Strategies for SERS Detection of Organochlorine Pesticides
Abstract
:1. Introduction
2. Brief Introduction to Surface Enhanced Raman Spectroscopy (SERS)
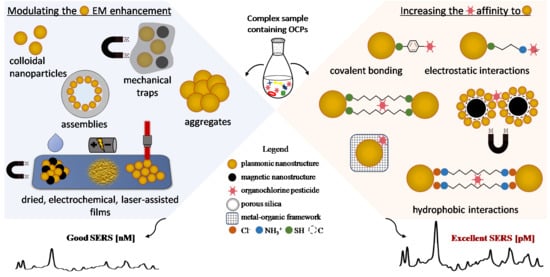
3. Increasing the Electromagnetic SERS Enhancement
4. Increasing the Affinity toward the Analyte
5. Sample Processing and Preconcentration
6. Multiplex Analysis
7. Future Outlook/Perspectives
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
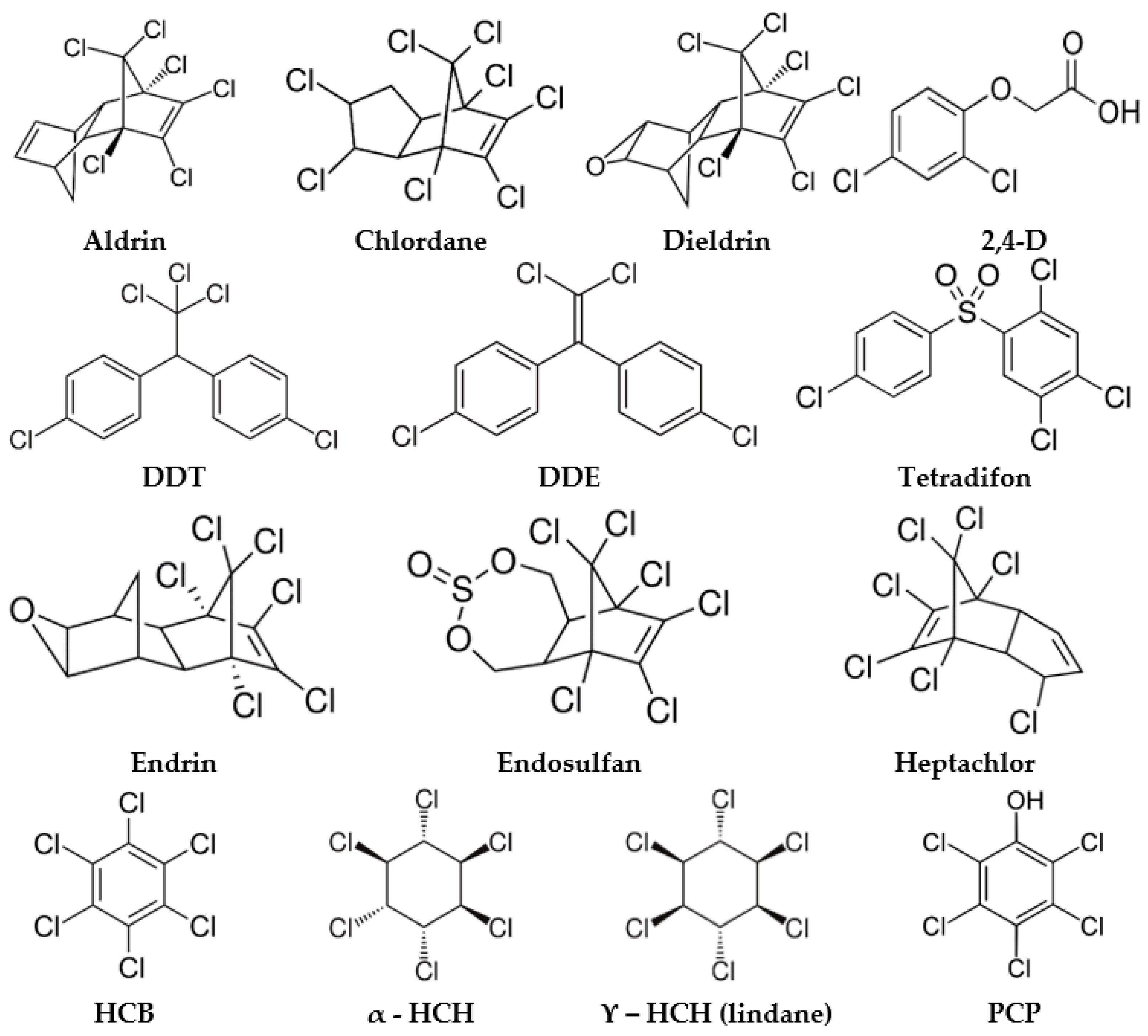
| 2,4-D | 2,4-dichloro-phenoxyacetic acid |
| DDE | dichloro-diphenyl-dichloro-ethylene |
| DDT | dichloro-diphenyl-trichloroethane |
| DT8 | 1,8-octanedithiol |
| EF | enhancement factor |
| F-NPs | ferro-nanoparticles |
| GC/ECD | gas chromatography/electron capture detection |
| GC/MS | gas chromatography/mass spectrometry |
| HCB | hexachlorobenzene |
| HCH | hexachlorocyclohexanes |
| ϒ-HCH | lindane |
| LOD | limit of detection |
| LSPR | localized surface plasmon resonance |
| MIPs | molecularly imprinted polymers |
| MOFs | metallic organic frameworks |
| OCPs | organochlorine pesticides |
| PAHs | polycyclic aromatic hydrocarbons |
| PCP | pentachlorophenol |
| pNIPAM | poly(N-isopropylacrylamide) |
| POPs | persistent organic pollutants |
| SAM | self-assembled monolayer |
| SERS | surface enhanced Raman spectroscopy |
References
- Kutz, F.W.; Wood, P.H.; Bottimore, D.P. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. Rev. Environ. Contam. Toxicol. 1991, 120, 1–82. [Google Scholar] [CrossRef]
- James, R.R.; Xu, J. Mechanisms by which pesticides affect insect immunity. J. Invertebr. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef]
- Idowu, G.; Aiyesanmi, A.; Owolabi, B.J. Organochlorine pesticide residue levels in river and sediment from cocoa producing areas of Ondo State central district, Nigeria. J. Environ. Chem. Ecotoxicol. 2013, 5, 242–249. [Google Scholar]
