Disclosing the Potential of Fluorinated Ionic Liquids as Interferon-Alpha 2b Delivery Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Conductivity Titration Experiments
2.3. Density Measurements
2.4. Tensiometry and Contact Angle Goniometry
2.5. Scanning Transmission Electron Microscopy with Energy-Dispersive X-ray Spectroscopy
2.6. Dynamic Light Scattering
2.7. Ultraviolet-Visible Spectroscopy
2.8. Fluorescence Spectroscopy
2.9. Circular Dichroism Spectroscopy
2.10. Microscale Thermophoresis
3. Results and Discussion
3.1. Influence of Interferon-Alpha 2b in the Aggregation Behaviour of Fluorinated Ionic Liquids
3.1.1. Critical Aggregation Concentrations
3.1.2. Surface Properties
3.1.3. Characterization of the Self-Assembled Aggregates
3.2. Interactions of the Fluorinated Ionic Liquids with Interferon-Alpha 2b
3.2.1. Spectroscopy Analysis
3.2.2. Binding Affinity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berdigaliyev, N.; Aljofan, M. An Overview of Drug Discovery and Development. Future Med. Chem. 2020, 12, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.S.B.; Hammou, A.; Poulsen, T.F.; Laursen, M.C.; Hansen, S.F. Definition, Categorization, and Environmental Risk Assessment of Biopharmaceuticals. Sci. Total Environ. 2021, 789, 147884. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G. Biopharmaceutical Benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Kesik-Brodacka, M. Progress in Biopharmaceutical Development. Biotechnol. Appl. Biochem. 2018, 65, 306–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voynov, V.; Caravella, J.A. (Eds.) Therapeutic Proteins; Humana Press: Totowa, NJ, USA, 2012; Volume 899, ISBN 978-1-61779-920-4. [Google Scholar]
- Akash, M.S.H.; Rehman, K.; Tariq, M.; Chen, S. Development of Therapeutic Proteins: Advances and Challenges. Turk. J. Biol. 2015, 39, 343–358. [Google Scholar] [CrossRef]
- Schuster, J.; Koulov, A.; Mahler, H.C.; Detampel, P.; Huwyler, J.; Singh, S.; Mathaes, R. In Vivo Stability of Therapeutic Proteins. Pharm. Res. 2020, 37, 23. [Google Scholar]
- Dingman, R.; Balu-Iyer, S.V. Immunogenicity of Protein Pharmaceuticals. J. Pharm. Sci. 2019, 108, 1637–1654. [Google Scholar] [CrossRef]
- Tourdot, S.; Hickling, T.P. Nonclinical Immunogenicity Risk Assessment of Therapeutic Proteins. Bioanalysis 2019, 11, 1631–1643. [Google Scholar] [CrossRef]
