Advances Achieved by Ionic-Liquid-Based Materials as Alternative Supports and Purification Platforms for Proteins and Enzymes
Abstract
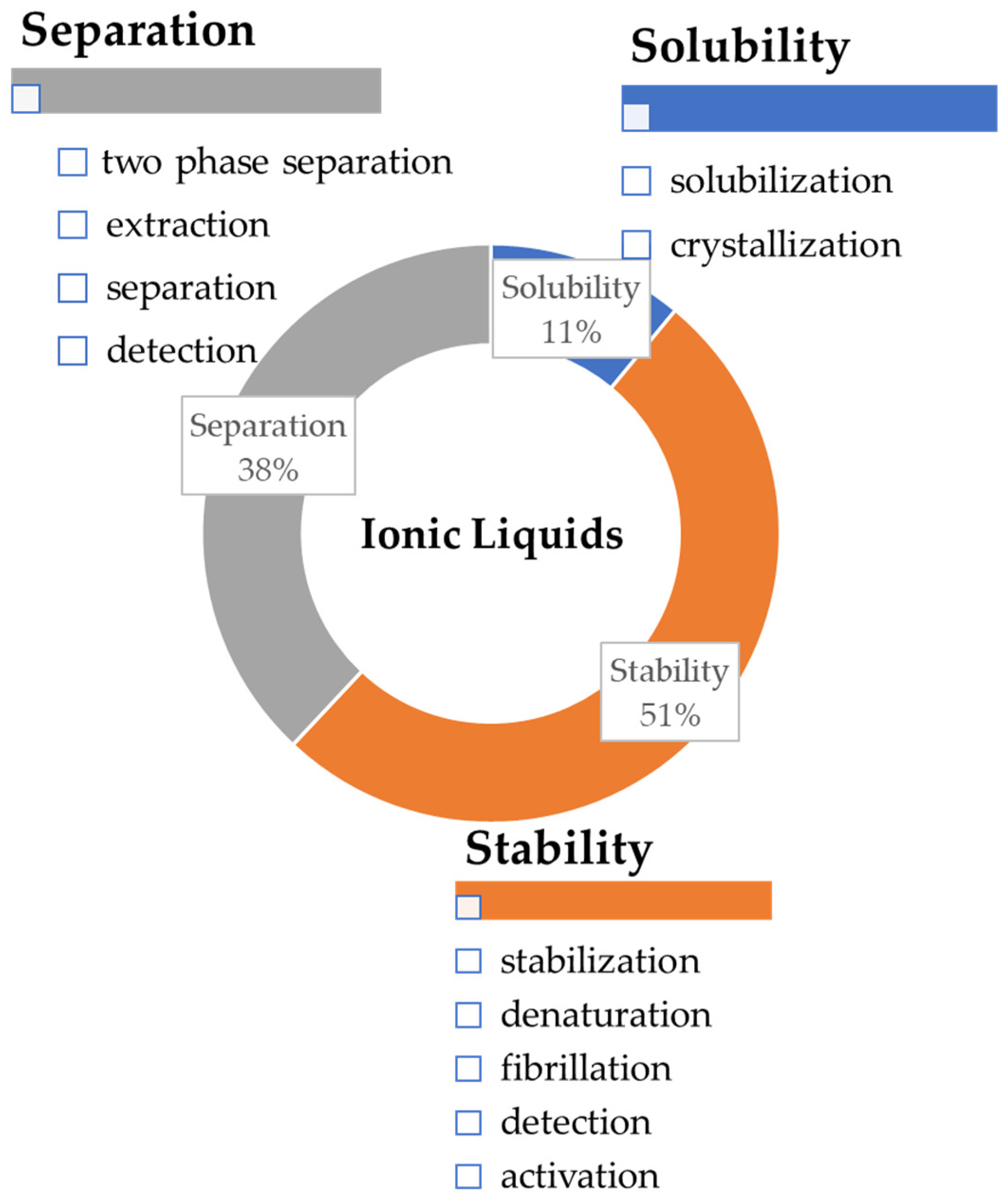
:1. Introduction
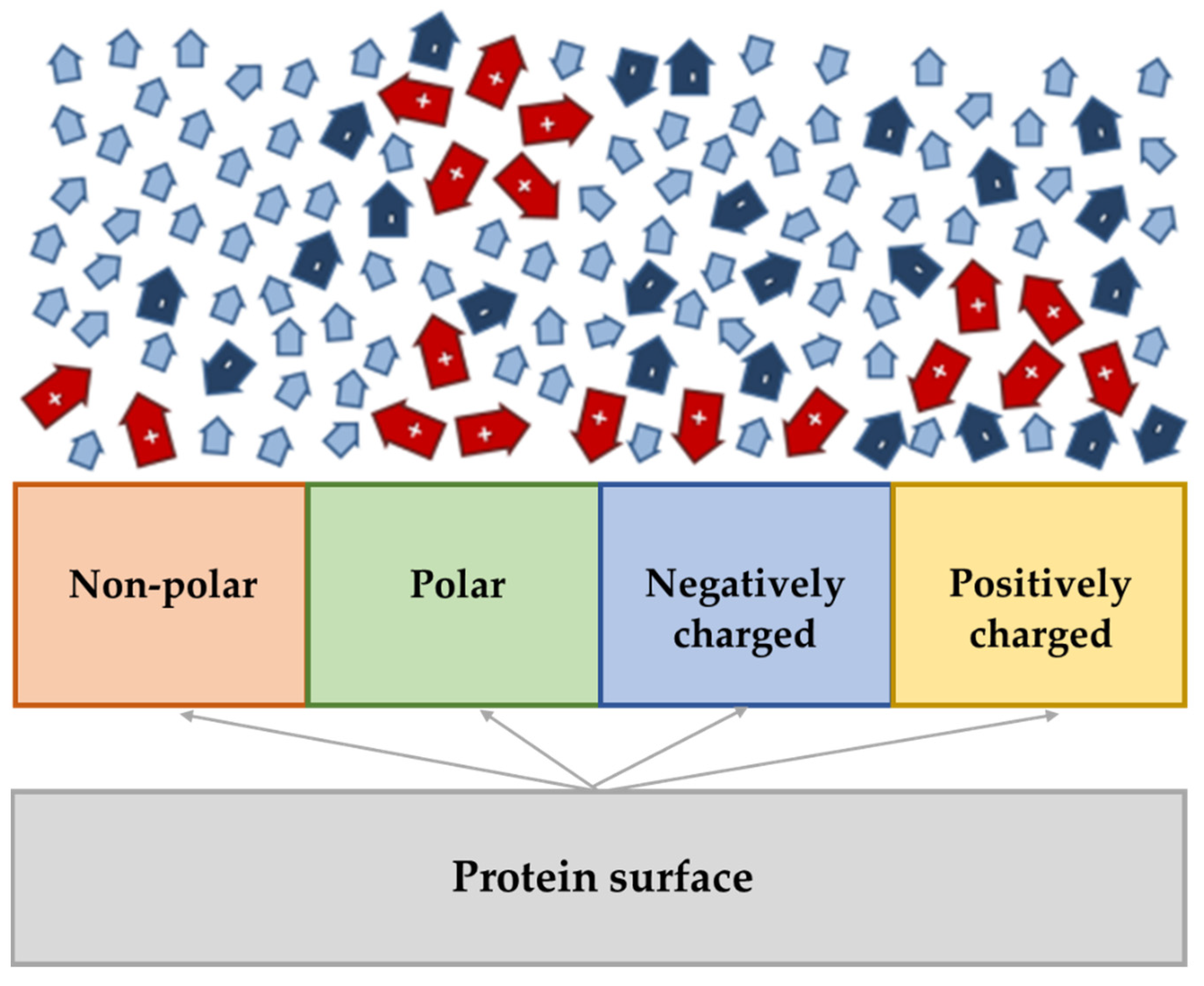
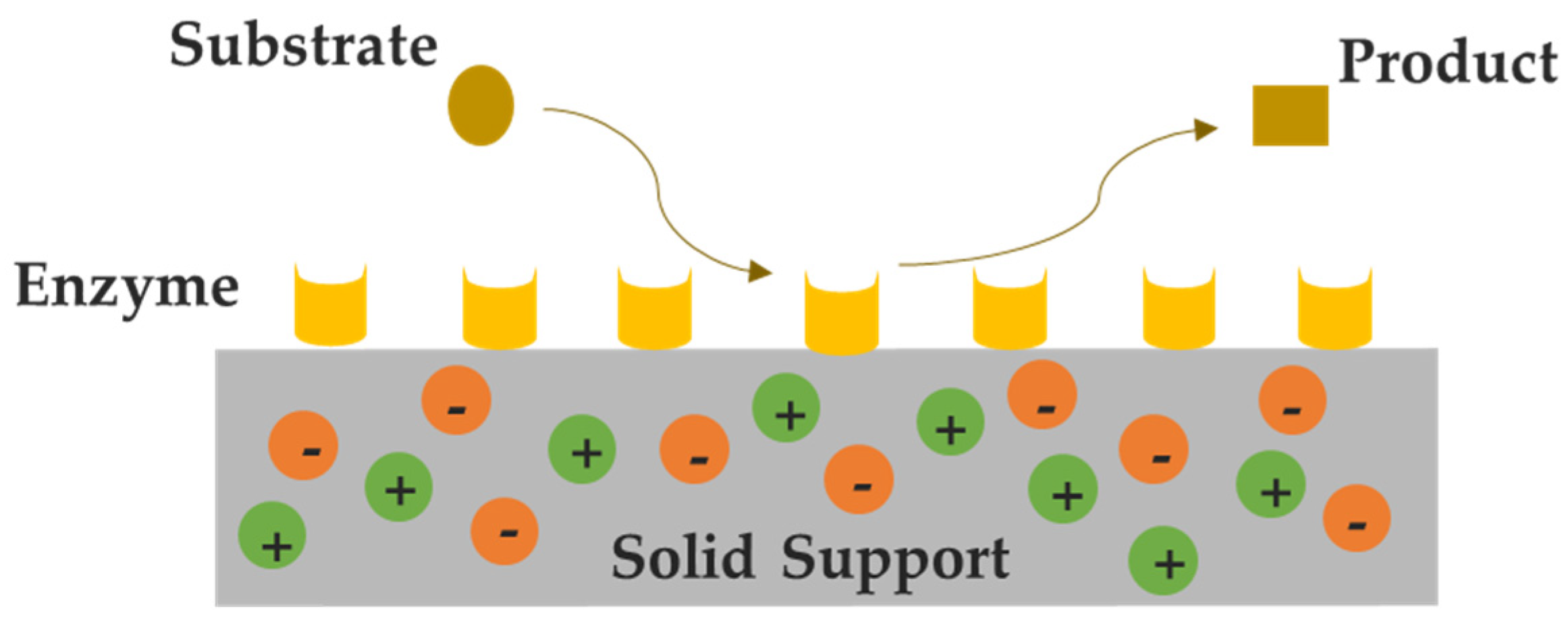
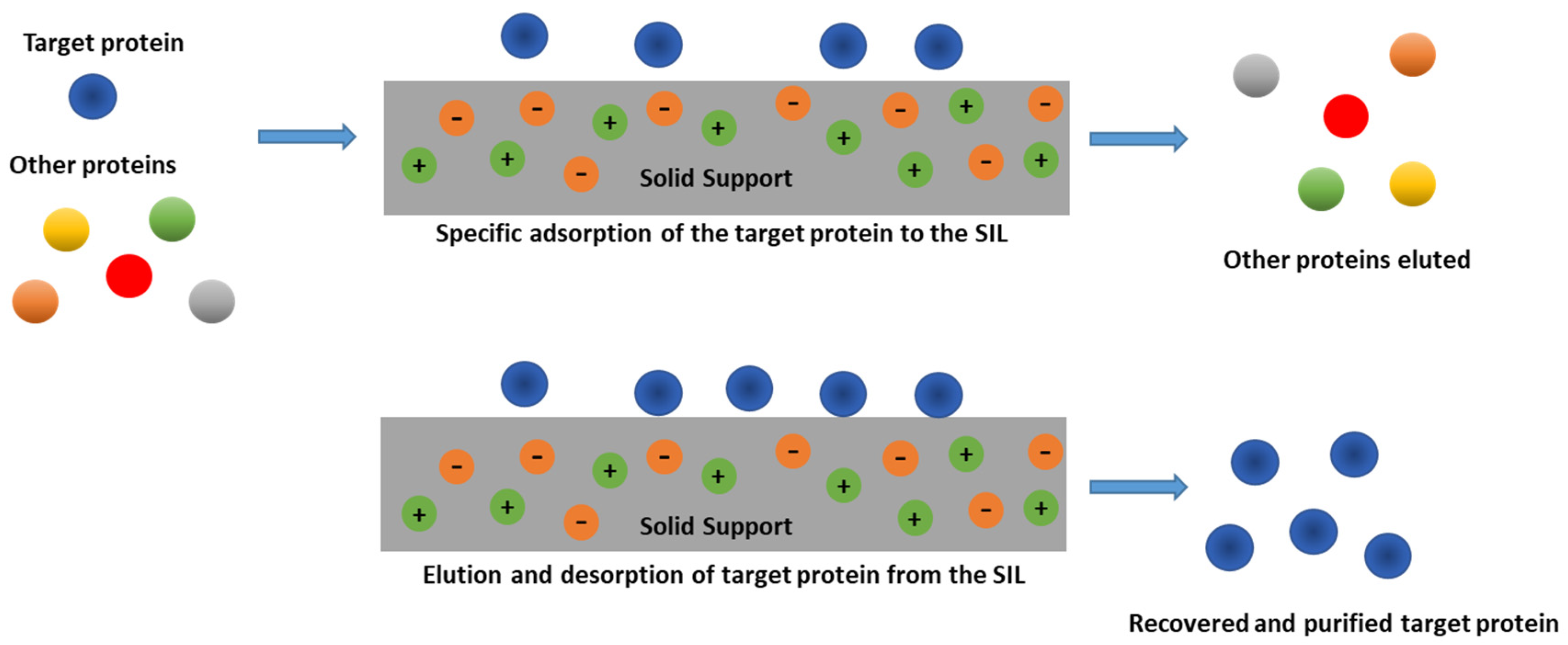
2. Supported Ionic Liquids (SILs)
3. SILs Applications in Proteins and Enzymes-Related Fields
3.1. SILs in Biocatalysis
3.2. SILs as Separation and Purification Platforms for Proteins and Enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yavir, K.; Marcinkowski, Ł.; Marcinkowska, R.; Namieśnik, J.; Kloskowski, A. Analytical applications and physicochemical properties of ionic liquid-based hybrid materials: A review. Anal. Chim. Acta 2019, 1054, 1–16. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents Synth. Catal. Chem. 1999, 99, 2071–2084. [Google Scholar]
- Letkarska, G.; Bogdanov, M.G. Effect of Ionic Liquids on Activity and Stability of Enzymes. Encycl. Ion. Liq. 2019, 1–5. [Google Scholar]
- Marullo, S.; D’Anna, F.; Billeci, F. Ionic liquids: “normal” solvents or nanostructured fluids? Org. Biomol. Chem. 2021, 19, 2076–2095. [Google Scholar] [CrossRef] [PubMed]
- Erbeldinger, M.; Mesiano, A.J.; Russell, A.J. Enzymatic Catalysis of Formation of Z -Aspartame in Ionic Liquid - An Alternative to Enzymatic Catalysis in Organic Solvents. Biotecnol. Prog. 2000, 6, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 23, 2399–2407. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, T.; Han, B. Preparation of Catalytic Materials Using Ionic Liquids as the Media and Functional Components. Adv. Mater. 2014, 26, 6810–6827. [Google Scholar] [CrossRef]
- Scholten, J.D.; Leal, B.C.; Dupont, J. Transition Metal Nanoparticle Catalysis in Ionic Liquids. ACS Catal. 2012, 2, 184–200. [Google Scholar] [CrossRef]
- Han, X.; Armstrong, D.W. Ionic Liquids in Separations. Acc. Chem. Res. 2007, 40, 1079–1086. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Luo, G.; Yang, S.; Wang, D. Extraction and Separation of Phycocyanin from Spirulina using Aqueous Two-Phase Systems of Ionic Liquid and Salt. J. Food Nutr. Res. 2019, 3, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Luo, H.; Dai, S. Ionic Liquids-Based Extraction: A Promising Strategy for the Advanced Nuclear Fuel Cycle. Chem. Rev. 2012, 112, 2100–2128. [Google Scholar] [CrossRef]
- Meng, H.; Chen, X.W.; Wang, J.H. One-pot synthesis of N, N-bis[2-methylbutyl] imidazolium hexafluorophosphate-TiO2 nanocomposites and application for protein isolation. J. Mater. Chem. 2011, 21, 14857–14863. [Google Scholar] [CrossRef]
- Xin, B.; Hao, J. Imidazolium-based ionic liquids grafted on solid surfaces. Chem. Soc. Rev. 2014, 43, 7171–7187. [Google Scholar] [CrossRef]
- Asefa, T.; Tao, Z. Biocompatibility of Mesoporous Silica Nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Li, W.W.; Wang, F.; Li, W.W.; Zhao, J.; Liang, X.; Liu, H. Immobilizing Penicillin G Acylase Using Silica-Supported Ionic Liquids: The Effects of Ionic Liquid Loadings. Ind. Eng. Chem. Res. 2012, 51, 13173–13181. [Google Scholar] [CrossRef]
