha, S.W.; Jeon, S.J.; Jin, J.I.; Yoon, J.S. Second-order nonlinear optical properties of unpoled bent molecules in powder and in vacuum-deposited film. J. Korean Phys. Soc. 2001, 39, 912–915. [Google Scholar]
- Kajzar, F.; Okada-Shudo, Y.; Merrit, C.; Kafafi, C. Second- and third-order non-linear optical properties of multilayered structures and composites of C60 with electron donors. Synth. Met. 2001, 117, 189–193. [Google Scholar] [CrossRef]
- Kubodera, K.; Kobayashi, H. Determination of Third-Order Nonlinear Optical Susceptibilities for Organic Materials by Third-Harmonic Generation. Mol. Cryst. Liq. Cryst. Inc. Nonlinear Opt. 1990, 182, 103–113. [Google Scholar] [CrossRef]
- Wang, X.H.; West, D.P.; McKeown, N.B.; King, T.A. Determining the cubic susceptibility χ(3) of films or glasses by the Maker fringe method: A representative study of spin-coated films of copper phthalocyanine derivation. J. Opt. Soc. Am. B 1998, 15, 1895–1903. [Google Scholar] [CrossRef]
- Sahraoui, B.; Luc, J.; Meghea, A.; Czaplicki, R.; Fillaut, J.-L.; Migalska-Zalas, A. Nonlinear optics and surface relief gratings in alkynyl-ruthenium complexes. J. Opt. A Pure Appl. Opt. 2009, 11, 024005. [Google Scholar] [CrossRef]
- Page, R.H.; Jurich, M.C.; Reck, B.; Sen, A.; Twieg, R.J.; Swalen, J.D.; Bjorklund, G.C.; Wilson, C.G. Electrochromic and optical waveguide studies of corona-poled electro-optic polymer films. J. Opt. Soc. Am. B 1990, 7, 1239–1250. [Google Scholar] [CrossRef]
- Kulyk, B.; Guichaoua, D.; Ayadi, A.; El-Ghayoury, A.; Sahraoui, B. Metal-induced efficient enhancement of nonlinear optical response in conjugated azo-based iminopyridine complexes. Org. Electron. 2016, 36, 1–6. [Google Scholar] [CrossRef]



| P1 | P2 | P3 | |
|---|---|---|---|
| initial monomers mole ratio n/m | 1:3 | ||
| ratio in copolymer n/m a | 1:2.6 | 1:3.2 | 1:2.8 |
| Mw b [g/mol] | 25,400 | 15,000 | 27,900 |
| Mw/Mn b | 1.84 | 2.0 | 1.9 |
| Tg c [°C] | 140 | 160 | 145 |
| Sample | d [nm] | α355 × 103 [cm−1] | χ(2) [pmV−1] | χ(3) × 10−22 [m2 V−2] | |||||
|---|---|---|---|---|---|---|---|---|---|
| s-p | p-p | s-p | p-p | ||||||
| P1 | CP | 368 | 4.89 | - | - | (36.29 ± 1.1) | (36.05 ± 1.1) | ||
| UV | (0.3004 ± 0.0188) | (0.7915 ± 0.0245) | ↑ | (32.97 ± 1.01) | (33.36 ± 1.02) | ↓ | |||
| NPs | 1237 | 51.04 | (0.0946 ± 0.0046) | (0.2355 ± 0.00396) | ↓ | (32.73 ± 0.538) | (32.86 ± 0.543) | ↓ | |
| P2 | CP | 1350 | 10.18 | (0.1992 ± 0.0029) | (1.763 ± 0.0213) | (12.56 ± 0.197) | (12.61 ± 0.198) | ||
| UV | (0.211 ± 0.003) | (2.867 ± 0.0347) | ↑ | (12.52 ± 0.197) | (12.3 ± 0.195) | ↓ | |||
| P3 | CP | 1482 | 12.52 | (0.0957 ± 0.0032) | (0.238 ± 0.00331) | (12.29 ± 0.192) | (12.34 ± 0.193) | ||
| UV | (0.1332 ± 0.0027) | (0.2563 ± 0.00343) | ↑ | (11.87 ± 0.189) | (11.78 ± 0.188) | ↓ | |||
| L2ZnCl2 [32] | 0.44 | 1.00 | 9.93 | ||||||
| L2AgNO3 [32] | 0.32 | 0.96 | 7.92 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waszkowska, K.; Chtouki, T.; Krupka, O.; Smokal, V.; Figà, V.; Sahraoui, B. Effect of UV-Irradiation and ZnO Nanoparticles on Nonlinear Optical Response of Specific Photochromic Polymers. Nanomaterials 2021, 11, 492. https://doi.org/10.3390/nano11020492
Waszkowska K, Chtouki T, Krupka O, Smokal V, Figà V, Sahraoui B. Effect of UV-Irradiation and ZnO Nanoparticles on Nonlinear Optical Response of Specific Photochromic Polymers. Nanomaterials. 2021; 11(2):492. https://doi.org/10.3390/nano11020492
Chicago/Turabian StyleWaszkowska, Karolina, Tarek Chtouki, Oksana Krupka, Vitaliy Smokal, Viviana Figà, and Bouchta Sahraoui. 2021. "Effect of UV-Irradiation and ZnO Nanoparticles on Nonlinear Optical Response of Specific Photochromic Polymers" Nanomaterials 11, no. 2: 492. https://doi.org/10.3390/nano11020492
APA StyleWaszkowska, K., Chtouki, T., Krupka, O., Smokal, V., Figà, V., & Sahraoui, B. (2021). Effect of UV-Irradiation and ZnO Nanoparticles on Nonlinear Optical Response of Specific Photochromic Polymers. Nanomaterials, 11(2), 492. https://doi.org/10.3390/nano11020492








