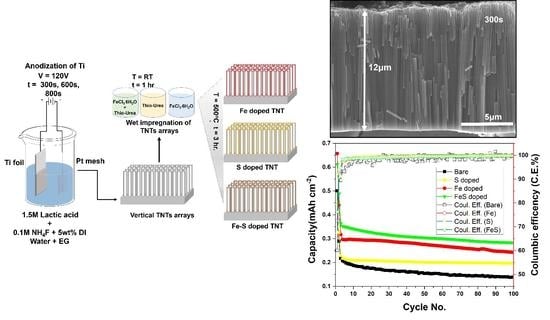
Vertically Aligned Binder-Free TiO2 Nanotube Arrays Doped with Fe, S and Fe-S for Li-ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
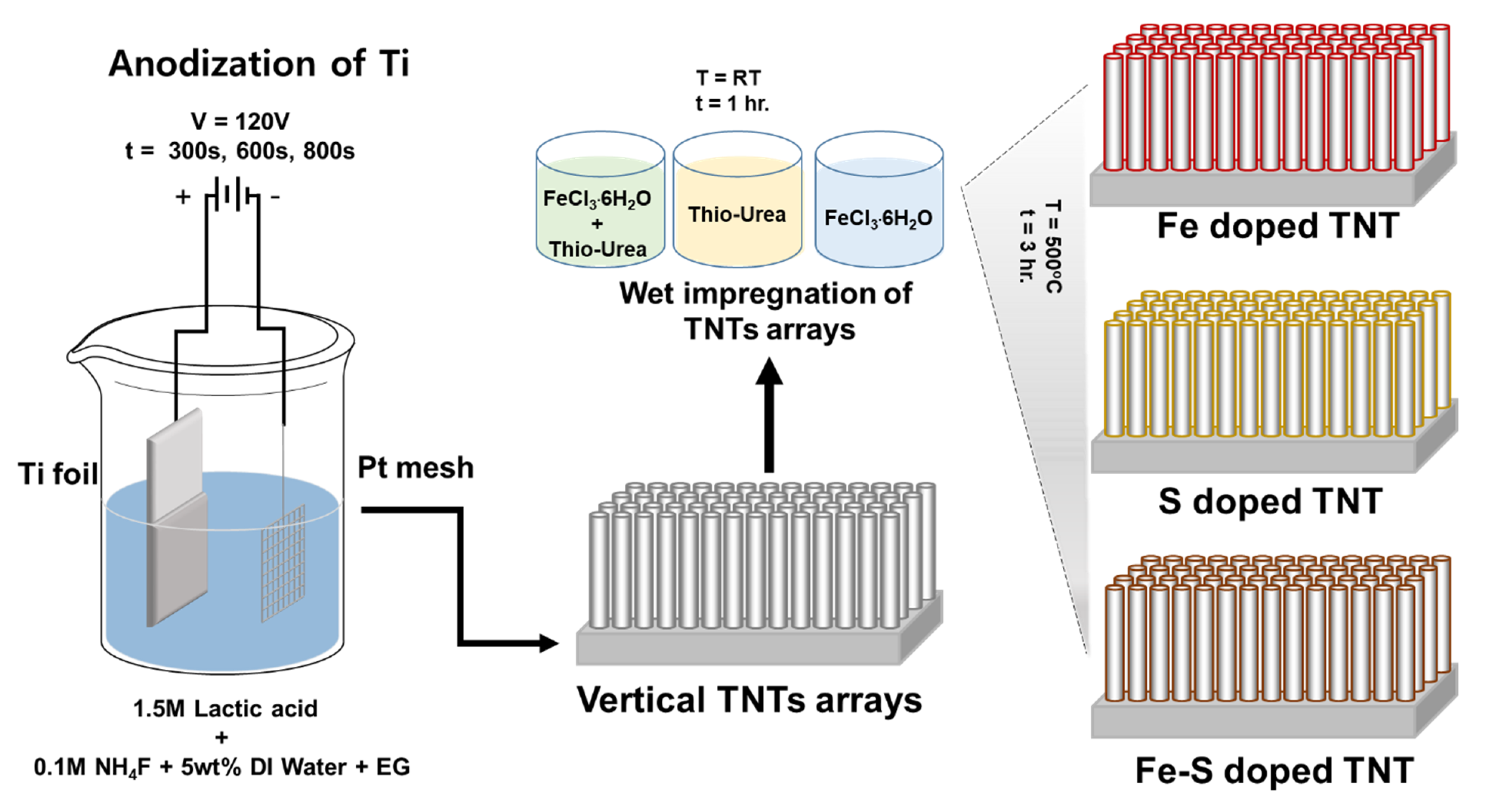
2.1. Preparation of TNTs
2.2. Preparation of Fe-S co-doped TNTs
2.3. Materials Characterization
2.4. Electrochemical Characterization
3. Results and Discussion
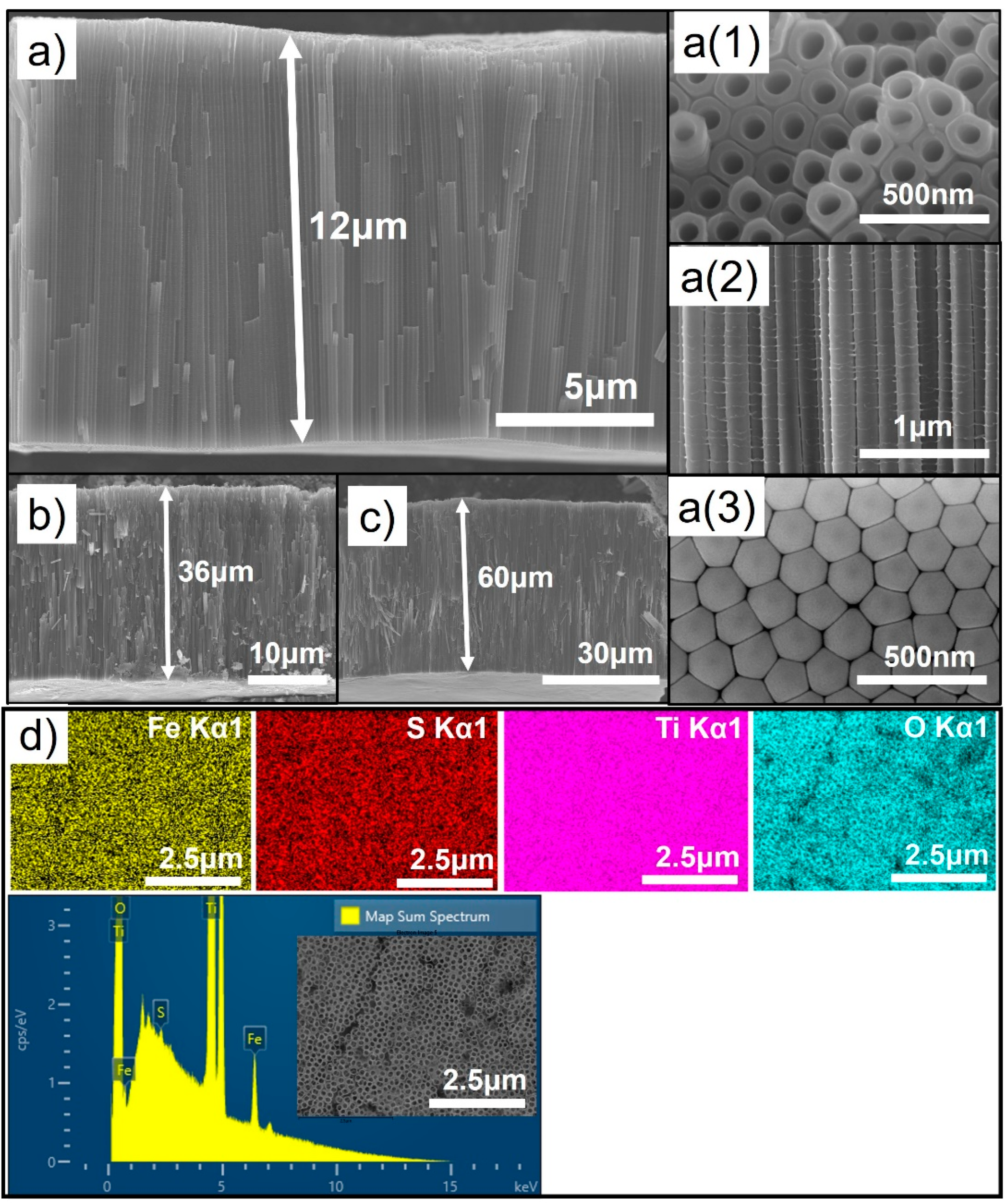
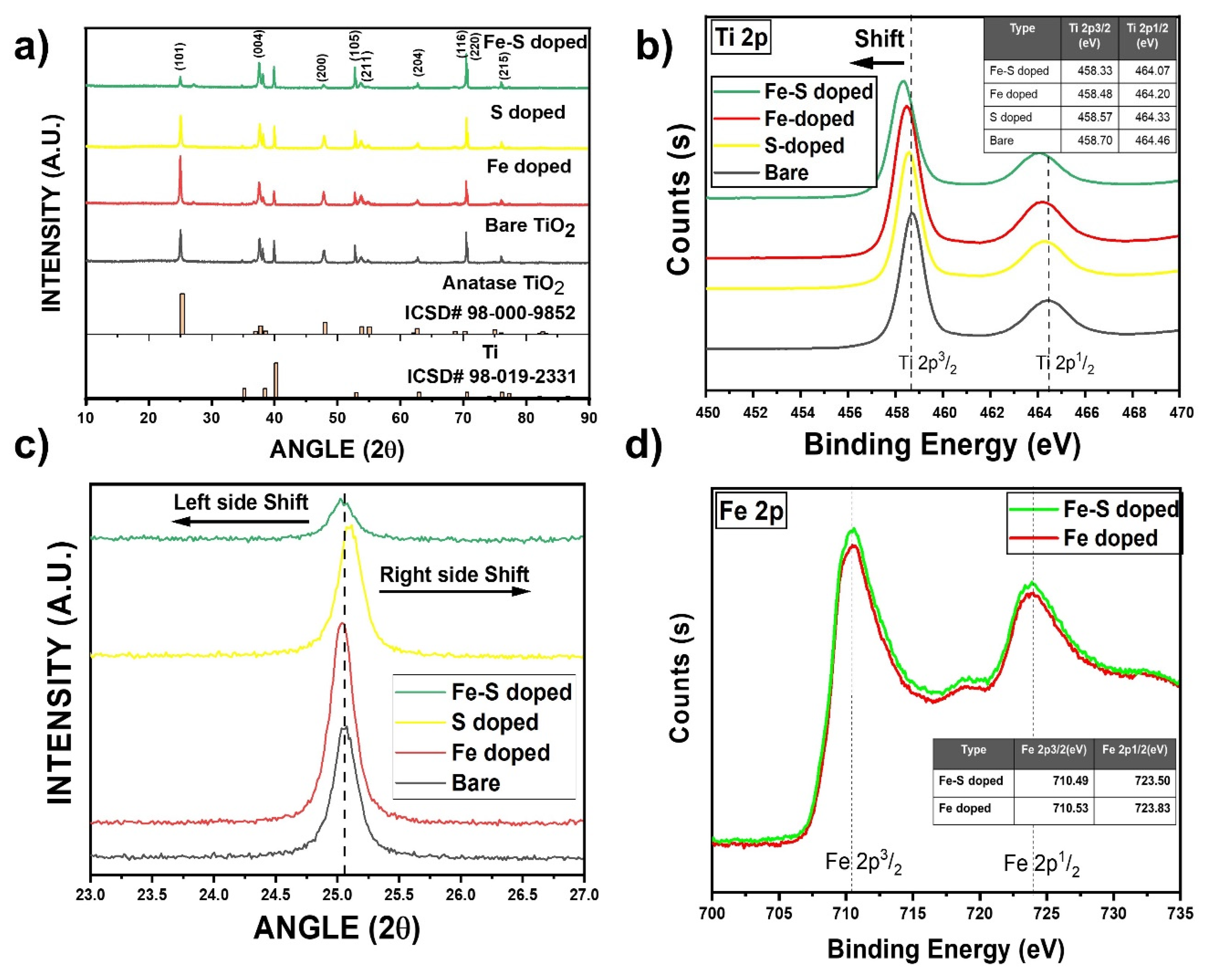
3.1. Morphology and Crystal Structure
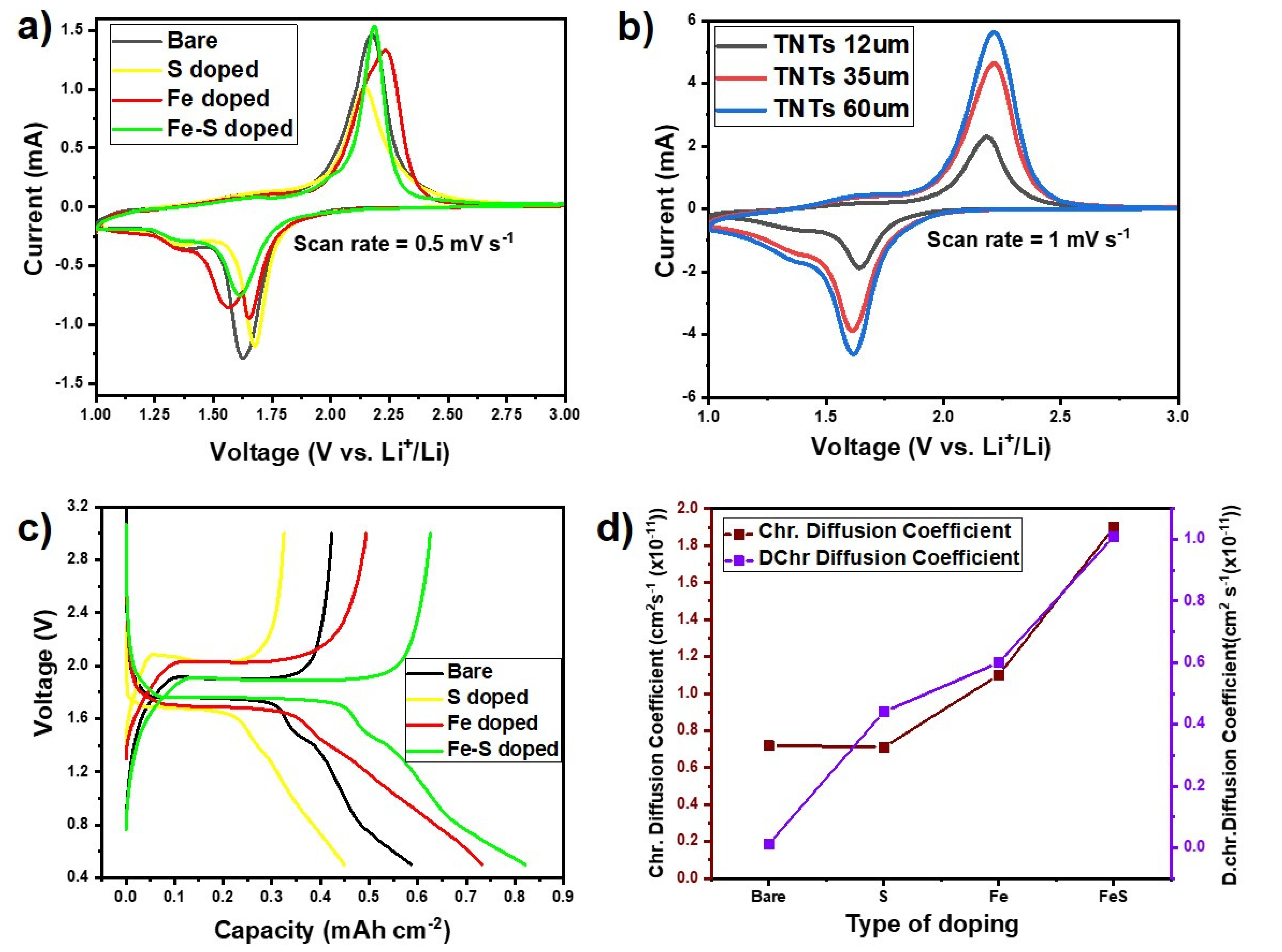
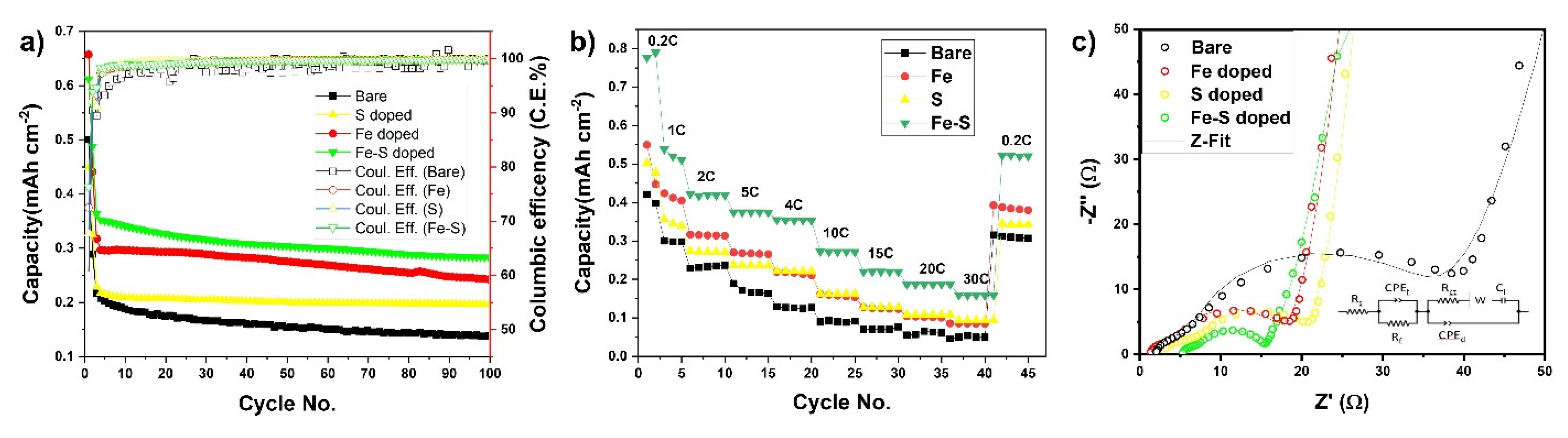
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Proietti Zaccaria, R.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.; Kim, H.; Cho, J. MoS2 Nanoplates consisting of disordered graphene-like layers for high rate lithium battery anode materials. Nano Lett. 2011, 11, 4826–4830. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Kim, G.T.; Bresser, D.; Renger, K.; Paillard, E.; Marassi, R.; Winter, M.; Passerini, S. Investigation of different binding agents for nanocrystalline anatase TiO2 anodes and its application in a novel, green lithium-ion battery. J. Power Sources 2013, 221, 419–426. [Google Scholar] [CrossRef]
- Chen, Z.; Belharouak, I.; Sun, Y.K.; Amine, K. Titanium-based anode materials for safe lithium-ion batteries. Adv. Funct. Mater. 2013, 23, 959–969. [Google Scholar] [CrossRef]
- Wagemaker, M.; Mulder, F.M. Properties and promises of nanosized insertion materials for li-ion batteries. Acc. Chem. Res. 2013, 46, 1206–1215. [Google Scholar] [CrossRef]