- Hlady, V.; Buijs, J. Protein adsorption on solid surfaces. Curr. Opin. Biotechnol. 1996, 7, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.; Kumari, M.; Khan, A.B. Recent advances in the applications of ionic liquids in protein stability and activity: A review. Appl. Biochem. Biotechnol. 2014, 172, 3701–3720. [Google Scholar] [CrossRef]
- Latour, R.A. Fundamental Principles of the Thermodynamics and Kinetics of Protein Adsorption to Material Surfaces. Colloids Surf. B Biointerfaces 2020, 191, 110992. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C. Proteins in Ionic Liquids: Current Status of Experiments and Simulations. Top. Curr. Chem. 2017, 375, 127–152. [Google Scholar]
- Elgharbawy, A.A.M.; Moniruzzaman, M.; Goto, M. Recent advances of enzymatic reactions in ionic liquids: Part II. Biochem. Eng. J. 2020, 154, 107426. [Google Scholar] [CrossRef]
- Bui-Le, L.; Clarke, C.J.; Bröhl, A.; Brogan, A.P.S.; Arpino, J.A.J.; Polizzi, K.M.; Hallett, J.P. Revealing the complexity of ionic liquid–protein interactions through a multi-technique investigation. Commun. Chem. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- Kumar, A.; Bisht, M.; Venkatesu, P. Biocompatibility of Ionic Liquids Towards Protein Stability: A Comprehensive Overview on the Current Understanding and Their Implications; Elsevier: Amsterdam, The Netherlands, 2017; Volume 96. [Google Scholar]
- Guncheva, M.; Paunova, K.; Yancheva, D.; Svinyarov, I.; Bogdanov, M. Effect of two series ionic liquids based on non-nutritive sweeteners on catalytic activity and stability of the industrially important lipases from Candida rugosa and Rhizopus delemar. J. Mol. Catal. B Enzym. 2015, 117, 62–68. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mohammadpourfard, M.; Zeinali Heris, S. Ionic liquids: Promising compounds for sustainable chemical processes and applications. Chem. Eng. Res. Des. 2020, 160, 264–300. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Xu, H.-X.; Chen, N.; Che, W.-C. The Application of Ionic Liquids in Enzyme Immobilization and Enzyme Modification. Austin J. Biotechnol. Bioeng. 2016, 3, 1060–1064. [Google Scholar]
- Klähn, M.; Lim, G.S.; Seduraman, A.; Wu, P. On the different roles of anions and cations in the solvation of enzymes in ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 1649–1662. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Kamiya, N.; Goto, M. Activation and stabilization of enzymes in ionic liquids. Org. Biomol. Chem. 2010, 8, 2887–2899. [Google Scholar] [CrossRef]
- Gao, W.-W.W.; Zhang, F.-X.X.; Zhang, G.-X.X.; Zhou, C.-H.H. Key factors affecting the activity and stability of enzymes in ionic liquids and novel applications in biocatalysis. Biochem. Eng. J. 2015, 99, 67–84. [Google Scholar] [CrossRef]
- Fujita, K.; MacFarlane, D.R.; Forsyth, M. Protein solubilising and stabilising ionic liquids. Chem. Commun. 2005, 70, 4804–4806. [Google Scholar] [CrossRef]
- Haberler, M.; Schröder, C.; Steinhauser, O. Hydrated ionic liquids with and without solute: The influence of water content and protein solutes. J. Chem. Theory Comput. 2012, 8, 3911–3928. [Google Scholar] [CrossRef] [PubMed]
