Abstract
Selective catalytic reduction (SCR) is the most efficient NOX removal technology, and the vanadium-based catalyst is mainly used in SCR technology. The vanadium-based catalyst showed higher NOX removal performance in the high-temperature range but catalytic efficiency decreased at lower temperatures, following exposure to SOX because of the generation of ammonium sulfate on the catalyst surface. To overcome these limitations, we coated an NH4+ layer on a vanadium-based catalyst. After silane coating the V2O5-WO3/TiO2 catalyst by vapor evaporation, the silanized catalyst was heat treated under NH3 gas. By decomposing the silane on the surface, an NH4+ layer was formed on the catalyst surface through a substitution reaction. We observed high NOX removal efficiency over a wide temperature range by coating an NH4+ layer on a vanadium-based catalyst. This layer shows high proton conductivity, which leads to the reduction of vanadium oxides and tungsten oxide; additionally, the NOX removal performance was improved over a wide temperature range. These findings provide a new mothed to develop SCR catalyst with high efficiency at a wide temperature range.
1. Introduction
Most energy is generated through the combustion of fossil fuels, which causes air pollution by emitting toxic pollutants, such as NOX, particulate matter, SOX, and CO [1,2]. Nitrogen oxides (NO, NO2, and N2O) are the most representative air pollutants because they are major causes of acid rain, photochemical smog, ozone depletion, and global warming [3,4,5]. NOX is emitted by automobiles, power stations, industrial heaters, and non-road vehicles. To reduce this form of pollution, NOX removal technologies must be developed. Many technologies have been developed to control NOX emissions, such as selective catalytic reduction (SCR), non-selective catalytic reduction (NSCR), and selective non-catalytic reduction (SNCR). Selective catalytic reduction (SCR), the most efficient NOX removal technology, selectively converts NOX in fuel gas into N2 and H2O. Compared with other technologies, such as non-selective catalytic reduction (NSCR) and selective non-catalytic reduction (SNCR), SCR shows a high NOX removal efficiency and no secondary pollutant emission and can be operated in the temperature range of 100–300 °C [4].
The V2O5-WO3/TiO2 catalyst has been commercially applied for SCR of NOX with NH3 because of its good thermal stability, N2 selectivity, and low SO2 oxidation activity for conversion to SO3 [6]. However, this catalyst shows high NOX removal performance only in the temperature range of 300–400 °C, and performance decreases at temperatures below 300 °C [7,8]. Therefore, many studies have been conducted to develop catalysts that function over a wide temperature range such as 200–420 °C. Djerad et al. increased the loading of V2O5 in WO3/TiO2 catalysts to improve NOX removal efficiency at lower temperatures [9,10]. A higher V2O5 content leads to increased catalytic activity but decreased N2 selectivity and a narrow activation temperature range. Other researchers modified catalysts using transition metal oxides (Co, Mn, Ce, Cu, etc.) and rare-earth oxides (Eu, Sm, Nd, and Ho) [3,11,12,13,14,15,16,17,18,19]. For example, Wang et al. investigated the effect of adding Cu to a V2O5-WO3/TiO2 catalyst on low-temperature NH3-SCR performance [20]. The added Cu oxides improved the catalytic activity of V2O5 by increasing the redox property of V2O5 (Cu2+ + V4+ ⇄ V5+ + Cu+), leading to a fast SCR reaction. GuO et al. modified Nd on MnOx catalysts to improve NH3 SCR performance [21]. The catalytic activity and N2 selectivity were enhanced by doping MnOx with an Nd catalyst. Although catalytic activity was improved at low temperatures (>300 °C), the catalytic performance decreased at temperatures above 400 °C. Therefore, covering a wide window temperature by modifying only the catalyst remains challenging. Another method for improving NOX removal technology at wide temperature ranges (200–420 °C) involves applying ammonium nitrate (NH4NO3) on the catalysts [22,23,24]. Chae et al. enhanced the catalytic performance of SCR of NOX at temperatures below 300 °C by injecting liquid ammonium nitrate on a V2O5-Sb2O3/TiO2 catalyst. The injected ammonium nitrate led to a fast SCR and prevented the formation of ammonia bisulfate on the catalyst surface and catalytic deactivation by SO2 at temperatures below 300 °C.
In this study, we enhanced the NOX removal efficiency and N2 selectivity over a wide temperature range of 200–420 °C by improving the catalytic activity at temperatures below 300 °C. The catalyst surface was coated with a silane layer, and the silane molecules evaporated with heat treatment at 500 °C in NH3 environment. The resulting catalyst surface was substituted with NH4+ layers present, and the NH4+ layers formed on the surface of the V2O5-WO3/TiO2 catalyst increased NOX removal efficiency at low temperatures (200–250 °C).