- Murray, K.E.; Thomas, S.M.; Bodour, A.A. Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environ. Pollut. 2010, 158, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- The 12 Initial POPs under the Stockholm Convention. Available online: http://chm.pops.int/TheConvention/ThePOPs/The12InitialPOPs/tabid/296/Default.aspx (accessed on 15 December 2020).
- All POPs Listed in the Stockholm Convention. Available online: http://chm.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx (accessed on 15 December 2020).
- Hu, G.; Dai, J.; Mai, B.; Luo, X.; Cao, H.; Wang, J.; Li, F.; Xu, M. Concentrations and Accumulation Features of Organochlorine Pesticides in the Baiyangdian Lake Freshwater Food Web of North China. Arch. Environ. Contam. Toxicol. 2010, 58, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, Y.; Hu, Y.; Sun, Y.; Xu, D. Endosulfan triggers epithelial-mesenchymal transition via PTP4A3-mediated TGF-β signaling pathway in prostate cancer cells. Sci. Total Environ. 2020, 731, 139234. [Google Scholar] [CrossRef]
- Parrón, T.; Requena, M.; Hernández, A.F.; Alarcón, R. Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicol. Appl. Pharmacol. 2011, 256, 379–385. [Google Scholar] [CrossRef]
- Hernández, A.F.; Parrón, T.; Alarcón, R. Pesticides and asthma. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 90–96. [Google Scholar] [CrossRef]
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent Organic Pollutants in Food: Contamination Sources, Health Effects and Detection Methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef] [Green Version]
- Taiwo, A.M. A review of environmental and health effects of organochlorine pesticide residues in Africa. Chemosphere 2019, 220, 1126–1140. [Google Scholar] [CrossRef]
- Weber, J.; Halsall, C.J.; Muir, D.; Teixeira, C.; Small, J.; Solomon, K.; Hermanson, M.; Hung, H.; Bidleman, T. Endosulfan, a global pesticide: A review of its fate in the environment and occurrence in the Arctic. Sci. Total Environ. 2010, 408, 2966–2984. [Google Scholar] [CrossRef] [PubMed]
- The New POPs under the Stockholm Convention. Available online: http://www.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx (accessed on 15 December 2020).