- Sivapragasam, M.; Moniruzzaman, M.; Goto, M. Recent advances in exploiting ionic liquids for biomolecules: Solubility, stability and applications. Biotechnol. J. 2016, 11, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, A.; Mogha, N.K.; Venkatesu, P. Insight into impact of choline-based ionic liquids on bovine β-lactoglobulin structural analysis: Unexpected high thermal stability of protein. Int. J. Biol. Macromol. 2019, 126, 1–10. [Google Scholar] [CrossRef]
- Guncheva, M.; Ossowicz, P.; Janus, E.; Todinova, S.; Yancheva, D. Elucidation of the effect of some cholinium amino acid ionic liquids on the thermal and the conformational stability of insulin. J. Mol. Liq. 2019, 283, 257–262. [Google Scholar] [CrossRef]
- Alves, M.M.S.; Araújo, J.M.M.; Martins, I.C.; Pereiro, A.B.; Archer, M. Insights into the interaction of Bovine Serum Albumin with Surface-Active Ionic Liquids in aqueous solution. J. Mol. Liq. 2021, 322, 114537. [Google Scholar] [CrossRef]
- Sundaram, V.; Ramanan, R.N.; Selvaraj, M.; Vijayaraghavan, R.; MacFarlane, D.R.; Ooi, C.W. Structural stability of insulin aspart in aqueous cholinium aminoate ionic liquids based on molecular dynamics simulation studies. J. Mol. Liq. 2021, 322, 114501. [Google Scholar] [CrossRef]
- Mogha, N.K.; Sindhu, A.; Venkatesu, P. A biophysical strategy to examine the impact of newly synthesized polymerizable ammonium-based ionic liquids on the structural stability and proteolytic activity of stem bromelain. Int. J. Biol. Macromol. 2020, 151, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Dotsenko, A.S.; Rozhkova, A.M.; Zorov, I.N.; Sinitsyn, A.P. Protein surface engineering of endoglucanase Penicillium verruculosum for improvement in thermostability and stability in the presence of 1-butyl-3-methylimidazolium chloride ionic liquid. Bioresour. Technol. 2020, 296, 122370. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Mojtabavi, S.; Faramarzi, M.A.; Mehrnejad, F.; Soleimani, M.; Mirjani, R. Molecular level insight into stability, activity, and structure of Laccase in aqueous ionic liquid and organic solvents: An experimental and computational research. J. Mol. Liq. 2020, 317, 113925. [Google Scholar] [CrossRef]
- Zou, B.; Yan, Y.; Xia, J.; Zhang, L.; Adesanya, I.O. Enhancing bio-catalytic activity and stability of lipase nanogel by functional ionic liquids modification. Colloids Surf. B Biointerfaces 2020, 195, 111275. [Google Scholar] [CrossRef]
- Alvarez-Guerra, E.; Irabien, A. Ionic liquid-based three phase partitioning (ILTPP) systems for whey protein recovery: Ionic liquid selection. J. Chem. Technol. Biotechnol. 2015, 90, 939–946. [Google Scholar] [CrossRef]
- Freire, M.G. (Ed.) Ionic-Liquid- Based Aqueous Biphasic Systems Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Pei, Y.; Wang, J.; Wu, K.; Xuan, X.; Lu, X. Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep. Purif. Technol. 2009, 64, 288–295. [Google Scholar] [CrossRef]
- Martínez-Aragón, M.; Burghoff, S.; Goetheer, E.L.V.; De Haan, A.B.B. Guidelines for solvent selection for carrier mediated extraction of proteins. Sep. Purif. Technol. 2009, 65, 65–72. [Google Scholar] [CrossRef]