- Meng, X.; Banis, M.N.; Geng, D.; Li, X.; Zhang, Y.; Li, R.; Abou-Rachid, H.; Sun, X. Controllable atomic layer deposition of one-dimensional nanotubular TiO2. Appl. Surf. Sci. 2013, 266, 132–140. [Google Scholar] [CrossRef]
- Acevedo-Peña, P.; Haro, M.; Rincón, M.E.; Bisquert, J.; Garcia-Belmonte, G. Facile kinetics of Li-ion intake causes superior rate capability in multiwalled carbon nanotube@TiO2 nanocomposite battery anodes. J. Power Sources 2014, 268, 397–403. [Google Scholar] [CrossRef]
- Guo, Y.G.; Hu, Y.S.; Sigle, W.; Maier, J. Superior electrode performance of nanostructured mesoporousTiO2 (Anatase) through efficient hierarchical mixed conducting networks. Adv. Mater. 2007, 19, 2087–2091. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, X.; Jing, M.; Hou, H.; Zhu, Y.; Fang, L.; Yang, X.; Chen, Q.; Banks, C.E. Carbon dots supported upon N-doped TiO2 nano rods applied into sodium and lithium ion batteries. J. Mater. Chem. A 2015, 3, 5648–5655. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Mazare, A.; Schmuki, P. One-Dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, Y.; Li, Z.; Zhang, W.F. Preparation and electrochemical properties of carbon-doped TiO2 nanotubes as an anode material for lithium-ion batteries. J. Power Sources 2008, 175, 903–908. [Google Scholar] [CrossRef]
- Andriamiadamanana, C.; Laberty-Robert, C.; Sougrati, M.T.; Casale, S.; Davoisne, C.; Patra, S.; Sauvage, F. Room-Temperature synthesis of iron-doped anatase TiO2 for lithium-ion batteries and photocatalysis. Inorg. chem. 2014, 53, 10129–10139. [Google Scholar] [CrossRef] [Green Version]