2. Materials and Methods
2.1. Catalyst Preparation
The NH4+-coated V2O5-WO3/TiO2 catalyst was prepared in three steps: first, V2O5-WO3/TiO2 was synthesized by the impregnation method, and then, the catalyst underwent vapor phase silane treatment at 180 °C for 1 h. Finally, the silanized V2O5-WO3/TiO2 catalysts were heat treated under NH3 gas with N2 gas.
To synthesize 2 wt.% V2O5 −10 wt.% WO3/TiO2, 8.800 g of TiO2 powder (8.800 g, NANO Co., Ltd., Seoul, Korea, NT-01), 0.256 g of NH4VO3 (Sigma-Aldrich, St. Louis, MO, USA, 99.99%), 1.062 g of (NH4)6H2W12O40 × H2O (Sigma-Aldrich, St. Louis, MO, USA, 99.99%), and 0.386 g of oxalic acid were mixed with 100 mL of deionized water and stirred for 2 h. The solution in the mixture was evaporated at 85 °C in an oil bath for impregnation of V and W atoms followed by drying in an oven at 110 °C for 12 h. The dried powder was placed in a furnace and calcinated at 500 °C under atmospheric pressure.
Vapor phase silanization was conducted. First, the catalyst was cleaned by ozone treatment to remove any impurities and to increase hydroxyl species on the surface. The catalyst was placed in a sealed Teflon jar with 1.0 mL of Trichloro (1H, 1H, 2H, 2H-perfluorooctyl) silane (Sigma-Aldrich, 97%). The jar was subjected to 180 °C to vary the time. To coat the NH4+ on the catalyst, the silanized V2O5-WO3/TiO2 catalyst underwent heat treatment at 500 °C for 2 h upon purging a of NH3 gas (300 ppm) for 2 h.
To observe the effect of the NH4+ layer on the commercial catalyst, we prepared commercial plate-type monoliths. The catalyst, consisting of V2O5, MoO3, and TiO2, was pasted on the wall of the plate, which had dimensions of 1mm × 34mm × 187 mm, and an NH4+ layer coating was performed. After silane coating by vapor deposition, the catalysts were heat treated at 500 °C under 300 ppm NH3 gas with N2 gas for 2 h.
2.2. Catalyst Characterization
The effect of each preparation step on the catalyst morphology was investigated by field emission scanning electron microscopy (Hitachi, Tokyo, Japan, SU8020) at an accelerating voltage of 15.0 kV. The surface properties were determined by measuring the water contact angle using the static sessile drop method with a Contact angle analyzer (Phoenix 300, SEO, Suwon, Korea). The contact angle of the prepared catalysts (thickness = about 0.2 cm) was measured within 10 s after dropping water
The crystallinity of and impurities in the catalysts were analyzed using X-ray diffraction (XRD, Rigaku, Tokyo, Japan, Ultima IV) with Cu Kα (λ = 0.15406 nm) radiation in the 2θ range of 10° to 80° at a scan rate of 1°/min. The Si and N elements in the catalysts were measured by X-ray photoelectron spectroscopy (XPS; Thermo VG Scientific, Waltham, MA, USA, K Alpha+) with Al Kα radiation; the binding energy of C1s was normalized to 284.8 eV. Fourier transform infrared spectroscopy (FT-IR, Varian Medical Systems, Palo Alto, CA, USA, 670 FTIR) was carried out over a wavelength range of 400–4000 cm−1.
H2-temperature-programmed reduction was carried out using an AutoChem II 2920 (Micromeritics Instrument Corp., Norcross, GA, USA). The samples were exposed to a current of 10% H2/Ar and measured in the 200–900 °C temperature range.
2.3. Catalytic Activity Measurement
The NOX removal efficiency of the catalysts was evaluated in a fixed-bed reactor under high atmospheric pressure, with the sample placed on a stainless-steel tube and analyzed using a powder catalyst. The analysis temperature was varied from 150 °C to 420 °C, and the reactive gas was composed of 300 ppm NOX, 300 ppm NH3 (NH3/NOX = 1.0), 300 ppm SO2, and 5 vol.% of O2, with a N2 balance under a total flow rate of 500 sccm. The powder catalyst (0.35 g) was tested, and the gas hourly space velocity was set at 60,000 h−1. Plate-type commercial catalysts were evaluated in a microreactor. The analysis temperature was varied from 200 °C to 450 °C, and the reactive gas was composed of 240 ppm NOX, 288 ppm NH3 (NH3/NOX = 1.2), 600 ppm SO2, and 3 vol.% of O2, with a N2 balance under a total flow rate of 1.2 m3/h; the area velocity was set to 25 m/h. The reactive gas concentration was determined by FT-IR (Gasmet, Vantaa, Finland, CX-4000) and gas analysis spectrometry (DSMXT, Heidelberg, Germany). NOX removal efficiency and N2 selectivity were calculated according to Equations (1) and (2), respectively.