- Quental, M.V.; Caban, M.; Pereira, M.M.; Stepnowski, P.; Coutinho, J.A.P.; Freire, M.G. Enhanced extraction of proteins using cholinium-based ionic liquids as phase-forming components of aqueous biphasic systems. Biotechnol. J. 2015, 10, 1457–1466. [Google Scholar] [CrossRef]
- Cheng, D.H.; Chen, X.W.; Shu, Y.; Wang, J.H. Selective extraction/isolation of hemoglobin with ionic liquid 1-butyl-3-trimethylsilylimidazolium hexafluorophosphate (BtmsimPF6). Talanta 2008, 75, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Koo, Y.-M. Application of Ionic Liquids in Biotechnology; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Canongia Lopes, J.N.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; E Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Khoiroh, I.; Ooi, C.W.; Ling, T.C.; Show, P.L. Recent Advances in Protein Extraction Using Ionic Liquid-based Aqueous Two-phase Systems. Sep. Purif. Rev. 2017, 46, 291–304. [Google Scholar] [CrossRef]
- Castro, L.; Pereira, P.; Freire, M.; Pedro, A. Progress in the Development of Aqueous Two-Phase Systems Comprising Ionic Liquids for the Downstream Processing of Protein- Based Biopharmaceuticals. Am. Pharm. Rev. 2019, 2019, 1–6. [Google Scholar]
- Song, C.P.; Ramanan, R.N.; Vijayaraghavan, R.; Macfarlane, D.R.; Chan, E.S.; Ooi, C.W. Green, Aqueous Two-Phase Systems Based on Cholinium Aminoate Ionic Liquids with Tunable Hydrophobicity and Charge Density. ACS Sustain. Chem. Eng. 2015, 3, 3291–3298. [Google Scholar] [CrossRef] [Green Version]
- Taha, M.; Quental, M.V.; Correia, I.; Freire, M.G.; Coutinho, J.A.P. Extraction and stability of bovine serum albumin (BSA) using cholinium-based Good’s buffers ionic liquids. Process Biochem. 2015, 50, 1158–1166. [Google Scholar] [CrossRef] [Green Version]
- Passos, H.; Luís, A.; Coutinho, J.A.P.; Freire, M.G. Thermoreversible (Ionic-Liquid-Based) Aqueous Biphasic Systems. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Fehrmann, R.; Haumann, M.; Riisager, A. Supported Ionic Liquids: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 1–9. [Google Scholar]
- Pedro, A.; Coutinho, J.A.P.; Freire, M.G. Immobilization of Ionic Liquids, Types of Materials, and Applications; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Campisciano, V.; Giacalone, F.; Gruttadauria, M. Supported Ionic Liquids: A Versatile and Useful Class of Materials. Chem. Rec. 2017, 17, 1–22. [Google Scholar] [CrossRef]
- Meijboom, R.; Haumann, M.; Thomas, E.M.; Müller, T.E.; Szesni, N. Synthetic Methodologies for Supported Ionic Liquid Materials. Support. Ion. Liq. Fundam. Appl. 2014, 22, 75–94. [Google Scholar]
- Giacalone, F.; Gruttadauria, M. Covalently Supported Ionic Liquid Phases: An Advanced Class of Recyclable Catalytic Systems. ChemCatChem 2016, 8, 664–684. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Yáñez, M.; Alves, V.D.; Palomar, J.; Moya, C.; Gorri, D.; Tomé, L.C.; Marrucho, I.M. CO2/H2 separation through poly(ionic liquid)–ionic liquid membranes: The effect of multicomponent gas mixtures, temperature and gas feed pressure. Sep. Purif. Technol. 2021, 259, 118113. [Google Scholar] [CrossRef]
- Santiago, R.; Moya, C.; Palomar, J. Siloxanes capture by ionic liquids: Solvent selection and process evaluation. Chem. Eng. J. 2020, 401, 126078. [Google Scholar] [CrossRef]