- Xu, X.; Yang, H.; Li, Q.; Yang, B.; Wang, X.; Lee, F.S.C. Residues of organochlorine pesticides in near shore waters of LaiZhou Bay and JiaoZhou Bay, Shandong Peninsula, China. Chemosphere 2007, 68, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kazmi, A.A.; Ahmed, N. Study on effects of temperature, moisture and pH in degradation and degradation kinetics of aldrin, endosulfan, lindane pesticides during full-scale continuous rotary drum composting. Chemosphere 2014, 102, 68–75. [Google Scholar] [CrossRef]
- Guillén, D.; Ginebreda, A.; Farré, M.; Darbra, R.M.; Petrovic, M.; Gros, M.; Barceló, D. Prioritization of chemicals in the aquatic environment based on risk assessment: Analytical, modeling and regulatory perspective. Sci. Total Environ. 2012, 440, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Aldeanueva-Potel, P.; Faoucher, E.; Alvarez-Puebla, R.A.; Liz-Marzán, L.M.; Brust, M. Recyclable Molecular Trapping and SERS Detection in Silver-Loaded Agarose Gels with Dynamic Hot Spots. Anal. Chem. 2009, 81, 9233–9238. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Q.; Liu, G.; Zhang, H.; Li, Y.; Cai, W. Kinetically-Controlled Growth of Chestnut-Like Au Nanocrystals with High-Density Tips and Their High SERS Performances on Organochlorine Pesticides. Nanomaterials 2018, 8, 560. [Google Scholar] [CrossRef] [Green Version]
- Farcau, C.; Sangeetha, N.M.; Decorde, N.; Astilean, S.; Ressier, L. Microarrays of gold nanoparticle clusters fabricated by Stop&Go convective self-assembly for SERS-based sensor chips. Nanoscale 2012, 4, 7870–7877. [Google Scholar] [CrossRef]
- Guerrini, L.; Izquierdo-Lorenzo, I.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Self-assembly of α,ω-aliphatic diamines on Ag nanoparticles as an effective localized surface plasmon nanosensor based in interparticle hot spots. Phys. Chem. Chem. Phys. 2009, 11, 7363–7371. [Google Scholar] [CrossRef]
- Guerrini, L.; Izquierdo Lorenzo, I.; Rodriguez-Oliveros, R.; Sánchez-Gil, J.; Sanchez-Cortes, S.; Garcia-Ramos, J.; Domingo, C. α,ω-Aliphatic Diamines as Molecular Linkers for Engineering Ag Nanoparticle Clusters: Tuning of the Interparticle Distance and Sensing Application. Plasmonics 2010, 5, 273–286. [Google Scholar] [CrossRef] [Green Version]
- Kubackova, J.; Fabriciova, G.; Miskovsky, P.; Jancura, D.; Sanchez-Cortes, S. Sensitive Surface-Enhanced Raman Spectroscopy (SERS) Detection of Organochlorine Pesticides by Alkyl Dithiol-Functionalized Metal Nanoparticles-Induced Plasmonic Hot Spots. Anal. Chem. 2015, 87, 663–669. [Google Scholar] [CrossRef]
- Guerrini, L.; Aliaga, A.; Cárcamo-Vega, J.; Gómez-Jeria, J.-S.; Sanchez-Cortes, S.; Vallette, M.; García-Ramos, J. V Functionalization of Ag nanoparticles with the bis-acridinium lucigenin as a chemical assembler in the detection of persistent organic pollutants by surface-enhanced Raman scattering. Anal. Chim. Acta 2008, 624, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, G.; Zhang, H.; Li, Y.; Cai, W. Porous zeolite imidazole framework-wrapped urchin-like Au-Ag nanocrystals for SERS detection of trace hexachlorocyclohexane pesticides via efficient enrichment. J. Hazard. Mater. 2019, 368, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Sigle, D.O.; Perkins, E.; Baumberg, J.J.; Mahajan, S. Reproducible Deep-UV SERRS on Aluminum Nanovoids. J. Phys. Chem. Lett. 2013, 4, 1449–1452. [Google Scholar] [CrossRef]
- Li, D.-W.; Zhai, W.-L.; Li, Y.-T.; Long, Y.-T. Recent progress in surface enhanced Raman spectroscopy for the detection of environmental pollutants. Microchim. Acta 2014, 181, 23–43. [Google Scholar] [CrossRef]
- Pang, S.; Yang, T.; He, L. Review of surface enhanced Raman spectroscopic (SERS) detection of synthetic chemical pesticides. TrAC Trends Anal. Chem. 2016, 85, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Ren, B.; Pérez-Jiménez, A.; Lyu, D.; Lu, Z.; Liu, G. Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem. Sci. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Bernat, A.; Samiwala, M.; Albo, J.; Jiang, X.; Rao, Q. Challenges in SERS-based pesticide detection and plausible solutions. J. Agric. Food Chem. 2019, 67, 12341–12347. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pang, S.; Zhou, G. Recent Advances in Spectroscopy Technology for Trace Analysis of Persistent Organic Pollutants. Appl. Sci. 2019, 9, 3439. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.-L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of Pesticide Residues in Food Using Surface-Enhanced Raman Spectroscopy: A Review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [CrossRef] [PubMed]