3. Results
Figure 1 illustrates the process of producing the NH4+ layer on the V2O5-WO3/TiO2 catalysts. The surface of the synthesized V2O5-WO3/TiO2 catalysts is hydrophilic because of the presence of numerous oxygen groups. After the catalysts were silanized at 180 °C for 1 h, the surface properties became hydrophobic because of the hydrophobic agent in silane [25]. When the catalysts were heat treated at 500 °C under 300 ppm NH3 gas with N2 gas for 2h, the silane molecules underwent thermal decomposition. Subsequently, the catalyst surface acquired NH4+ on their active sites. As a result, the V2O5-WO3/TiO2 catalysts were covered with NH4+ layers, generating hydrophilic surface properties.
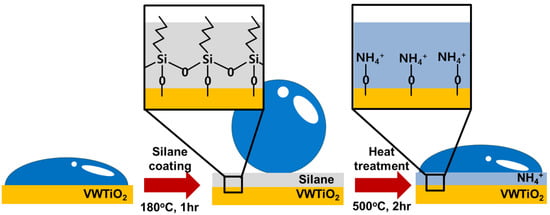
Figure 1.
Schematic illustration of V2O5-WO3/TiO2, silanized V2O5-WO3/TiO2, and heat-treated V2O5-WO3/TiO2 with NH4+ layer coating.
The surface properties and morphology of the prepared catalysts were investigated using a scanning electron microscopy, and contact angle measurements. Figure 2a,d show the V2O5-WO3/TiO2 catalysts without treatment. Figure 2b,c,e,f show the results obtained for the catalyst after silane treatment and the following heat treatment, respectively. To observe the surface properties, we dropped water on the surface of the prepared SCR catalyst powders; the microscope observation results are shown in Figure 2a–c. Water that dropped on the surface was immediately absorbed into the original V2O5-WO3/TiO2 catalyst. The inset image shows the results of static contact angle measurement. The observed contact angle was approximately 2°, indicating that the catalysts were hydrophilic. After silane treatment of the V2O5-WO3/TiO2 catalyst, the water did not spread on the surface but rather formed a drop on the surface with a contact angle of approximately 160°. As the hydrophobic agent in silane covered the catalyst, the surface became hydrophobic [26]. However, the surface properties became hydrophilic after heat treating the catalyst at 500 °C for 2h under NH3 gas. This is because the silane molecules are thermally decomposed at a high temperature (e.g., 500 °C). Subsequently, NH3 can react to the active sites of catalyst and form NH4+ on the surface as a monolayer. Although the surface properties were changed by each process, the catalyst morphologies (Figure 2d–f), catalyst contents, and the textural properties (Table 1) were not changed because the prepared silane and NH4+ layers are quite thin.
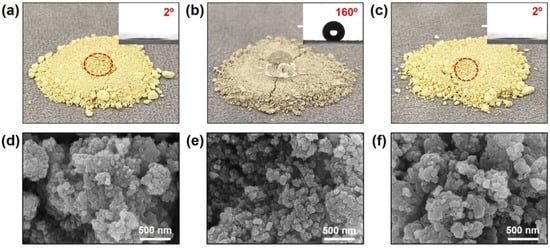
Figure 2.
Microscope images of V2O5-WO3/TiO2 (a), silanized V2O5-WO3/TiO2 (b), and heat-treated V2O5-WO3/TiO2 (c) (insets are contact angle measurement). The red circle marks the location where the water is absorbed on the catalyst. SEM images of V2O5-WO3/TiO2 (d), silanized V2O5-WO3/TiO2 (e), and heat-treated V2O5-WO3/TiO2 (f).

Table 1.
X-ray fluorescence analysis and Brunauer-Emmet-Teller (BET) results of the V2O5-WO3/TiO2, silanized V2O5-WO3/TiO2, and heat-treated V2O5-WO3/TiO2 catalyst.
The crystalline structures of the prepared catalysts were determined using XRD (Figure 3a), which clearly showed anatase TiO2, whereas peaks for V2O5 and WO3 were not observed in any samples. This is because the V2O5 and WO3 catalysts with small nanoparticle sizes were uniformly coated on the TiO2 support, and the peaks of the V2O5 and WO3 catalysts were hard to detect using XRD [27]. Furthermore, the peak intensities and positions were similar for all catalysts, indicating that silane and heat treatments under NH3 gas did not affect the crystallinity of the V2O5-WO3/TiO2 catalyst.
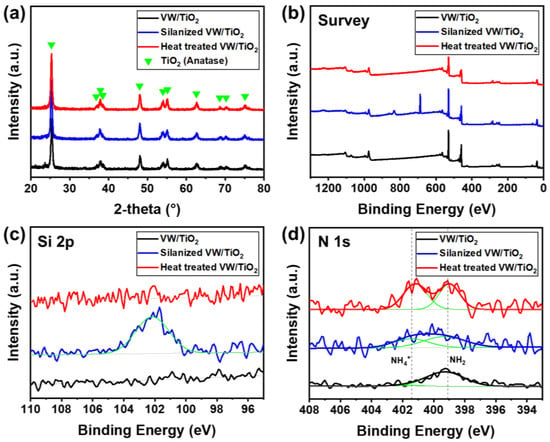
Figure 3.