- Lemus, J.; Santiago, R.; Hospital-Benito, D.; Welton, T.; Hallett, J.P.; Palomar, J. Process Analysis of Ionic Liquid-Based Blends as H2S Absorbents: Search for Thermodynamic/Kinetic Synergies. ACS Sustain. Chem. Eng. 2021, 9, 2080–2088. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Zhang, Y.; Deng, Y. Nanoconfined Ionic Liquids. Chem. Rev. 2017, 117, 6755–6833. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, Y.; Sun, Y.; Niu, C.; Qiao, F.; Yan, H. An ionic liquid-magnetic graphene composite for magnet dispersive solid-phase extraction of triazine herbicides in surface water followed by high performance liquid chromatography. Analyst 2017, 143, 175–181. [Google Scholar] [CrossRef]
- Mehnert, C.P.; Cook, R.A.; Dispenziere, N.C.; Afeworki, M. Supported ionic liquid catalysis - A new concept for homogeneous hydroformylation catalysis. J. Am. Chem. Soc. 2002, 124, 12932–12933. [Google Scholar] [CrossRef]
- Riisager, A.; Fehrmann, R.; Haumann, M.; Wasserscheid, P. Supported ionic liquids: Versatile reaction and separation media. Top. Catal. 2006, 40, 91–102. [Google Scholar] [CrossRef]
- Valkenberg, M.H.; DeCastro, C.; Hölderich, W.F. Immobilisation of ionic liquids on solid supports. Green Chem. 2002, 4, 88–93. [Google Scholar] [CrossRef]
- Mehnert, C.P. Supported ionic liquid catalysis. Chem.-Eur. J. 2005, 11, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Sandig, B.; Buchmeiser, M.R.; Haumann, M. Supported ionic liquid phase (SILP) facilitated gas-phase enzyme catalysis-CALB catalyzed transesterification of vinyl propionate. Catal. Sci. Technol. 2018, 8, 2460–2466. [Google Scholar] [CrossRef]
- Lozano, P.; García-Verdugo, E.; Piamtongkam, R.; Karbass, N.; De Diego, T.; Burguete, M.I.; Luis, S.V.; Iborra, J.L. Bioreactors based on monolith-supported ionic liquid phase for enzyme catalysis in supercritical carbon dioxide. Adv. Synth. Catal. 2007, 349, 1077–1084. [Google Scholar] [CrossRef]
- Lozano, P.; García-Verdugo, E.; Karbass, N.; Montague, K.; De Diego, T.; Burguete, M.I.; Luis, S.V. Supported Ionic Liquid-Like Phases (SILLPs) for enzymatic processes: Continuous KR and DKR in SILLP–scCO2 systems. Green Chem. 2010, 12, 1803–1810. [Google Scholar] [CrossRef]
- Du, P.; Liu, S.; Wu, P.; Cai, C. Preparation and characterization of room temperature ionic liquid/single-walled carbon nanotube nanocomposites and their application to the direct electrochemistry of heme-containing proteins/enzymes. Electrochim. Acta 2007, 52, 6534–6547. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, B.; Wu, P.; Zhang, H.; Cai, C. Effects of ionic liquids on enzymatic catalysis of the glucose oxidase toward the oxidation of glucose. J. Phys. Chem. B 2009, 113, 13365–13373. [Google Scholar] [CrossRef]
- Wan, X.; Tang, S.; Xiang, X.; Huang, H.; Hu, Y. Immobilization of Candida antarctic Lipase B on Functionalized Ionic Liquid Modified MWNTs. Appl. Biochem. Biotechnol. 2017, 183, 807–819. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Santos, A.J.; Carvalho, N.B.; Figueiredo, R.T.; Pereira, M.M.; Lima, Á.S.; Freire, M.G.; Cabrera-Padilla, R.Y.; Soares, C.M.F. Enhanced Activity of Immobilized Lipase by Phosphonium-Based Ionic Liquids Used in the Support Preparation and Immobilization Process. ACS Sustain. Chem. Eng. 2019, 7, 15648–15659. [Google Scholar] [CrossRef]