- Lübke, M.; Johnson, I.; Makwana, N.M.; Brett, D.; Shearing, P.; Liu, Z.; Darr, J.A. High power TiO2 and high capacity Sn-doped TiO2 nanomaterial anode for lithium-ion batteries. J. Power Sources 2015, 294, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Du, F.; Yan, X.; Jin, Y.; Zhu, K.; Wang, X.; Li, H.; Chen, G.; Wang, C.; Wei, Y. Improvements in the electrochemical kinetic properties and rate capability of anatase titanium dioxide nanoparticles by nitrogen doping. ACS Appl. Mater. Interfaces 2014, 6, 4458–4465. [Google Scholar] [CrossRef] [PubMed]
- Lübke, M.; Shin, J.; Marchand, P.; Brett, D.; Shearing, P.; Liu, Z.; Darr, J.A. Highly pseudo capacitive Nb-doped TiO2 high power anodes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 22908–22914. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.N.; Wong, C.L.; Mohamed, A.R. An Overview on the photocatalytic activity of nano-doped-TiO2 in the degradation of organic pollutants. ISRN Mater. Sci. 2011, 2011, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Wang, Z.; Zhang, L.; Wu, H.; Bin, X.; Lou, W. TiO2 nanotube arrays grafted with Fe2O3 hollow nanorods as integrated electrodes for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 122–127. [Google Scholar] [CrossRef]
- Chamanzadeh, Z.; Noormohammadi, M. Self-organized and uniform TiO2 nanotube arrays with optimized NH4F concentration in electrolyte by high voltage electrochemical anodization. Mater. Res. Express 2018, 5, 055025. [Google Scholar] [CrossRef]