(a) XRD patterns and XPS spectra for (b) survey, (c) Si 2p, and (d) N 1s of V2O5-WO3/TiO2 (black line), silanized V2O5-WO3/TiO2 (blue line), and heat-treated V2O5-WO3/TiO2 (red line).
To observe the silane layer and difference in chemical bonding in the catalyst after the heat-treatment process, XPS analysis was conducted (Figure 3b–d). Figure 3b shows the survey peaks in the XPS spectra. The XPS spectra of the Si 2p peak of the V2O5-WO3/TiO2 catalyst (black line), silanized V2O5-WO3/TiO2 catalyst (blue line), and following catalyst after heat treatment (red and green lines) are shown in Figure 3c. In the V2O5-WO3/TiO2 catalyst, no Si 2p peak was observed. After silane treatment, silicon atoms appeared at low binding energy (102 eV), indicating that silane was coated on the V2O5-WO3/TiO2 catalyst. However, the peaks at 102 eV disappeared after heat treatment of the silanized V2O5-WO3/TiO2 catalyst, indicating that the coated silane had decomposed at temperatures over 500 °C. By contrast, binding energies near 401 and 399 eV were observed for the heat-treated V2O5-WO3/TiO2 catalyst (Figure 3d). The intensity of V2O5-WO3/TiO2 catalyst and silanized V2O5-WO3/TiO2 catalyst were lower, but the intensity was larger for the heat-treated V2O5-WO3/TiO2 catalyst. The binding energies were attributed to the neutral amine’s residual on the surface and NH4+, respectively, from the flowing NH3 gas during the heat-treatment process [28,29]. The NH2 peak at around 399 eV came from the use of ammonium-based precursors of V and W, and the NH2 peak is observed in all samples. Table 2 and S1 show the atomic contents of the prepared catalysts obtained from XPS spectra. Heat-treated V2O5-WO3/TiO2 catalyst had increased N concentration of 0.79 at.% compared with the V2O5-WO3/TiO2 catalyst (0.40 at.%) and the Silanized V2O5-WO3/TiO2 catalyst (0.31 at.%). The results indicate that the NH4+ layer is formed on the heat-treated V2O5-WO3/TiO2 catalyst.

Table 2.
Atomic percent of element obtained from XPS spectra of the V2O5-WO3/TiO2, silanized V2O5-WO3/TiO2, heat-treated V2O5-WO3/TiO2 catalyst.
The NOX removal efficiency and N2 selectivity following the formation of the NH4+ layer on the surface of the V2O5-WO3/TiO2 catalyst were measured in a fixed bed (Figure 4). Figure 4a shows the NOX removal efficiency of the prepared samples, which were higher for the silanized catalyst and heated silanized catalyst than for the original V2O5-WO3/TiO2 catalyst below 300 °C. The catalytic performance of the silanized V2O5-WO3/TiO2 catalyst improved from 45% to 60%, particularly at 200 °C. Figure 4b shows the N2 selectivity; a trace amount of N2O in the prepared catalyst was produced at 300 °C. The trend in N2 selectivity was similar to the NOX removal efficiency, and the heat-treated V2O5-WO3/TiO2 catalyst showed a higher N2 selectivity at 420 °C. Sulfur in the fuel deactivated the catalyst through the poisoning effect, specifically in the low-temperature range (below 300 °C). When vanadium catalysts are exposed to SOx, ammonium bisulfate (NH4HSO4) and ammonium sulfate ((NH4)2SO4) are formed on the catalyst surface, decreasing efficiency [24]. The hydrophobic properties of the coated silane prevent the formation of ammonium sulfate on the V2O5-WO3/TiO2 catalyst surface, enhancing the NOX removal efficiency at low temperatures. Interestingly, the NOX removal efficiency of the silanized catalyst after heat treatment was higher than that of the silanized and original catalysts. The NH4+ intermediates in the catalyst exhibit high catalytic activity and proton conductivity in NH3 SCR, particularly in the low-temperature range. Therefore, the NH4+ layer on the catalyst produced by silane and heat treatments leads to enhanced NOX removal efficiency at low temperatures, specifically at 250 °C, because of the higher proton conductivity and reactivity [30], whereas silane is decomposed and reacts with NH3 at over 300 °C in the reaction gas during fixed bed evaluation, and some NH4+ is produced on the catalyst surface. Therefore, the silanized and heat-treated V2O5-WO3/TiO2 catalysts show similar catalytic performances at high temperatures (>300 °C). Note that there was no significant difference in the NOX removal efficiency and N2 selectivity between V2O5-WO3/TiO2 and heat-treated V2O5-WO3/TiO2 without a silane coating process in the temperature range of 150–420 °C (Figure S1). This results demonstrate that silane coating is a key process to prepare ammonium ion enhanced V2O5-WO3/TiO2 catalysts.