- Yang, J.; Hu, Y.; Jiang, L.; Zou, B.; Jia, R.; Huang, H. Enhancing the catalytic properties of porcine pancreatic lipase by immobilization on SBA-15 modified by functionalized ionic liquid. Biochem. Eng. J. 2013, 70, 46–54. [Google Scholar] [CrossRef]
- Suo, H.; Xu, L.; Xue, Y.; Qiu, X.; Huang, H.; Hu, Y. Ionic liquids-modified cellulose coated magnetic nanoparticles for enzyme immobilization: Improvement of catalytic performance. Carbohydr. Polym. 2020, 234, 115914. [Google Scholar] [CrossRef]
- Suo, H.; Xu, L.; Xu, C.; Qiu, X.; Chen, H.; Huang, H.; Hu, Y. Graphene Oxide Nanosheets Shielding of Lipase Immobilized on Magnetic Composites for the Improvement of Enzyme Stability. ACS Sustain. Chem. Eng. 2019, 7, 4486–4494. [Google Scholar] [CrossRef]
- Lozano, P.; García-Verdugo, E.; Bernal, J.M.; Izquierdo, D.F.; Burguete, M.I.; Sánchez-Gómez, G.; Luis, S.V. Immobilised lipase on structured supports containing covalently attached ionic liquids for the continuous synthesis of biodiesel in scCO2. ChemSusChem 2012, 5, 790–798. [Google Scholar] [CrossRef]
- Hara, P.; Mikkola, J.P.; Murzin, D.Y.; Kanerva, L.T. Supported ionic liquids in Burkholderia cepacia lipase-catalyzed asymmetric acylation. J. Mol. Catal. B Enzym. 2010, 67, 129–134. [Google Scholar] [CrossRef]
- Abd, A.; Ahmed, A.; Xiashi, Z. Developments / Application of Ionic Liquids / Poly Ionic Liquids in Magnetic Solid-Phase Extraction and Solid Phase Microextraction. Colloid Surf. Sci. 2017, 2, 162–170. [Google Scholar]
- Wang, H.; He, Y.; He, X.; Li, W.; Chen, L.; Zhang, Y. BSA-imprinted synthetic receptor for reversible template recognition. J. Sep. Sci. 2009, 32, 1981–1986. [Google Scholar] [CrossRef]
- Jiang, T.F.; Gu, Y.L.; Liang, B.; Li, J.B.; Shi, Y.P.; Ou, Q.Y. Dynamically coating the capillary with 1-alkyl-3-methylimidazolium-based ionic liquids for separation of basic proteins by capillary electrophoresis. Anal. Chim. Acta 2003, 479, 249–254. [Google Scholar] [CrossRef]
- Wu, X.; Wei, W.; Su, Q.; Xu, L.; Chen, G. Simultaneous separation of basic and acidic proteins using 1-butyl-3-methylimidazolium-based ion liquid as dynamic coating and background electrolyte in capillary electrophoresis. Electrophoresis 2008, 29, 2356–2362. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Wang, E. Microchip micellar electrokinetic chromatography based on one functionalized ionic liquid and its excellent performance on proteins separation. J. Chromatogr. A 2008, 1207, 175–180. [Google Scholar] [CrossRef]
- Shu, Y.; Chen, X.W.; Wang, J.H. Ionic liquid-polyvinyl chloride ionomer for highly selective isolation of basic proteins. Talanta 2010, 81, 637–642. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, S.; Chen, X.W.; He, R.H. Selective isolation of hemoglobin by use of imidazolium-modified polystyrene as extractant. Anal. Bioanal. Chem. 2013, 405, 5353–5358. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, R.; Deng, Q.; Zhang, L.; Liu, C.; Wang, S. Preparation of ionic liquid polymer materials and their recognition properties for proteins. RSC Adv. 2014, 4, 52147–52154. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Ding, X.; Huang, Y.; Xu, K. Magnetic solid-phase extraction of proteins based on hydroxy functional ionic liquid-modified magnetic nanoparticles. Anal. Methods 2014, 6, 8358–8367. [Google Scholar] [CrossRef]