- Sabaghi, D.; Madian, M.; Omar, A.; Oswald, S.; Uhlemann, M.; Maghrebi, M.; Baniadam, M.; Mikhailova, D. Directly anodized sulfur-doped TiO2 nanotubes as improved anodes for Li-ion batteries. Batteries 2020, 6, 51. [Google Scholar] [CrossRef]
- Fang, J.; Liu, W.; Yu, F.; Qin, F.; Wang, M.; Zhang, K.; Lai, Y. Fe, S co-doped anatase TiO2 nanotubes as anodes with improved electrochemical performance for lithium ion batteries. RSC Adv. 2016, 6, 70133–70140. [Google Scholar] [CrossRef]
- Macák, J.M.; Tsuchiya, H.; Schmuki, P. High-Aspect-Ratio TiO2 nanotubes by anodization of titanium. Angew. Chem Int. Ed. 2005, 44, 2100–2102. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Albu, S.P.; Ghicov, A.; Macak, J.M.; Schmuki, P. 250 µm long anodic TiO2 nanotubes with hexagonal self-ordering. Physic. Stat. Solid. 2007, 1, R65–R67. [Google Scholar] [CrossRef]
- Macák, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- So, S.; Lee, K.; Schmuki, P. Ultrafast growth of highly ordered anodic TiO2 nanotubes in lactic acid electrolytes. J. Am. Chem. Soc. 2012, 134, 11316–11318. [Google Scholar] [CrossRef]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Macák, J.M.; Tsuchiya, H.; Taveira, L.; Aldabergerova, S.; Schmuki, P. Smooth Anodic TiO2 nanotubes. Angew. Chem. Int. Ed. 2005, 44, 7463–7465. [Google Scholar] [CrossRef]
- Bayati, M.R.; Moshfegh, A.Z.; Golestani-Fard, F. Micro-arc oxidized S-TiO2 nanoporous layers: Cationic or anionic doping? Mater. Lett. 2010, 64, 2215–2218. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, J.; Cheng, B. Effects of Fe-doping on the photocatalytic activity of mesoporous TiO2 powders prepared by an ultrasonic method. J. Hazard. Mater. 2006, 137, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Hiroyuki, U.; Sho, Y.; Kuniaki, W.; Masahiro, S.; Hiroki, S. Nb-doped rutile TiO2: A potential anode material for Na-ion battery. ACS Appl. Mater. Interfaces 2015, 7, 6567–6573. [Google Scholar]