- Pilot, R. SERS detection of food contaminants by means of portable Raman instruments. J. Raman Spectrosc. 2018, 49. [Google Scholar] [CrossRef]
- Mehta, J.; Kumar, R.; Dhaka, S.; Deep, A. Biofunctionalized Nanostructured Materials for Sensing of Pesticides. In Nanosensors for Environmental Applications; Kumar Tuteja, S., Arora, D., Dilbaghi, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 29–86. ISBN 978-3-030-38101-1. [Google Scholar]
- Kalyani, N.; Goel, S.; Jaiswal, S. Point-of-Care Sensors for On-Site Detection of Pesticides. In Nanosensors for Environmental Applications; Kumar Tuteja, S., Arora, D., Dilbaghi, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 197–224. ISBN 978-3-030-38101-1. [Google Scholar]
- Santos Costa, J.C.; Ando, R.A.; Sant’Ana, A.C.; Rossi, L.M.; Santos, P.S.; Temperini, M.L.A.; Corio, P. High performance gold nanorods and silver nanocubes in surface-enhanced Raman spectroscopy of pesticides. Phys. Chem. Chem. Phys. 2009, 11, 7491–7498. [Google Scholar] [CrossRef]
- Zhu, C.; Meng, G.; Zheng, P.; Huang, Q.; Li, Z.; Hu, X.; Wang, X.; Huang, Z.; Li, F.; Wu, N. A Hierarchically Ordered Array of Silver-Nanorod Bundles for Surface-Enhanced Raman Scattering Detection of Phenolic Pollutants. Adv. Mater. 2016, 28, 4871–4876. [Google Scholar] [CrossRef]
- Mariño-Lopez, A.; Sousa-Castillo, A.; Blanco-Formoso, M.; Furini, L.N.; Rodríguez-Lorenzo, L.; Pazos-Perez, N.; Guerrini, L.; Pérez-Lorenzo, M.; Correa-Duarte, M.A.; Alvarez-Puebla, R.A. Microporous plasmonic capsules as stable molecular sieves for direct SERS quantification of small pollutants in natural waters. Chem. Nanomater. Energy Biol. More 2019, 5, 46–50. [Google Scholar] [CrossRef]
- Nedyalkov, N.; Nikov, R.; Nikov, R.; Nikolov, A.; Atanasov, P.; Nakajima, Y.; Terakawa, M.; Sawczak, M.; Grochowska, K.; Sliwinski, G. Gold nanostructures for detection of pesticides, nitrates and drugs using Surface Enhanced Raman spectroscopy. In Proceedings of the 19th International Conference and School on Quantum Electronics: Laser Physics and Applications; Dreischuh, T., Gateva, S., Daskalova, A., Serafetinides, A., Eds.; SPIE: Washington, DC, USA, 2017; Volume 10226, pp. 85–92. [Google Scholar]
- Cai, L.; Deng, Z.; Dong, J.; Song, S.; Wang, Y.; Chen, X. Fabrication of Non-Woven Fabric-Based SERS Substrate for Direct Detection of Pesticide Residues in Fruits. J. Anal. Test. 2017, 1, 322–329. [Google Scholar] [CrossRef]
- Chi, H.; Wang, C.; Wang, Z.; Zhu, H.; Mesias, V.S.D.; Dai, X.; Chen, Q.; Liu, W.; Huang, J. Highly reusable nanoporous silver sheet for sensitive SERS detection of pesticides. Analyst 2020, 145, 5158–5165. [Google Scholar] [CrossRef]
- Hernández-Castillo, M.I.; Zaca-Morán, O.; Zaca-Morán, P.; Orduña-Diaz, A.; Delgado-Macuil, R.; Rojas-López, M. Surface-enhanced Raman scattering of the adsorption of pesticide endosulfan on gold nanoparticles. J. Environ. Sci. Health. B 2015, 50, 584–589. [Google Scholar] [CrossRef]
- Gong, T.; Huang, Y.; Wei, Z.; Huang, W.; Wei, X.; Zhang, X. Magnetic assembled 3D SERS substrate for sensitive detection of pesticide residue in soil. Nanotechnology 2020, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, Q.; Liu, G.; Zhang, H.; Li, Y.; Cai, W. Temperature regulation growth of Au nanocrystals: From concave trisoctahedron to dendritic structures and their ultrasensitive SERS-based detection of lindane. J. Mater. Chem. C 2017, 5, 10399–10405. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Ma, Q.; Zhang, Q.; Bai, H.; Yi, W.; Liu, J.; Han, J.; Xi, G. A metallic molybdenum dioxide with high stability for surface enhanced Raman spectroscopy. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Cáceres, R.; Abalde-Cela, S.; Guardia-Girós, P.; Fernández-Barbero, A.; Pérez-Juste, J.; Alvarez-Puebla, R.A.; Liz-Marzán, L.M. Multifunctional Microgel Magnetic/Optical Traps for SERS Ultradetection. Langmuir 2011, 27, 4520–4525. [Google Scholar] [CrossRef]