- Zaman, R.; Islam, R.A.; Ibnat, N.; Othman, I.; Zaini, A.; Lee, C.Y.; Chowdhury, E.H. Current Strategies in Extending Half-Lives of Therapeutic Proteins. J. Control. Release 2019, 301, 176–189. [Google Scholar] [CrossRef]
- Yin, L.; Yuvienco, C.; Montclare, J.K. Protein Based Therapeutic Delivery Agents: Contemporary Developments and Challenges. Biomaterials 2017, 134, 91–116. [Google Scholar] [CrossRef]
- Zeb, A.; Rana, I.; Choi, H.-I.; Lee, C.-H.; Baek, S.-W.; Lim, C.-W.; Khan, N.; Arif, S.T.; Sahar, N.; Alvi, A.M.; et al. Potential and Applications of Nanocarriers for Efficient Delivery of Biopharmaceuticals. Pharmaceutics 2020, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Wang, W.; Liu, X.; Wang, Y.; Li, K.; Song, X.; Zhang, J.; Yu, L.; He, Z. Sustained Release Systems for Delivery of Therapeutic Peptide/Protein. Biomacromolecules 2021, 22, 2299–2324. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, Z.; Xu, D. Advance in Oral Delivery Systems for Therapeutic Protein. J. Drug Target. 2019, 27, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Moncalvo, F.; Martinez Espinoza, M.I.; Cellesi, F. Nanosized Delivery Systems for Therapeutic Proteins: Clinically Validated Technologies and Advanced Development Strategies. Front. Bioeng. Biotechnol. 2020, 8, 89. [Google Scholar] [CrossRef]
- Paul, F.; Pellegrini, S.; Uzé, G. IFNA2: The Prototypic Human Alpha Interferon. Gene 2015, 567, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Asmana Ningrum, R. Human Interferon Alpha-2b: A Therapeutic Protein for Cancer Treatment. Scientifica 2014, 2014, 970315. [Google Scholar] [CrossRef] [Green Version]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in Clinical Cancer Immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Young, H.A. Interferons: Success in Anti-Viral Immunotherapy. Cytokine Growth Factor Rev. 2014, 25, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Antonelli, G.; Scagnolari, C.; Moschella, F.; Proietti, E. Twenty-Five Years of Type I Interferon-Based Treatment: A Critical Analysis of Its Therapeutic Use. Cytokine Growth Factor Rev. 2015, 26, 121–131. [Google Scholar] [CrossRef]
- Chkhis, A.; Abdulrazzaq, N.; Mokhtar, S.; al Jasmi, A. al Efficacy of High-Dose Nebulized Interferon α 2b in Severe COVID-19 Pneumonia. Turk. Thorac. J. 2021, 22, 199–204. [Google Scholar] [CrossRef]