- Wen, Q.; Wang, Y.; Xu, K.; Li, N.; Zhang, H.; Yang, Q.; Zhou, Y. Magnetic solid-phase extraction of protein by ionic liquid-coated Fe@graphene oxide. Talanta 2016, 160, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Deng, Q.; Fang, G.; Pan, M.; Zhai, X.; Wang, S. A novel ionic liquid polymer material with high binding capacity for proteins. J. Mater. Chem. 2012, 22, 3965–3972. [Google Scholar] [CrossRef]
- Song, H.; Yang, C.; Yohannes, A.; Yao, S. Acidic ionic liquid modified silica gel for adsorption and separation of bovine serum albumin (BSA). RSC Adv. 2016, 6, 107452–107462. [Google Scholar] [CrossRef]
- Jia, X.; Hu, X.; Wang, W.; Du, C. Non-covalent loading of ionic liquid-functionalized nanoparticles for bovine serum albumin: Experiments and theoretical analysis. RSC Adv. 2019, 9, 19114–19120. [Google Scholar] [CrossRef] [Green Version]
- Dang, M.; Deng, Q.; Fang, G.; Zhang, D.; Liu, J.; Wang, S. Preparation of novel anionic polymeric ionic liquid materials and their potential application to protein adsorption. J. Mater. Chem. B 2017, 5, 6339–6347. [Google Scholar] [CrossRef]
- Tang, Z.; Lu, L.; Dai, Z.; Xie, W.; Shi, L.; Lu, X. CO2 Absorption in the Ionic Liquids Immobilized on Solid Surface by Molecular Dynamics Simulation. Langmuir 2017, 33, 11658–11669. [Google Scholar] [CrossRef]
- Budhathoki, S.; Shah, J.K.; Maginn, E.J. Molecular Simulation Study of the Performance of Supported Ionic Liquid Phase Materials for the Separation of Carbon Dioxide from Methane and Hydrogen. Ind. Eng. Chem. Res. 2017, 56, 6775–6784. [Google Scholar] [CrossRef]
| Support(s) | Immobilization Type | Enzyme(s) | Reaction/Application | Reference |
|---|---|---|---|---|
| Polymeric hybrid monolith | Physisorption | CALB | Vinyl propionate and 2-propanol continuous gas-phase transesterification | [69] |
| Polymeric monolith | Physisorption | CALB | Continuous flow synthesis of citronellyl propionate in supercritical CO2 conditions | [70] |
| Polymers | Physisorption | CALB | Biocatalysts for a stereospecific reaction model | [71] |
| Single-walled carbon nanotubes (SWNT) | Physisorption | Myoglobin, cyt-c and horseradish peroxidase | Bioelectrocatalysis | [72] |
| Single-walled carbon nanotubes (SWNTs) | Physisorption | Glucose oxidase (GOx) | Oxidation of glucose | [73] |
| Multiwalled carbon nanotubes (MWNTs) | Physisorption | CALB | Hydrolysis of triacetin | [74] |
| Silica | Physisorption | Burkholderia cepacia lipase | Supports for lipase immobilization | [75] |
| Mesoporous silica SBA-15 | Physisorption and chemisorption | Porcine pancreatic lipase | Hydrolysis of triacetin | [76] |
| Magnetic carboxymethyl cellulose (IL-MCMC) | Chemisorption | Lipase and penicillin G acylase (PGA) | Supports for enzyme immobilization | [77] |