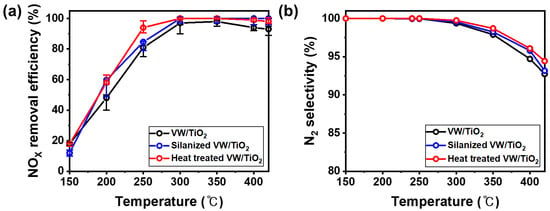
Figure 4.
NOx removal efficiency (a) and N2 selectivity (b) of V2O5-WO3/TiO2 (black line), silanized V2O5-WO3/TiO2 (blue line), and heat-treated V2O5-WO3/TiO2 (red line). Reaction conditions: [NO] = 300 ppm, [NH3] = 300 ppm, [SO2] = 600 ppm, [O2] = 5 vol.%, and [gas hourly space velocity] = 60,000 h−1.
The formed NH4+ layer and acid sites were analyzed using FT-IR spectra, as shown in Figure 5a–c. Si-CH2 and Si-O-Si bond peaks only appeared at 1250 and 1216 cm−1, respectively, in the silanized V2O5-WO3/TiO2 catalyst (Figure 5b). After heat treatment, the peaks related to Si (e.g., 1250 and 1216 cm−1) disappeared while various peaks related to NH4+ started to appear in the range of 1400 to 1600 cm−1. Furthermore, the peak near 3300 cm−1 corresponds to N–H stretching (Figure 5c). According to these results, the coated silane layer disappeared during heat treatment, and a new NH4+ layer was generated after the decomposition of Si atoms on the surface. To investigate the redox properties of the prepared catalysts depending on the silane treatment and formation of the NH4+ layer, we analyzed the H2-temperature-programmed reduction profiles (Figure 5d). V2O5-WO3/TiO2 exhibited three reduction peaks at 397.1 °C, 480.2 °C, and 800.8 °C, which were assigned to the reduction of V5+ to V3+ in the vanadium oxides, the reduction of W6+ to W4+, and the reduction of W4+ to W0 in the tungsten oxide, respectively [31,32,33]. After coating the NH4+ layer on the V2O5-WO3/TiO2 catalyst surface, the reduction peaks shifted to lower temperatures of 385.6 °C, 461.2 °C, and 778.7 °C, respectively. As the NH4+ layer on the surface has high proton conductivity and activity, the active materials reduced a larger amount of NOX.
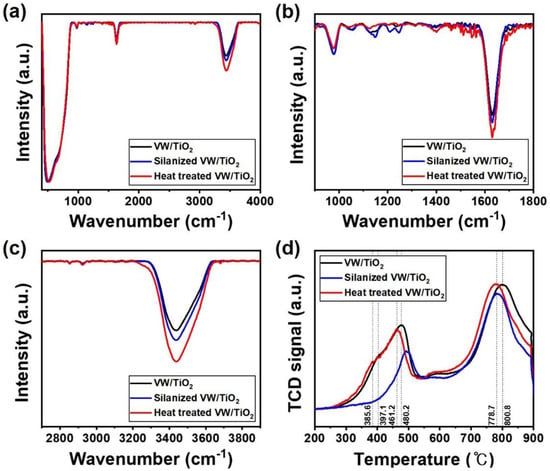
Figure 5.
Fourier transform infrared spectra (a–c) and H2-temperature-programmed reduction (d) of V2O5-WO3/TiO2 (black line), silanized V2O5-WO3/TiO2 (blue line), and heat-treated V2O5-WO3/TiO2 (red line).
To investigate the effect of the NH4+ layer under actual conditions, we applied the NH4+ layer on the commercial catalyst (Figure 6). Figure 6a shows a plate-type commercial catalyst containing V2O5, MoO3, TiO2, etc. (Table S2), which is used in electric power stations to reduce NOX gas emissions. The catalyst surface was hydrophilic, similar to the power type of the catalyst (Figure 6b). Silane treatment was conducted by the vapor evaporation method, and the surface became hydrophobic (Figure 6c). Finally, after heat treating the silanized catalysts at 500 °C for 2h under NH3 gas with N2 gas, the surface properties were hydrophilic because of decomposition of the silane layer and because of coating the NH4+ layer on the catalyst surface (Figure 6d).
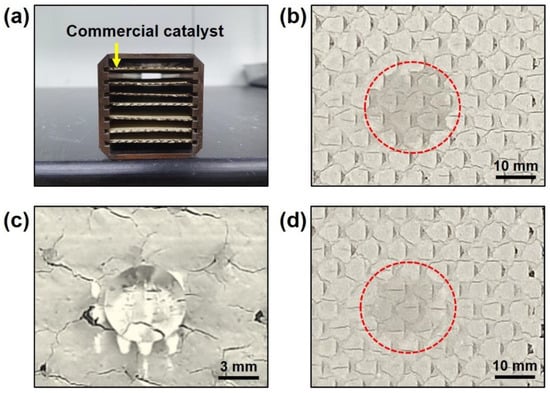
Figure 6.