- Yu, J.; Lu, X.; Tong, L.; Shi, X.; Ma, J.; Lv, F.; Wu, J.; Pan, Q.; Yang, J.; Cao, H.; et al. Interferon-α-2b Aerosol Inhalation Is Associated with Improved Clinical Outcomes in Patients with Coronavirus Disease-2019. Br. J. Clin. Pharmacol. 2021, 87, 4737–4746. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.I.; Villacis-Aguirre, C.A.; Santiago Vispo, N.; Santiago Padilla, L.; Pedroso Santana, S.; Parra, N.C.; Alonso, J.R.T. Forms and Methods for Interferon’s Encapsulation. Pharmaceutics 2021, 13, 1533. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, R.N.; de Castro Ferreira, L.E.V.V.; de Lima Pace, F.H. Health-Related Quality of Life and Fatigue in Patients with Chronic Hepatitis C with Therapy with Direct-Acting Antivirals Agents Interferon-Free. PLoS ONE 2020, 15, e0237005. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Zhuang, W.; Hachem, K.; Bokov, D.; Javed Ansari, M.; Taghvaie Nakhjiri, A. Ionic Liquids in Pharmaceutical Industry: A Systematic Review on Applications and Future Perspectives. J. Mol. Liq. 2022, 349, 118145. [Google Scholar] [CrossRef]
- Jadhav, N.R.; Bhosale, S.P.; Bhosale, S.S.; Mali, S.D.; Toraskar, P.B.; Kadam, T.S. Ionic Liquids: Formulation Avenues, Drug Delivery and Therapeutic Updates. J. Drug Deliv. Sci. Technol. 2021, 65, 102694. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic Liquids as a Potential Tool for Drug Delivery Systems. Medchemcomm 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Moshikur, R.M.; Ali, M.K.; Moniruzzaman, M.; Goto, M. Recent Advances in Surface-Active Ionic Liquid-Assisted Self-Assembly Systems for Drug Delivery. Curr. Opin. Colloid Interface Sci. 2021, 56, 101515. [Google Scholar] [CrossRef]
- Buettner, C.S.; Cognigni, A.; Schröder, C.; Bica-Schröder, K. Surface-Active Ionic Liquids: A Review. J. Mol. Liq. 2022, 347, 118160. [Google Scholar] [CrossRef]
- Vieira, N.S.M.; Ferreira, M.L.; Castro, P.J.; Araújo, J.M.M.; Pereiro, A.B. Fluorinated Ionic Liquids as Task-Specific Materials: An Overview of Current Research. In Ionic Liquids-Thermophysical Properties and Applications; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Vieira, N.S.M.; Castro, P.J.; Vega, L.F.; Araújo, J.M.M.; Pereiro, A.B. Understanding the Phase and Solvation Behavior of Fluorinated Ionic Liquids. J. Mol. Liq. 2022, 359, 119285. [Google Scholar] [CrossRef]