| Magnetic chitosan (MCS) composites | Physisorption | Porcine pancreatic lipase (PPL) | Supports for PLL adsorption | [78] |
| Polystyrene divinylbenzene porous matrix | Physisorption | CALB | Synthesis of biodiesel | [79] |
| Kynol™ ACC 507-15 | Physisorption | B. cepacia lipase | 1-Phenylethanol acylation with vinyl acetate | [80] |
| Poly(styrene-co-divinylbenzene) | Physisorption | CALB | Catalysis | [58] |
| Material(s) | Protein(s) | Observation | Reference |
|---|---|---|---|
| [Nmim]Cl/polyvinyl chloride (PVC) | lyz, cyt-c and Hb | Materials were able to adsorb proteins with high efficiency | [86] |
| Imidazolium-modified polystyrene | Hb | High adsorption efficiencies for Hb, with almost no protein denaturation using these materials | [87] |
| 1-Allyl-3-butylimidazolium chloride ([aC4im]Cl) and 1-vinyl-3-octyl-imidazolium bromide ([VC8im]Br) based polymers | BSA, bovine hemoglobin (BHb), lyz, and cyt-c | Different materials can be designed to adsorb and separate specific proteins | [88] |
| IL-modified magnetic nanoparticles (ILs-MNPs) | BSA | High adsorption capacity, selective adsorption, and stability of BSA | [89] |
| Amino functional dicationic IL (AFDCIL)/magnetic graphene oxide (Fe@GO) | BHb | Excellent adsorption and high extraction capacity for BHb | [90] |
| 1-vinyl-3-butylimidazolium chloride ([ViBuIm]Cl) macroporous polymer | BSA, BHb, equine myoglobin, cyt-c and lys | The macroporous IL polymer material presents an excellent adsorption capacity for proteins, especially for lyz | [91] |
| Silica gel functionalized with IL (SiO2@IL) IL: 1-methylimidazolium hydrogen sulphate ([mim][HSO4−]) | BSA, BHb | At the optimized conditions, a selective adsorption of BSA and BHb was successfully obtained from cow blood | [92] |
| Silane-coupling IL (SiO2@IL) IL: 1-(3-trimethoxysilylpropyl)-3-methylimidazolium chloride ([tmim]Cl | BSA | The SiO2@IL is able to adsorb BSA, being the main driving force of electrostatic interactions | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bento, R.M.F.; Almeida, C.A.S.; Neves, M.C.; Tavares, A.P.M.; Freire, M.G. Advances Achieved by Ionic-Liquid-Based Materials as Alternative Supports and Purification Platforms for Proteins and Enzymes. Nanomaterials 2021, 11, 2542. https://doi.org/10.3390/nano11102542
Bento RMF, Almeida CAS, Neves MC, Tavares APM, Freire MG. Advances Achieved by Ionic-Liquid-Based Materials as Alternative Supports and Purification Platforms for Proteins and Enzymes. Nanomaterials. 2021; 11(10):2542. https://doi.org/10.3390/nano11102542
Chicago/Turabian StyleBento, Rui M. F., Catarina A. S. Almeida, Márcia C. Neves, Ana P. M. Tavares, and Mara G. Freire. 2021. "Advances Achieved by Ionic-Liquid-Based Materials as Alternative Supports and Purification Platforms for Proteins and Enzymes" Nanomaterials 11, no. 10: 2542. https://doi.org/10.3390/nano11102542
APA StyleBento, R. M. F., Almeida, C. A. S., Neves, M. C., Tavares, A. P. M., & Freire, M. G. (2021). Advances Achieved by Ionic-Liquid-Based Materials as Alternative Supports and Purification Platforms for Proteins and Enzymes. Nanomaterials, 11(10), 2542. https://doi.org/10.3390/nano11102542