- Yan, D.; Yu, C.; Bai, Y.; Zhang, W.; Chen, T.; Hu, B.; Sun, Z.; Pan, L. Sn-doped TiO2 nanotubes as superior anode materials for sodium ion batteries. Chem. Commun. 2015, 51, 8261–8264. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Fu, S.; Wu, C.; Maier, J.; Yu, Y.; Li, L. Self-Supported nanotube arrays of sulfur-doped TiO2 enabling ultrastable and robust sodium storage. Adv. Mater. 2016, 28, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Piatkowska, A.; Janus, M.; Szymanski, K.; Mozia, S. C-, N- and S-doped TiO2 photo catalysts: A review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Band gap narrowing of titanium dioxide by sulfur doping. Appl. Phys. Lett. 2002, 81, 454–456. [Google Scholar] [CrossRef]
- Ohno, T.; Akiyoshi, M.; Umebayashi, T.; Asai, K.; Mitsui, T.; Matsumura, M. Preparation of S-doped TiO2 photocatalysts and their photocatalytic activities under visible light. Appl. Catal. A Gen. 2004, 265, 115–121. [Google Scholar] [CrossRef]
- Brumbarov, J.; Vivek, J.P.; Leonardi, S.; Valero-Vidal, C.; Portenkirchner, E.; Kunze-Liebhäuser, J. Oxygen deficient, carbon coated self-organized TiO2 nanotubes as anode material for Li-ion intercalation. J. Mater. Chem. A 2015, 3, 16469–16477. [Google Scholar] [CrossRef]