- Spencer, K.; Clauson, S.; Spencer, S.; Sylvia, J.; Vallejos, Q.; Quandt, S.; Arcury, T. Development of a Fieldable Rapid Pesticide Exposure Analysis Sensing System. Proc. SPIE Int. Soc. Opt. Eng. 2010, 7673. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, P.; Yu, Z.; Xia, J.; Ni, D.; Wang, D.; Zhou, Y.; Cao, Y.; Chen, J.; Chen, J.; et al. Self-assembled “bridge” substance for organochlorine pesticides detection in solution based on Surface Enhanced Raman Scattering. J. Hazard. Mater. 2020, 382, 121023. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Q.; Liu, G.; Cai, W. 4-Mercaptophenylboronic Acid modified Au Nanosheets-built Hollow Sub-microcubes for Active Capture and Ultrasensitive SERS-based Detection of Hexachlorocyclohexane Pesticides. Sens. Actuators B Chem. 2019, 293. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, M.; Meng, Y.; Jiang, W.; Zhan, J. Cysteamine-Modified Silver Nanoparticle Aggregates for Quantitative SERS Sensing of Pentachlorophenol with a Portable Raman Spectrometer. ACS Appl. Mater. Interfaces 2013, 5, 6902–6908. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, H.; Liu, W.; Ge, J.; Wu, J.; Wang, S.; Wang, P. Surface-enhanced Raman scattering substrate based on cysteamine-modified gold nanoparticle aggregation for highly sensitive pentachlorophenol detection. RSC Adv. 2016, 6, 85285–85292. [Google Scholar] [CrossRef]
- Bian, W.; Zhu, S.; Qi, M.; Xiao, L.; Liu, Z.; Zhan, J. Electrostatic-driven solid phase microextraction coupled with surface enhanced Raman spectroscopy for rapid analysis of pentachlorophenol. Anal. Methods 2017, 9, 459–464. [Google Scholar] [CrossRef]
- An, Q.; Zhang, P.; Li, J.-M.; Ma, W.-F.; Guo, J.; Hu, J.; Wang, C.C. Silver-coated magnetite–carbon core–shell microspheres as substrate-enhanced SERS probes for detection of trace persistent organic pollutants. Nanoscale 2012, 4, 5210–5216. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; He, L. Development of a facile rolling method to amplify an analyte’s weak SERS activity and its application for chlordane detection. Anal. Methods 2020, 12, 433–439. [Google Scholar] [CrossRef]
- Xie, X.; Pu, H.; Sun, D.-W. Recent advances in nanofabrication techniques for SERS substrates and their applications in food safety analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 2800–2813. [Google Scholar] [CrossRef] [PubMed]
- Mosier-Boss, P.A. Review of SERS Substrates for Chemical Sensing. Nanomater 2017, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Ren, W.; Zhu, L.; Irudayaraj, J. Prosperity to challenges: Recent approaches in SERS substrate fabrication. Rev. Anal. Chem. 2016, 36. [Google Scholar] [CrossRef]
- Lin, X.-M.; Cui, Y.; Xu, Y.-H.; Ren, B.; Tian, Z.-Q. Surface-enhanced Raman spectroscopy: Substrate-related issues. Anal. Bioanal. Chem. 2009, 394, 1729–1745. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wang, Y. Plasmonic Nanostructures as Surface-Enhanced Raman Scattering (SERS) Substrate for Protein Biomarker Sensing. In Nanoplasmonics—Fundamentals and Applications; INTECH: Longon, UK, 2017; pp. 341–359. [Google Scholar]
- Cong, S.; Yuan, Y.; Chen, Z.; Hou, J.; Yang, M.; Su, Y.; Zhang, Y.; Li, L.; Li, Q.; Geng, F.; et al. Noble metal-comparable SERS enhancement from semiconducting metal oxides by making oxygen vacancies. Nat. Commun. 2015, 6, 7800. [Google Scholar] [CrossRef]
- Kim, A.; Barcelo, S.J.; Li, Z. SERS-based pesticide detection by using nanofinger sensors. Nanotechnology 2014, 26, 15502. [Google Scholar] [CrossRef]
- Goodacre, R.; Graham, D.; Faulds, K. Recent developments in quantitative SERS: Moving towards absolute quantification. TrAC Trends Anal. Chem. 2018, 102, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Bell, S.E.J.; Charron, G.; Cortés, E.; Kneipp, J.; de la Chapelle, M.L.; Langer, J.; Procházka, M.; Tran, V.; Schlücker, S. Towards Reliable and Quantitative Surface-Enhanced Raman Scattering (SERS): From Key Parameters to Good Analytical Practice. Angew. Chem. Int. Ed. 2020, 59, 5454–5462. [Google Scholar] [CrossRef] [Green Version]