Microscope image of plate-type commercial catalyst (a), commercial catalyst surface (b), silanized commercial catalyst surface (c), and heat-treated commercial catalyst surface (d) after dropping water.
The prepared plate-type commercial catalysts were evaluated in a microreactor from 200 °C to 450 °C, as shown in Figure 7. The commercial catalysts exhibited a lower NOX removal efficiency at 200 °C, which gradually increased to 400 °C. The commercial catalyst specialized in high temperature range over 300 °C because it used an electric power plant. Therefore, the NOX removal efficiency is not largely changed at high temperature. After forming the NH4+ layer on the catalyst, the NOX removal efficiency was enhanced over a wide window range from 200 °C to 450 °C, particularly at 200 °C. Usually, the catalytic efficiency deteriorated at lower temperature due to generating ammonium sulfate on the catalyst surface. However, the formed NH4+ layer has high proton conductivity, which leads to the reduction of vanadium oxides and tungsten oxide, and improved the NOX removal efficiency.
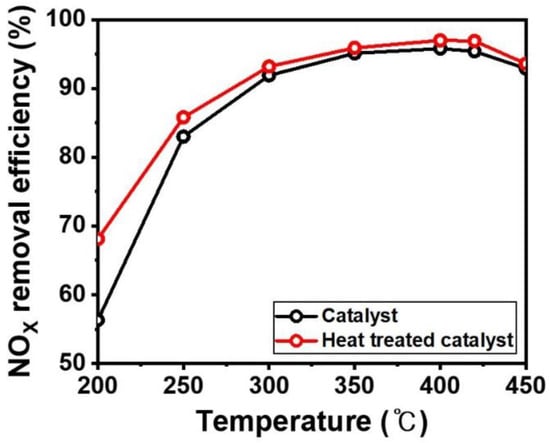
Figure 7.
NOx removal efficiency of V2O5-WO3/TiO2 (black line) and heat-treated V2O5-WO3/TiO2 (red line). Reaction conditions: [NO] = 240 ppm [NH3] = 288 ppm, [SO2] = 600 ppm, [O2] = 3 vol.%, [area velocity] = 25 m/h.
4. Conclusions
We investigated the effect of coating of V2O5-WO3/TiO2 catalysts with an NH4+ layer through heat treatment of silanized catalysts at 500 °C under NH3 gas with N2 gas. The silane molecules were substituted with NH4+ by decomposing the silane layer on the surface. V2O5-WO3/TiO2 catalysts with an NH4+ layer showed an increased NOX removal efficiency over a wide temperature range (150–420 °C) compared with the V2O5-WO3/TiO2 catalysts. Particularly, the performance was improved at low temperatures (<300 °C). The coated NH4+ layer led to enhanced proton conductivity and promoted further reduction of vanadium oxides and tungsten oxide. The V2O5-WO3/TiO2 catalysts with an NH4+ layer exhibited improved SCR performance. These results may contribute to future studies on SCR catalysts and other catalyst systems.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nano11102677/s1, Figure S1: NOx removal efficiency (a) and N2 selectivity (b) of V2O5-WO3/TiO2 (black line) and heat-treated V2O5-WO3/TiO2 without the silane coating process (red line). Reaction conditions: [NO] = 300 ppm, [NH3] = 300 ppm, [SO2] = 600 ppm, [O2] = 5 vol.%, and GHSV = 60,000 h−1., Table S1: Atomic percent of elements obtained from the XPS spectra of the heat-treated V2O5-WO3/TiO2 catalyst without silane coating process., Table S2: X-ray fluorescence analysis of the commercial plate-type monoliths.
Author Contributions
Conceptualization, G.K. and D.H.L.; methodology, B.J., J.-W.P., and T.K.; validation, J.W.L.; writing—original draft preparation, M.S.L. and S.-I.K.; writing—review and editing, G.K. and D.H.L.; project administration, D.H.L. All authors have read and agreed to the published version of the manuscript.