- Guan, D.; Wang, Y. Electrodeposition of Ag nanoparticles onto bamboo-type TiO2 nanotube arrays to improve their lithium-ion intercalation performance. Ionics 2013, 19, 879–885. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Barclay, J.E.; Butt, J.N.; Lomonossoff, G.P.; Evans, D. Redox-active ferrocene-modified cowpea mosaic virus nanoparticles. J. Dalt. Trans. 2010, 39, 7569–7574. [Google Scholar] [CrossRef] [Green Version]
- Arrouvel, C.; Parker, S.C.; Saiful Islam, M. Lithium insertion and transport in the TiO2-B anode Material: A Computational Study. Chem. Mater. 2009, 21, 4778–4783. [Google Scholar] [CrossRef]
- Lee, C.W.; Pang, Q.; Ha, S.; Cheng, L.; Han, S.D.; Zavadil, K.R.; Gallagher, K.G.; Nazar, L.F.; Balasubramanian, M. Directing the lithium−sulfur reaction pathway via sparingly solvating electrolytes for high energy density batteries. ACS Cent. Sci. 2017, 3, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wang, Q.; Kim, J.; Pesaran, A.; Frank, A.J. Pseudocapacitive lithium-ion storage in oriented anatase TiO2 nanotube arrays. J. Phys. Chem. C 2012, 116, 11895–11899. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.P.; Shao, Z. Intercalation pseudocapacitance in electrochemical energy storage: Recent advances in fundamental understanding and materials development. Mater. Today Adv. 2020, 7, 100072. [Google Scholar] [CrossRef]
- Pervez, S.; Kim, D.; Doh, C.H.; Farooq, U.; Choi, H.Y.; Choi, J.H. Anodic WO3 mesosponge @ carbon: A novel binder-less electrode for advanced energy storage devices. ACS Appl. Mater. Interface 2015, 7, 7635–7643. [Google Scholar] [CrossRef] [PubMed]