- Madasamy, K.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.-C. Viologen-based electrochromic materials and devices. J. Mater. Chem. C 2019, 7, 4622–4637. [Google Scholar] [CrossRef]
- Striepe, L.; Baumgartner, T. Viologens and Their Application as Functional Materials. Chem. A Eur. J. 2017, 23. [Google Scholar] [CrossRef] [PubMed]
- López-Tocón, I.; Otero, J.C.; Arenas, J.F.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Multicomponent Direct Detection of Polycyclic Aromatic Hydrocarbons by Surface-Enhanced Raman Spectroscopy Using Silver Nanoparticles Functionalized with the Viologen Host Lucigenin. Anal. Chem. 2011, 83, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Bruckbauer, A.; Chen, Y.X. On the chloride activation in SERS and single molecule SERS. J. Mol. Struct. 2003, 661–662, 501–514. [Google Scholar] [CrossRef]
- Vikrant, K.; Tsang, D.C.W.; Raza, N.; Giri, B.S.; Kukkar, D.; Kim, K.-H. Potential Utility of Metal–Organic Framework-Based Platform for Sensing Pesticides. ACS Appl. Mater. Interfaces 2018, 10, 8797–8817. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-H.; Ho, C.-H.; Wu, C.-Y.; Chien, C.-H.; Lin, C.-H.; Lee, S. Metal–organic frameworks: A novel SERS substrate. J. Raman Spectrosc. 2013, 44, 1506–1511. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Lai, K.; Fan, Y.; Rasco, B.A. Trace analysis of organic compounds in foods with surface-enhanced Raman spectroscopy: Methodology, progress, and challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 622–642. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.C.; Bilon, N.; Hay, M. Analytical Methods for Pesticide Residues. Water Environ. Res. 2014, 86. [Google Scholar] [CrossRef]
- Correia-Sá, L.; Fernandes, V.C.; Carvalho, M.; Calhau, C.; Domingues, V.F.; Delerue-Matos, C. Optimization of QuEChERS method for the analysis of organochlorine pesticides in soils with diverse organic matter. J. Sep. Sci. 2012, 35, 1521–1530. [Google Scholar] [CrossRef]
- Salihovic, S.; Mattioli, L.; Lindström, G.; Lind, L.; Lind, M.; Bavel, B. A rapid method for screening of the Stockholm Convention POPs in small amounts of human plasma using SPE and HRGC/HRMS. Chemosphere 2011, 86, 747–753. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.; Fabris, L.; Alvarez-Puebla, R.A. Multiplex optical sensing with surface-enhanced Raman scattering: A critical review. Anal. Chim. Acta 2012, 745, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Huang, Z.; Li, S.; Zhao, C. Surface-Enhanced Raman Spectroscopy on Self-Assembled Au Nanoparticles Arrays for Pesticides Residues Multiplex Detection under Complex Environment. Nanomaterials 2019, 9, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Tian, S.; Zhou, Q.; Adkins, J.; Gu, Z.; Li, X.; Zheng, J. SERS detection of polycyclic aromatic hydrocarbons on a bowl-shaped silver cavity substrate. RSC Adv. 2013, 3, 25989. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Zheng, J.; He, L. Surface-enhanced Raman spectroscopy (SERS) combined techniques for high-performance detection and characterization. TrAC Trends Anal. Chem. 2017, 90, 1–13. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Sandulescu, R.; Cristea, C.; Bodoki, E.; Oprean, R. Recent advances in the analysis of bioactive compunds based on molecular recognition. In Frontiers in Bioactive Compounds, Volume 1, Natural Sources, Physicochemical Characterization and Applications; Apetrei, C., Ed.; Bentham Science: Sharjah, UAE, 2016; pp. 69–110. ISBN 978-1-68108-342-1. [Google Scholar]
- Xue, J.-Q.; Li, D.-W.; Qu, L.-L.; Long, Y.-T. Surface-imprinted core–shell Au nanoparticles for selective detection of bisphenol A based on surface-enhanced Raman scattering. Anal. Chim. Acta 2013, 777, 57–62. [Google Scholar] [CrossRef]
- Holthoff, E.L.; Stratis-Cullum, D.N.; Hankus, M.E. A nanosensor for TNT detection based on molecularly imprinted polymers and surface enhanced Raman scattering. Sensors 2011, 11, 2700–2714. [Google Scholar] [CrossRef] [Green Version]