- Hollõczki, O.; Macchiagodena, M.; Weber, H.; Thomas, M.; Brehm, M.; Stark, A.; Russina, O.; Triolo, A.; Kirchner, B. Triphilic Ionic-Liquid Mixtures: Fluorinated and Non-Fluorinated Aprotic Ionic-Liquid Mixtures. ChemPhysChem 2015, 16, 3325–3333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russina, O.; lo Celso, F.; di Michiel, M.; Passerini, S.; Appetecchi, G.B.; Castiglione, F.; Mele, A.; Caminiti, R.; Triolo, A. Mesoscopic Structural Organization in Triphilic Room Temperature Ionic Liquids. Faraday Discuss. 2013, 167, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Kennedy, D.F.; Greaves, T.L.; Weerawardena, A.; Mulder, R.J.; Kirby, N.; Song, G.; Drummond, C.J. Protic Ionic Liquids with Fluorous Anions: Physicochemical Properties and Self-Assembly Nanostructure. Phys. Chem. Chem. Phys. 2012, 14, 7981–7992. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Freitas, A.A.; Canongia Lopes, J.N. Structural Characterization of the [CnC1im][C4F9SO3] Ionic Liquid Series: Alkyl versus Perfluoroalkyl Side Chains. J. Mol. Liq. 2017, 226, 28–34. [Google Scholar] [CrossRef]
- Almantariotis, D.; Gefflaut, T.; Pádua, A.A.H.; Coxam, J.Y.; Costa Gomes, M.F. Effect of Fluorination and Size of the Alkyl Side-Chain on the Solubility of Carbon Dioxide in 1-Alkyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl) Amide Ionic Liquids. J. Phys. Chem. B 2010, 114, 3608–3617. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Araújo, J.M.M.; Teixeira, F.S.; Marrucho, I.M.; Piñeiro, M.M.; Rebelo, L.P.N. Aggregation Behavior and Total Miscibility of Fluorinated Ionic Liquids in Water. Langmuir 2015, 31, 1283–1295. [Google Scholar] [CrossRef]
- Patinha, D.J.S.; Tomé, L.C.; Florindo, C.; Soares, H.R.; Coroadinha, A.S.; Marrucho, I.M. New Low-Toxicity Cholinium-Based Ionic Liquids with Perfluoroalkanoate Anions for Aqueous Biphasic System Implementation. ACS Sustain. Chem. Eng. 2016, 4, 2670–2679. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Vieira, N.S.M.; Araújo, J.M.M.; Pereiro, A.B. Unveiling the Influence of Non-Toxic Fluorinated Ionic Liquids Aqueous Solutions in the Encapsulation and Stability of Lysozyme. Sustain. Chem. 2021, 2, 149–166. [Google Scholar] [CrossRef]
- Alves, M.; Vieira, N.S.M.; Rebelo, L.P.N.; Araújo, J.M.M.; Pereiro, A.B.; Archer, M. Fluorinated Ionic Liquids for Protein Drug Delivery Systems: Investigating Their Impact on the Structure and Function of Lysozyme. Int. J. Pharm. 2017, 526, 309–320. [Google Scholar] [CrossRef]
- Vieira, N.S.M.; Castro, P.J.; Marques, D.F.; Araújo, J.M.M.; Pereiro, A.B. Tailor-Made Fluorinated Ionic Liquids for Protein Delivery. Nanomaterials 2020, 10, 1594. [Google Scholar] [CrossRef]