- Pervez, S.; Kim, D.; Farooq, U.; Yaqub, A.; Choi, J.H.; Lee, Y.J.; Doh, C.-H. Comparative electrochemical analysis of crystalline and amorphous anodized iron oxide nanotube layers as negative electrode for LIB. ACS Appl. Mater. Interfaces 2014, 6, 11219–11224. [Google Scholar] [CrossRef] [PubMed]
- Pervez, S.; Farooq, U.; Yaqub, A.; Doh, C.-H.; Kim, D.; Sim, S.; Hwang, M.; Choi, J.H.; Lee, Y.J. Improved performance of Ag-nanoparticle-decorated TiO2 nanotube Arrays in li-ion batteries. J. Korean Phys. Soc. 2013, 63, 1809–1814. [Google Scholar] [CrossRef]
- Pervez, S.; Kim, D.; Doh, C.H.; Farooq, U.; Yaqub, A.; Choi, J.H.; Lee, Y.J.; Saleem, M. High areal capacity for battery anode using rapidly growing self-ordered TiO2 nanotubes with a high aspect ratio. Mater. Lett. 2014, 137, 347–350. [Google Scholar] [CrossRef]
- Pervez, S.; Kim, D.; Farooq, U.; Yaqub, A.; Choi, J.H.; Lee, Y.J.; Muhammad, S.; Doh, C.-H. Crystalline iron oxide nanotube arrays with high aspect ratio as binder free anode for li-ion batteries. Phys. Status Solidi 2014, 211, 1889–1894. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, M.; Lin, Z.; Li, N.; Liu, Y.; Zhao, B.; Pang, H.; Cao, J.; He, P.; Shi, Y. Activated carbon with ultrahigh specific surface area synthesized from natural plant material for lithium–sulfur batteries. J. Mater. Chem. A 2014, 2, 15889–15896. [Google Scholar] [CrossRef]
- Gauthier, M.; Carney, T.J.; Grimaud, A.; Giordano, L.; Pour, N.; Chang, H.H.; Fenning, D.P.; Lux, S.F.; Paschos, O.; Bauer, C.; et al. The electrode-electrolyte interface in li-ion batteries: Current understanding and new Insights. J. Phys. Chem. Lett. 2015, 6, 4653–4672. [Google Scholar] [CrossRef]