- Yao, G.-H.; Liang, R.-P.; Huang, C.-F.; Wang, Y.; Qiu, J.-D. Surface plasmon resonance sensor based on magnetic molecularly imprinted polymers amplification for pesticide recognition. Anal. Chem. 2013, 85, 11944–11951. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, X. Rapid Detection of Melamine in Tap Water and Milk Using Conjugated “One-Step” Molecularly Imprinted Polymers-Surface Enhanced Raman Spectroscopic Sensor. J. Food Sci. 2016, 81, N1272–N1280. [Google Scholar] [CrossRef]
- Beltran, A.; Borrull, F.; Marcé, R.; Cormack, P.A.G. Molecularly-Imprinted Polymers: Useful Sorbents for Selective Extractions. TrAC Trends Anal. Chem. 2010, 29, 1363–1375. [Google Scholar] [CrossRef]
- Kamińska, A.; Winkler, K.; Kowalska, A.; Witkowska, E.; Szymborski, T.; Janeczek, A.; Waluk, J. SERS-based Immunoassay in a Microfluidic System for the Multiplexed Recognition of Interleukins from Blood Plasma: Towards Picogram Detection. Sci. Rep. 2017, 7, 10656. [Google Scholar] [CrossRef] [PubMed]
- Gezer, P.G.; Liu, G.L.; Kokini, J.L. Development of a biodegradable sensor platform from gold coated zein nanophotonic films to detect peanut allergen, Ara h1, using surface enhanced raman spectroscopy. Talanta 2016, 150, 224–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Ji, X.; Xu, W.; Li, X.; Wang, L.; Bai, Y.; Zhao, B.; Ozaki, Y. Immunoassay using probe-labelling immunogold nanoparticles with silver staining enhancement via surface-enhanced Raman scattering. Analyst 2004, 129, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Driskell, J.D.; Kwarta, K.M.; Lipert, R.J.; Porter, M.D.; Neill, J.D.; Ridpath, J.F. Low-Level Detection of Viral Pathogens by a Surface-Enhanced Raman Scattering Based Immunoassay. Anal. Chem. 2005, 77, 6147–6154. [Google Scholar] [CrossRef] [PubMed]
- Grubisha, D.S.; Lipert, R.J.; Park, H.-Y.; Driskell, J.; Porter, M.D. Femtomolar Detection of Prostate-Specific Antigen: An Immunoassay Based on Surface-Enhanced Raman Scattering and Immunogold Labels. Anal. Chem. 2003, 75, 5936–5943. [Google Scholar] [CrossRef]
- Lisa, M.; Chouhan, R.S.; Vinayaka, A.C.; Manonmani, H.K.; Thakur, M.S. Gold nanoparticles based dipstick immunoassay for the rapid detection of dichlorodiphenyltrichloroethane: An organochlorine pesticide. Biosens. Bioelectron. 2009, 25, 224–227. [Google Scholar] [CrossRef]
- Jahn, I.J.; Žukovskaja, O.; Zheng, X.-S.; Weber, K.; Bocklitz, T.W.; Cialla-May, D.; Popp, J. Surface-enhanced Raman spectroscopy and microfluidic platforms: Challenges, solutions and potential applications. Analyst 2017, 142, 1022–1047. [Google Scholar] [CrossRef]
- Fu, C.; Wang, Y.; Chen, G.; Yang, L.; Xu, S.; Xu, W. Aptamer-Based Surface-Enhanced Raman Scattering-Microfluidic Sensor for Sensitive and Selective Polychlorinated Biphenyls Detection. Anal. Chem. 2015, 87, 9555–9558. [Google Scholar] [CrossRef]
- Abalde-Cela, S.; Abell, C.; Alvarez-Puebla, R.; Liz-Marzán, L. Real Time Dual-Channel Multiplex SERS Ultradetection. J. Phys. Chem. Lett. 2014, 5, 73–79. [Google Scholar] [CrossRef]
- Lacharmoise, P.D.; Le Ru, E.C.; Etchegoin, P.G. Guiding Molecules with Electrostatic Forces in Surface Enhanced Raman Spectroscopy. ACS Nano 2009, 3, 66–72. [Google Scholar] [CrossRef]
- Li, D.; Li, D.-W.; Fossey, J.S.; Long, Y.-T. Portable Surface-Enhanced Raman Scattering Sensor for Rapid Detection of Aniline and Phenol Derivatives by On-Site Electrostatic Preconcentration. Anal. Chem. 2010, 82, 9299–9305. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhu, Z.; Zhou, S.; Zhu, C.; Dong, S. Recent advances in spectroelectrochemistry. Nanoscale 2018, 10, 3089–3111. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.; Wang, S.; Li, T.; Wang, B.; Ma, X.; Huang, B.; Zhu, L.; Guo, J. Detection of Pesticide Residues Using Nano-SERS Chip and a Smartphone-Based Raman Sensor. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–6. [Google Scholar] [CrossRef]


| Strategy | Analyte | SERS Substrate/Method | LOD | EF | Metal | Laser (nm) | Incubation Time | Ref. |