- Alves, M.M.S.; Araújo, J.M.M.; Martins, I.C.; Pereiro, A.B.; Archer, M. Insights into the Interaction of Bovine Serum Albumin with Surface-Active Ionic Liquids in Aqueous Solution. J. Mol. Liq. 2021, 322, 114537. [Google Scholar] [CrossRef]
- Alves, M.M.S.; Leandro, P.; Mertens, H.D.T.; Pereiro, A.B.; Archer, M. Impact of Fluorinated Ionic Liquids on Human Phenylalanine Hydroxylase—A Potential Drug Delivery System. Nanomaterials 2022, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.S.; Pereira, P.; Passarinha, L.A.; Freire, M.G.; Pedro, A.Q. Enhanced Performance of Polymer-Polymer Aqueous Two-Phase Systems Using Ionic Liquids as Adjuvants towards the Purification of Recombinant Proteins. Sep. Purif. Technol. 2020, 248, 117051. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Ferreira, A.S.D.; Araújo, J.M.M.; Cabrita, E.J.; Pereiro, A.B. The Impact of Fluorinated Ionic Liquids Aggregation in the Interactions with Proteins. Fluid Phase Equilibria 2022, 559, 113488. [Google Scholar] [CrossRef]
- Louis-Jeune, C.; Andrade-Navarro, M.A.; Perez-Iratxeta, C. Prediction of Protein Secondary Structure from Circular Dichroism Using Theoretically Derived Spectra. Proteins: Struct. Funct. Bioinform. 2012, 80, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.N. The Energetics of Micelle Formation. Trans. Faraday Soc. 1955, 51, 561–569. [Google Scholar] [CrossRef]
- Zana, R. Critical Micellization Concentration of Surfactants in Aqueous Solution and Free Energy of Micellization. Langmuir 1996, 12, 1208–1211. [Google Scholar] [CrossRef]
- Doogue, M.P.; Polasek, T.M. The ABCD of Clinical Pharmacokinetics. Ther. Adv. Drug Saf. 2013, 4, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Kharazi, M.; Saien, J.; Asadabadi, S. Review on Amphiphilic Ionic Liquids as New Surfactants: From Fundamentals to Applications. Top. Curr. Chem. 2022, 380, 5. [Google Scholar] [CrossRef]
- Das, S.; Mondal, S.; Ghosh, S. Physicochemical Studies on the Micellization of Cationic, Anionic, and Nonionic Surfactants in Water–Polar Organic Solvent Mixtures. J. Chem. Eng. Data 2013, 58, 2586–2595. [Google Scholar] [CrossRef]
- Wadekar, M.N.; Boekhoven, J.; Jager, W.F.; Koper, G.J.M.; Picken, S.J. Micellization Behavior of Aromatic Moiety Bearing Hybrid Fluorocarbon Sulfonate Surfactants. Langmuir 2012, 28, 3397–3402. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Dong, B.; Zhang, H.; Zhao, Y.; Wang, X.; Wang, R.; Yu, L. Aggregation Behaviors of Dodecyl Sulfate-Based Anionic Surface Active Ionic Liquids in Water. J. Phys. Chem. B 2012, 116, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Sharma, R.; Mahajan, R.K. An Investigation of Drug Binding Ability of a Surface Active Ionic Liquid: Micellization, Electrochemical, and Spectroscopic Studies. Langmuir 2012, 28, 17238–17246. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of Self-Assembly of Hydrocarbon Amphiphiles into Micelles and Bilayers. J. Chem. Soc. Faraday Trans. 2 1976, 72, 1525. [Google Scholar] [CrossRef]
- Tanford, C. Micelle Shape and Size. J. Phys. Chem. 1972, 76, 3020–3024. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Walter, L.J.; Hruza, A.; Reichert, P.; Trotta, P.P.; Nagabhushan, T.L.; Walter, M.R. Zinc Mediated Dimer of Human Interferon-α(2b) Revealed by X-Ray Crystallography. Structure 1996, 4, 1453–1463. [Google Scholar] [CrossRef] [Green Version]
- Mudalige, T.; Qu, H.; van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of Nanomaterials: Tools and Challenges. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. [Google Scholar]
- Kueltzo, L.A.; Midddaugh, C.R. Ultraviolet Absorption Spectroscopy. In Methods for Structural Analysis of Protein Pharmaeuticals; Jiskoot, W., Crommelin, D.J.A., Eds.; AAPS Press: Arlington, VA, USA, 2005. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Nikolaiev, R.; Stepanenko, Y.; Chernykh, S.; Melnichuk, N.; Tkachuk, Z. Conformational, Fluorescence and Energy Parameters of Interferon A2b with Different Forms of Oligoribonucleotides and Adenosine Monophosphate. In Proceedings of the the 1st International Electronic Conference on Pharmaceutics, Online, 1 December 2020; MDPI: Basel, Switzerland, 2020; p. 16. [Google Scholar]
- Ware, W.R. Oxygen quenching of fluorescence in solution: An experimental study of the diffusion process. J. Phys. Chem. 1962, 66, 455–458. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using Circular Dichroism Spectra to Estimate Protein Secondary Structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- Salmannejad, F.; Nafissi-Varcheh, N. Ectoine and Hydroxyectoine Inhibit Thermal-Induced Aggregation and Increase Thermostability of Recombinant Human Interferon Alfa2b. Eur. J. Pharm. Sci. 2017, 97, 200–207. [Google Scholar] [CrossRef]