Funding
D.H.L. was supported by the Korea Institute of Industrial Technology (KITECH), (grant number IZ200073) and the Ministry of Trade, Industry, and Energy, South Korea (MOTIE) (grant number 20005721). G.K. was supported by Hanwha Solution Corporation (grant number 43041).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A review of low temperature NH3-SCR for removal of NOX. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.J.; Kim, D.H.; Lee, M.; Ye, B.; Jeong, B.; Lee, D.; Kim, H.D.; Lee, H. Enhanced NOX removal efficiency for SCR catalyst of well-dispersed Mn-Ce nanoparticles on hexagonal boron nitride. Environ. Sci. Pollut. Res. 2019, 26, 36107–36116. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.; Liu, B.; Sun, S. A review of Mn-containing oxide catalysts for low temperature selective catalytic reduction of NOX with NH3: Reaction mechanism and catalyst deactivation. RSC Adv. 2017, 7, 26226–26242. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.; Li, C.; Lu, P.; Qu, L.; Zhang, M.; Zhou, Y.; Yu, M.; Fang, Y. A review on selective catalytic reduction of NOX by supported catalysts at 100–300 C—catalysts, mechanism, kinetics. Catal. Sci. Technol. 2014, 4, 14–25. [Google Scholar] [CrossRef]
- Ye, B.; Lee, M.; Jeong, B.; Kim, J.; Lee, D.H.; Baik, J.M.; Kim, H.D. Partially reduced graphene oxide as a support of Mn-Ce/TiO2 catalyst for selective catalytic reduction of NOX with NH3. Catal. Today 2019, 328, 300–306. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, S.I.; Lee, M.J.; Ye, B.; Kim, T.; Kim, H.D.; Lee, J.W.; Lee, D.H. Effect of Catalyst Crystallinity on V-Based Selective Catalytic Reduction with Ammonia. Nanomaterials 2021, 11, 1452. [Google Scholar] [CrossRef]
- Zhang, X.; Diao, Q.; Hu, X.; Wu, X.; Xiao, K.; Wang, J. Modification of V2O5-WO3/TiO2 Catalyst by Loading of MnOx for Enhanced Low-Temperature NH3-SCR Performance. Nanomaterials 2020, 10, 1900. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Ye, B.; Kim, E.-S.; Kim, H.-D. Characteristics of selective catalytic reduction (SCR) catalyst adding graphene-tungsten nanocomposite. Catal. Commun. 2017, 93, 15–19. [Google Scholar] [CrossRef]
- Lai, J.-K.; Wachs, I.E. A Perspective on the Selective Catalytic Reduction (SCR) of NO with NH3 by Supported V2O5-WO3/TiO2 Catalysts. ACS Catal. 2018, 8, 6537–6551. [Google Scholar] [CrossRef]
- Djerad, S.; Tifouti, L.; Crocoll, M.; Weisweiler, W. Effect of vanadia and tungsten loadings on the physical and chemical characteristics of V2O5-WO3/TiO2 catalysts. J. Mol. Catal. A Chem. 2004, 208, 257–265. [Google Scholar] [CrossRef]
- Chen, J.; Peng, G.; Liang, T.; Zhang, W.; Zheng, W.; Zhao, H.; Guo, L.; Wu, X. Catalytic Performances of Cu/MCM-22 Zeolites with Different Cu loadings in NH3-SCR. Nanomaterials 2020, 10, 2170. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, C.; Li, X.; Tan, P.; Zhou, A.; Fang, Q.; Chen, G. Ho-modified Mn-Ce/TiO2 for low-temperature SCR of NOX with NH3: Evaluation and characterization. Chin. J. Catal. 2018, 39, 1653–1663. [Google Scholar] [CrossRef]
- Huang, J.; Huang, H.; Jiang, H.; Liu, L. The promotional role of Nd on Mn/TiO2 catalyst for the low-temperature NH3-SCR of NOX. Catal. Today 2019, 332, 49–58. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G. A highly effective catalyst of Sm-MnOx for the NH3-SCR of NOX at low temperature: Promotional role of Sm and its catalytic performance. ACS Catal. 2015, 5, 5973–5983. [Google Scholar] [CrossRef]
- Sun, P.; Guo, R.-T.; Liu, S.-M.; Wang, S.-X.; Pan, W.-G.; Li, M.-Y. The enhanced performance of MnOx catalyst for NH3-SCR reaction by the modification with Eu. Appl. Catal. A Gen. 2017, 531, 129–138. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tran, D.; Burton, S.D.; Szanyi, J.; Lee, J.H.; Peden, C.H.F. Effects of hydrothermal aging on NH3-SCR reaction over Cu/zeolites. J. Catal. 2012, 287, 203–209. [Google Scholar] [CrossRef]