- Wagemaker, M.; Borghols, W.J.H.; Mulder, F.M. Large impact of particle size on insertion reactions. A Case for anatase LixTiO2. J. Am. Chem. Soc. 2007, 129, 4323–4327. [Google Scholar] [CrossRef]
- Auer, A.; Portenkirchner, E.; Götsch, T.; Valero-Vidal, C.; Penner, S.; Kunze Liebhäuser, H. Preferentially oriented TiO2 nanotubes as anode material for li-ion batteries: Insight into li-ion storage and lithiation kinetics. ACS Appl. Mater. Interfaces 2017, 9, 36828–36836. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasarathan, S.; Ali, M.; Jung, T.-J.; Sung, J.; Ha, Y.-C.; Park, J.-W.; Kim, D. Vertically Aligned Binder-Free TiO2 Nanotube Arrays Doped with Fe, S and Fe-S for Li-ion Batteries. Nanomaterials 2021, 11, 2924. https://doi.org/10.3390/nano11112924
Dasarathan S, Ali M, Jung T-J, Sung J, Ha Y-C, Park J-W, Kim D. Vertically Aligned Binder-Free TiO2 Nanotube Arrays Doped with Fe, S and Fe-S for Li-ion Batteries. Nanomaterials. 2021; 11(11):2924. https://doi.org/10.3390/nano11112924
Chicago/Turabian StyleDasarathan, Suriyakumar, Mukarram Ali, Tai-Jong Jung, Junghwan Sung, Yoon-Cheol Ha, Jun-Woo Park, and Doohun Kim. 2021. "Vertically Aligned Binder-Free TiO2 Nanotube Arrays Doped with Fe, S and Fe-S for Li-ion Batteries" Nanomaterials 11, no. 11: 2924. https://doi.org/10.3390/nano11112924
APA StyleDasarathan, S., Ali, M., Jung, T.-J., Sung, J., Ha, Y.-C., Park, J.-W., & Kim, D. (2021). Vertically Aligned Binder-Free TiO2 Nanotube Arrays Doped with Fe, S and Fe-S for Li-ion Batteries. Nanomaterials, 11(11), 2924. https://doi.org/10.3390/nano11112924