- Jerabek-Willemsen, M.; André, T.; Wanner, R.; Roth, H.M.; Duhr, S.; Baaske, P.; Breitsprecher, D. MicroScale Thermophoresis: Interaction Analysis and Beyond. J. Mol. Struct. 2014, 1077, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Wienken, C.J.; Baaske, P.; Rothbauer, U.; Braun, D.; Duhr, S. Protein-Binding Assays in Biological Liquids Using Microscale Thermophoresis. Nat. Commun. 2010, 1, 100. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, S.F.; Custódio, M.H.; Pereiro, A.B.; Araújo, J.M.M. Towards Enhanced Tunability of Aqueous Biphasic Systems: Furthering the Grasp of Fluorinated Ionic Liquids in the Purification of Proteins. J. Mol. Liq. Submitt. 2022. [Google Scholar]
- Zahradník, J.; Kolářová, L.; Pařízková, H.; Kolenko, P.; Schneider, B. Interferons Type II and Their Receptors R1 and R2 in Fish Species: Evolution, Structure, and Function. Fish Shellfish Immunol. 2018, 79, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Tosstorff, A.; Svilenov, H.; Peters, G.H.J.; Harris, P.; Winter, G. Structure-Based Discovery of a New Protein-Aggregation Breaking Excipient. Eur. J. Pharm. Biopharm. 2019, 144, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Corin, K.; Baaske, P.; Ravel, D.B.; Song, J.; Brown, E.; Wang, X.; Wienken, C.J.; Jerabek-Willemsen, M.; Duhr, S.; Luo, Y.; et al. Designer Lipid-Like Peptides: A Class of Detergents for Studying Functional Olfactory Receptors Using Commercial Cell-Free Systems. PLoS ONE 2011, 6, e25067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, D.M.; Fernandes, L.C.; Fernandes, M.M.; Hermenegildo, B.; Meira, R.M.; Ribeiro, C.; Ribeiro, S.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid-Based Materials for Biomedical Applications. Nanomaterials 2021, 11, 2401. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Lee, K.; Nam, M.W.; Jeong, K.M.; Lee, J.E.; Kim, N.W.; Yin, Y.; Lim, S.Y.; Yoo, D.E.; Lee, J.; et al. Natural Deep Eutectic Solvents as a Storage Medium for Human Interferon-A2: A Green and Improved Strategy for Room-Temperature Biologics. J. Ind. Eng. Chem. 2018, 65, 343–348. [Google Scholar] [CrossRef]

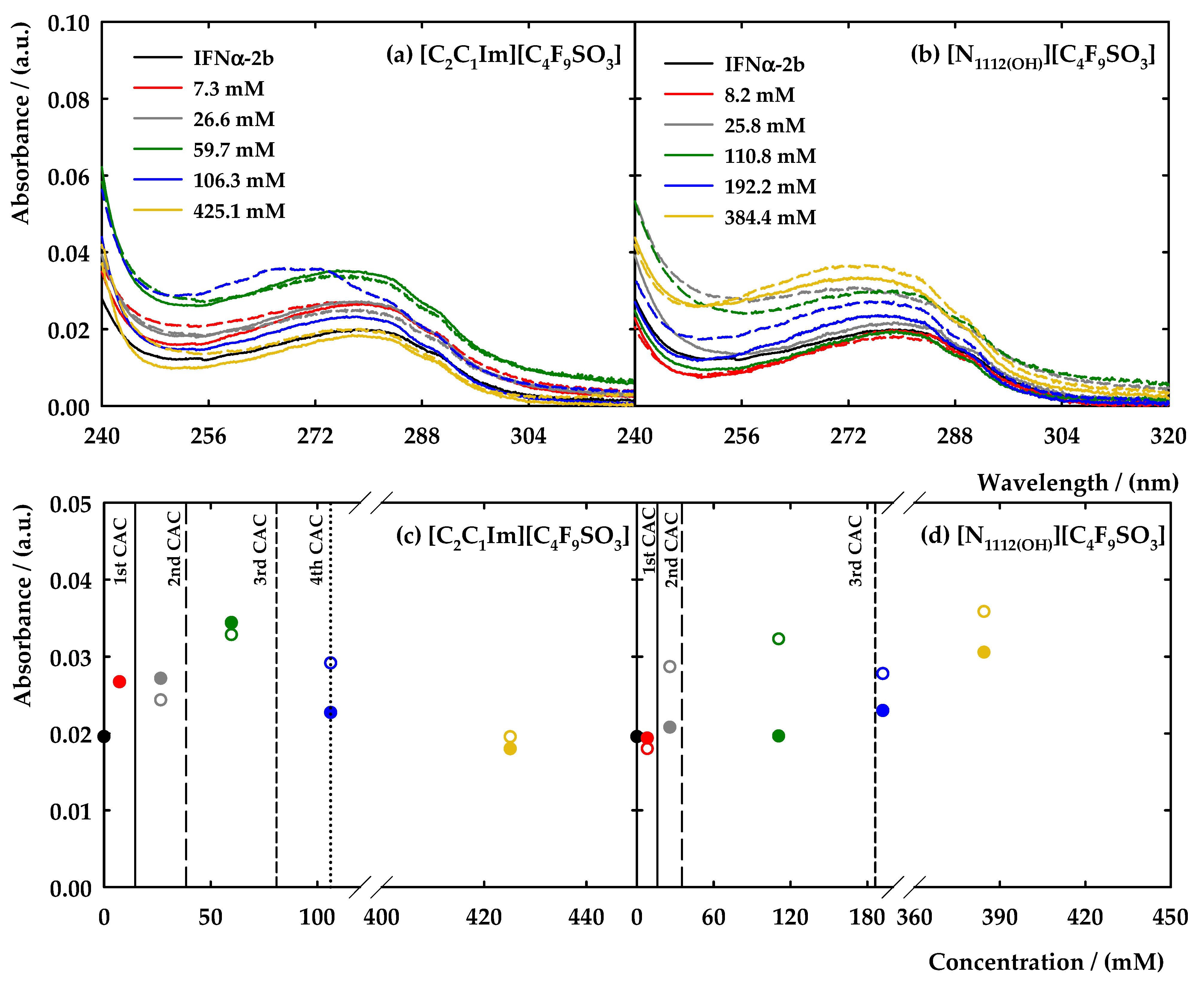
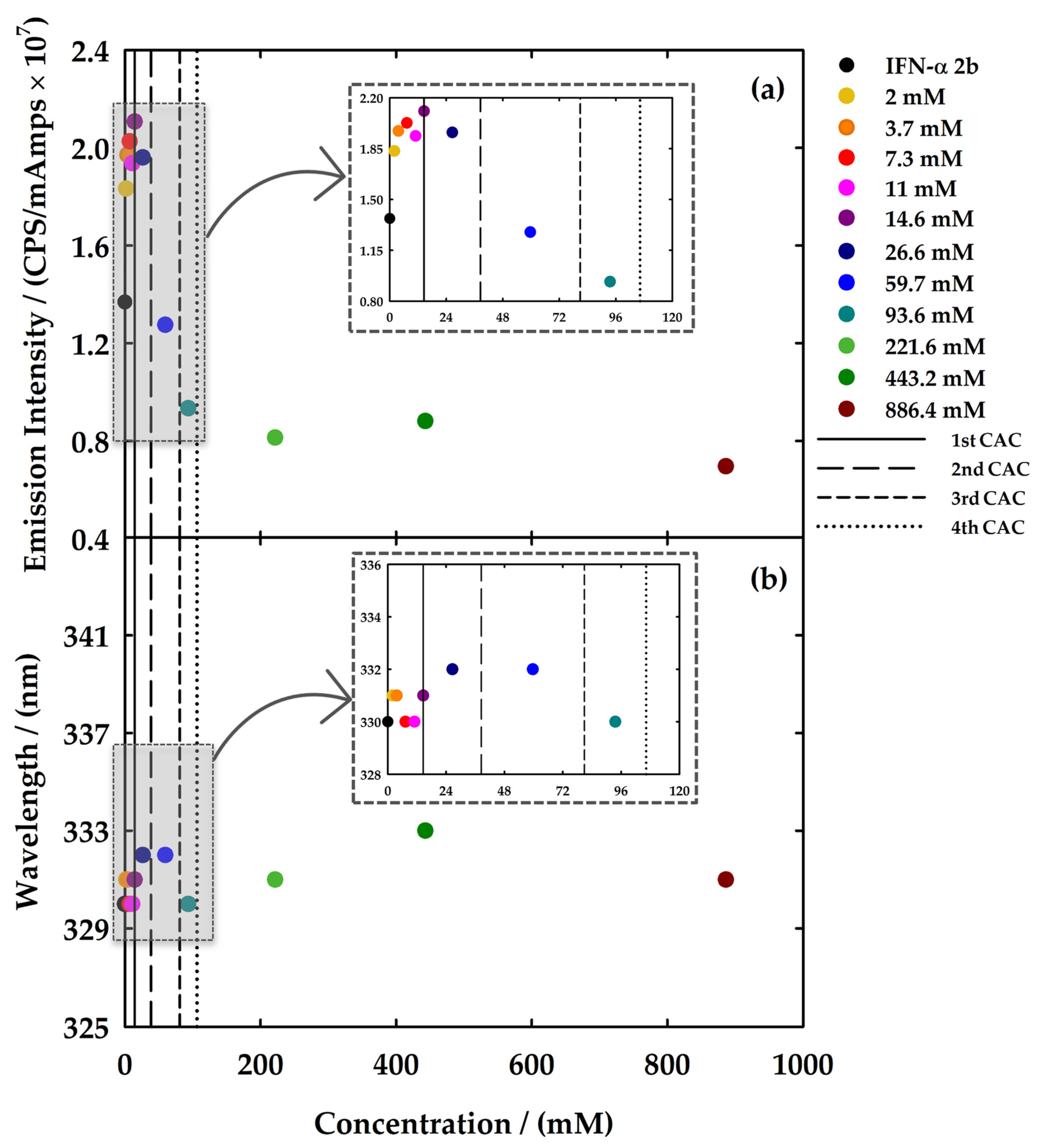
 ) and with 5 μg/mL IFN-α 2b in 5 mM NaH2PO4 (pH = 7.4) (red,
) and with 5 μg/mL IFN-α 2b in 5 mM NaH2PO4 (pH = 7.4) (red,  ). The vertical dotted line in (a) represents the value of CAC determined by surface tension in water on Langmuir 2015, 31, 1283–1295 [38]. The solid and dashed lines represent the fittings to obtain the value of FIL concentration where several breakpoints occurred.