- Guo, R.-T.; Li, M.-Y.; Sun, P.; Pan, W.-G.; Liu, S.-M.; Liu, J.; Sun, X.; Liu, S.-W. Mechanistic Investigation of the Promotion Effect of Bi Modification on the NH3–SCR Performance of Ce/TiO2 Catalyst. J. Phys. Chem. C 2017, 121, 27535–27545. [Google Scholar] [CrossRef]
- Hu, H.; Xie, J.L.; Fang, D.; He, F. Study of Co-Mn/TiO2 SCR catalyst at low temperature. In Advanced Materials Research; Trans Tech Publications Ltd.: Zürich, Switzerland, 2015; Volume 1102, pp. 11–16. [Google Scholar]
- Ou, X.; Chen, K.; Wei, L.; Deng, Y.; Li, J.; Li, B.; Dong, L. Effect of Co Doping on Magnetic and CO-SCR Properties of γ-Fe2O3. Ind. Eng. Chem. Res. 2021, 60, 5744–5757. [Google Scholar] [CrossRef]
- Shen, M.; Li, C.; Wang, J.; Xu, L.; Wang, W.; Wang, J. New insight into the promotion effect of Cu doped V2O5/WO3–TiO2 for low temperature NH3-SCR performance. RSC Adv. 2015, 5, 35155–35165. [Google Scholar] [CrossRef]
- Guo, R.T.; Sun, P.; Pan, W.G.; Li, M.Y.; Liu, S.M.; Sun, X.; Liu, S.W.; Liu, J. A Highly Effective MnNdOx Catalyst for the Selective Catalytic Reduction of NOX with NH3. Ind. Eng. Chem. Res. 2017, 56, 12566–12577. [Google Scholar] [CrossRef]
- Liu, K.; Liu, F.; Xie, L.; Shan, W.; He, H. DRIFTS study of a Ce–W mixed oxide catalyst for the selective catalytic reduction of NOX with NH3. Catal. Sci. Technol. 2015, 5, 2290–2299. [Google Scholar] [CrossRef]
- Forzatti, P.; Nova, I.; Tronconi, E. New enhanced NH3-SCR reaction for NOX emission control. Ind. Eng. Chem. Res. 2010, 49, 10386–10391. [Google Scholar] [CrossRef]
- Bae, Y.K.; Kim, T.-W.; Kim, J.-R.; Kim, Y.; Ha, K.-S.; Chae, H.-J. Enhanced SO2 tolerance of V2O5-Sb2O3/TiO2 catalyst for NO reduction with co-use of ammonia and liquid ammonium nitrate. J. Ind. Eng. Chem. 2021, 96, 277–283. [Google Scholar] [CrossRef]
- Kobaku, S.P.R.; Kota, A.K.; Lee, D.H.; Mabry, J.M.; Tuteja, A. Patterned Superomniphobic-Superomniphilic Surfaces: Templates for Site-Selective Self-Assembly. Angew. Chem. 2012, 124, 10256–10260. [Google Scholar] [CrossRef]
- Golovin, K.; Lee, D.H.; Mabry, J.M.; Tuteja, A. Transparent, Flexible, Superomniphobic Surfaces with Ultra-Low Contact Angle Hysteresis. Angew. Chem. 2013, 125, 13245–13249. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Huang, W.; Xu, H.; Chen, T.; Ke, Y.; Qu, Z.; Yan, N. Alkali-induced deactivation mechanism of V2O5-WO3/TiO2 catalyst during selective catalytic reduction of NO by NH3 in aluminum hydrate calcining flue gas. Appl. Catal. B Environ. 2020, 270, 118872. [Google Scholar] [CrossRef]
- Kang, H.; Hong, S.; Lee, J.; Lee, K. Electrostatically Self-Assembled Nonconjugated Polyelectrolytes as an Ideal Interfacial Layer for Inverted Polymer Solar Cells. Adv. Mater. 2012, 24, 3005–3009. [Google Scholar] [CrossRef]
- Liu, G.; Tao, C.; Zhang, M.; Gu, X.; Meng, F.; Zhang, X.; Chen, Y.; Ruan, S. Effects of surface self-assembled NH4+ on the performance of TiO2-based ultraviolet photodetectors. J. Alloy. Compd. 2014, 601, 104–107. [Google Scholar] [CrossRef]
- Chen, P.; Jabłońska, M.; Weide, P.; Caumanns, T.; Weirich, T.E.; Muhler, M.; Moos, R.; Palkovits, R.; Simon, U. Formation and Effect of NH4+ Intermediates in NH3–SCR over Fe-ZSM-5 Zeolite Catalysts. ACS Catal. 2016, 6, 7696–7700. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.; Wang, L.; Song, M.; Yang, L.; Shen, K.; Xu, H.; Zhou, C. Characterization and activity of V2O5-CeO2/TiO2-ZrO2 catalysts for NH3-selective catalytic reduction of NOX. Chin. J. Catal. 2015, 36, 1701–1710. [Google Scholar] [CrossRef]
- Wu, X.; Yu, W.; Si, Z.; Weng, D. Chemical deactivation of V2O5-WO3/TiO2 SCR catalyst by combined effect of potassium and chloride. Front. Environ. Sci. Eng. 2013, 7, 420–427. [Google Scholar] [CrossRef]
- Lee, M.; Ye, B.; Jeong, B.; Chun, H.-Y.; Lee, D.H.; Park, S.-S.; Lee, H.; Kim, H.-D. Reduced graphene oxide supported V2O5-WO3-TiO2 catalysts for selective catalytic reduction of NOX. Korean J. Chem. Eng. 2018, 35, 1988–1993. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).