|---|---|---|---|---|---|---|---|---|
| Increasing the substrate’s performance | 2,4-D | Au nanorods and Ag nanocubes | - | - | Au, Ag | 632.8 | 1 h | [40] |
| vertically ordered arrays of Ag nanorod bundles | 61.9 nM | 1.4 × 108 | Ag + Au | 633 | hours | [41] | ||
| DDT | void@AuNPs@SiO2 microporous capsules | - | - | Au | 785 | 15 min | [42] | |
| AgNP@composite agarose gels | - | - | Ag | 785 | 2 h | [18] | ||
| AuNP array fabricated by laser annealing of gold film | - | - | Au | 785 | - | [43] | ||
| AgNPs prepared by self-assembly/ in situ growing method | - | 3.5 × 106 | Ag | 785 | <1 min | [44] | ||
| AgNPs sheet | - | - | Ag | 632.8 | 20 min | [45] | ||
| Endosulfan | AuNPs | - | - | Au | 785 | - | [46] | |
| HCB | 3D Ag F-NPs | - | - | Ag | 785 | 30 min | [47] | |
| Lindane | chestnut-like Au nanocrystals-built film | 34.38 nM | > 107 | Au | 785 | 8h | [19] | |
| concave trisoctahedral and calyptriform Au nanocrystals-built films | 0.1 µM | 107 | Au | 785 | 8h | [48] | ||
| AgNPs sheet | 0.3 µM | - | Ag | 632.8 | 20 min | [45] | ||
| PCP | MoO2nanodumbbells | 0.1 µM | 3.75 × 106 | MoO2 | 532.8 | 20 min | [49] | |
| Fe3O4xAgNPs@pNIPAM | 1 nM | - | Ag | 785 | 2 h | [50] | ||
| >100 pesticides | electrochemically roughened silver oxide SERS sensor | - | - | Ag | 785 | - | [51] | |
| Increasing the affinity of the analyte | Aldrin | AgNPs@ α, ω-aliphatic diamines | 13.7 nM | 2 × 104 | Ag | 514.5 | - | [21] |
| AuNPs/AgNPs@alkyl dithiols | 0.12 µM | - | Au, Ag | 785 | 10 min | [23] | ||
| AgNPs clusters by α, ω-aliphatic diamines | 10 nM | - | Ag | 785 514.5 | - | [22] | ||
| flower like AgNPs@ diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | ||
| Dieldrin | AuNPs/AgNPs@alkyl dithiols | 0.82 µM | - | Au, Ag | 785 | 10 min | [23] | |
| op’-DDT | flower like AgNPs@ diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | |
| pp’-DDE | flower like AgNPs@ diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | |
| Endosulfan (α) | AuNPs/AgNPs@alkyl dithiols | 0.41 µM | - | Au, Ag | 785 | 10 min | [23] | |
| Endosulfan (α, β) | AgNP clusters by α, ω-aliphatic diamines | 10nM | - | Ag | 785 514.5 | - | [22] | |
| Endosulfan | AgNP@ bis-acridinium lucigenin | 49.15 nM | - | Ag | 785 | - | [24] | |
| Endosulfan (α, β) | flower like AgNPs@diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | |
| HCH | Au nanosheets built hollow sub-microcubes@4-MPBA | 1.03 nM | - | Au | 785 | 4h | [53] | |
| HCH (α, ϒ) | urchin-like Au–Ag nanocrystals@ porous zeolite imidazole framework | 15.15 nM | 3 × 107 | Au + Ag | 785 | 10 h | [25] | |
| HCH (α, β) | flower like AgNPs@diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | |
| HCH (ϒ) | AuNPs/AgNPs@alkyl dithiols | 3.53 µM | - | Au, Ag | 785 | 10 min | [23] | |
| Heptachlor | flower like AgNPs@ diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | |
| PCP | AgNPs aggregates@cysteamine SAM | 0.20 μM | - | Ag | 785 | 3 h | [54] | |
| AuNPs@cysteamine | 1 nM | 5.7 × 105 | Au | 785 | - | [55] | ||
| nanoporous Ag coating@cysteamine | 6.4 nM | 3.7 × 105 | Ag | 785 | 5 h | [56] | ||
| Fe3O4@carbon@AgNPs core–shell microspheres | 1 pM | - | Ag | 633 | 1 h | [57] | ||
| Tetradifon | flower like AgNPs@ diquat/lucigenin | - | - | Ag | 532 | 5 min | [52] | |
| Preconcentra-tion | Chlordane | citrate coated AuNPs/rolling method and prediction model | 1 ppm | - | Au | 780 | - | [58] |
| PCP | nanoporous Ag coating modified by cysteamine | 6.4 nM | 3.7 ×105 | Ag | 785 | 5 h | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, R.; Iacob, B.-C.; Farcău, C.; Bodoki, E.; Oprean, R. Strategies for SERS Detection of Organochlorine Pesticides. Nanomaterials 2021, 11, 304. https://doi.org/10.3390/nano11020304
Moldovan R, Iacob B-C, Farcău C, Bodoki E, Oprean R. Strategies for SERS Detection of Organochlorine Pesticides. Nanomaterials. 2021; 11(2):304. https://doi.org/10.3390/nano11020304
Chicago/Turabian StyleMoldovan, Rebeca, Bogdan-Cezar Iacob, Cosmin Farcău, Ede Bodoki, and Radu Oprean. 2021. "Strategies for SERS Detection of Organochlorine Pesticides" Nanomaterials 11, no. 2: 304. https://doi.org/10.3390/nano11020304