). The vertical dotted line in (a) represents the value of CAC determined by surface tension in water on Langmuir 2015, 31, 1283–1295 [38]. The solid and dashed lines represent the fittings to obtain the value of FIL concentration where several breakpoints occurred.
 ) and with 5 μg/mL IFN-α 2b in 5 mM NaH2PO4 (pH = 7.4) (red,
) and with 5 μg/mL IFN-α 2b in 5 mM NaH2PO4 (pH = 7.4) (red,  ). The vertical dotted line in (a) represents the value of CAC determined by surface tension in water on Langmuir 2015, 31, 1283–1295 [38]. The solid and dashed lines represent the fittings to obtain the value of FIL concentration where several breakpoints occurred.
). The vertical dotted line in (a) represents the value of CAC determined by surface tension in water on Langmuir 2015, 31, 1283–1295 [38]. The solid and dashed lines represent the fittings to obtain the value of FIL concentration where several breakpoints occurred.




 [C2C1Im][C4F9SO3] 1-Ethyl-3-methylimidazolium perfluorobutanesulfonate |  [N1112(OH)][C4F9SO3] (2-Hydroxyethyl)trimethylammonium perfluorobutanesulfonate |
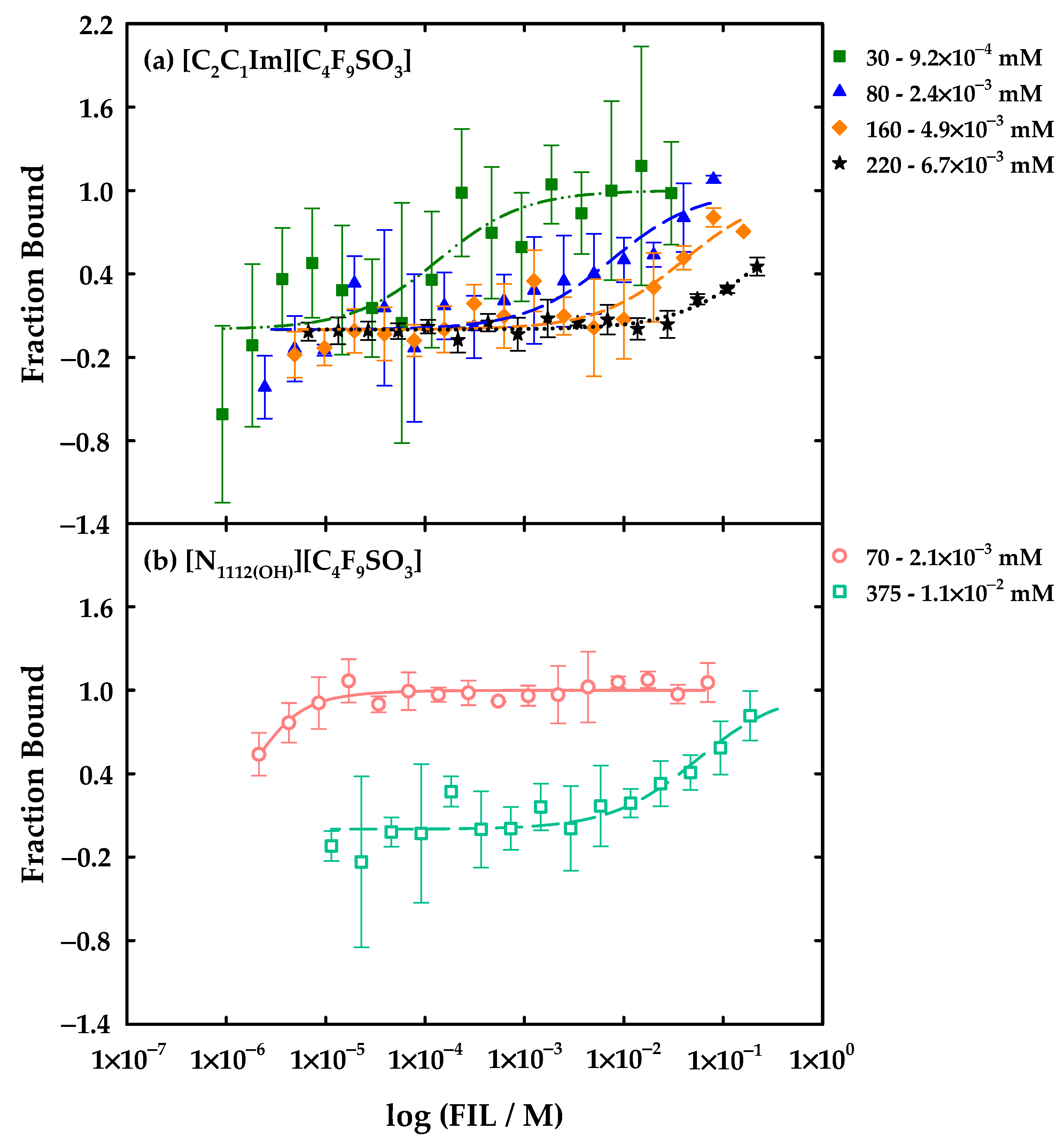
| FIL | Assay | Kd (mM) |
|---|---|---|
| [C2C1Im][C4F9SO3] | 30 − 9.2 × 10−4 mM | 0.136 ± 0.117 a |
| 80 − 2.4 × 10−3 mM | 7.53 ± 5.61 a | |
| 160 − 4.9 × 10−3 mM | 40.5 ± 29.5 a | |
| 220 − 6.7 × 10−3 mM | 263 ± 197 a | |
| [N1112(OH)][C4F9SO3] | 70 − 2.1 × 10−3 mM | 5.97 × 10−4 ± 3.93 × 10−4 a |
| 375 − 1.1 × 10−2 mM | 54.7 ± 36.9 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.L.; Vieira, N.S.M.; Oliveira, A.L.S.; Araújo, J.M.M.; Pereiro, A.B. Disclosing the Potential of Fluorinated Ionic Liquids as Interferon-Alpha 2b Delivery Systems. Nanomaterials 2022, 12, 1851. https://doi.org/10.3390/nano12111851
Ferreira ML, Vieira NSM, Oliveira ALS, Araújo JMM, Pereiro AB. Disclosing the Potential of Fluorinated Ionic Liquids as Interferon-Alpha 2b Delivery Systems. Nanomaterials. 2022; 12(11):1851. https://doi.org/10.3390/nano12111851
Chicago/Turabian StyleFerreira, Margarida L., Nicole S. M. Vieira, Ana L. S. Oliveira, João M. M. Araújo, and Ana B. Pereiro. 2022. "Disclosing the Potential of Fluorinated Ionic Liquids as Interferon-Alpha 2b Delivery Systems" Nanomaterials 12, no. 11: 1851. https://doi.org/10.3390/nano12111851
APA StyleFerreira, M. L., Vieira, N. S. M., Oliveira, A. L. S., Araújo, J. M. M., & Pereiro, A. B. (2022). Disclosing the Potential of Fluorinated Ionic Liquids as Interferon-Alpha 2b Delivery Systems. Nanomaterials, 12(11), 1851. https://doi.org/10.3390/nano12111851








