Covalent Immobilization of Polyaniline Doped with Ag+ or Cu2+ on Carbon Nanotubes for Ethylene Chemical Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Polyaniline (PANI) Oligomers
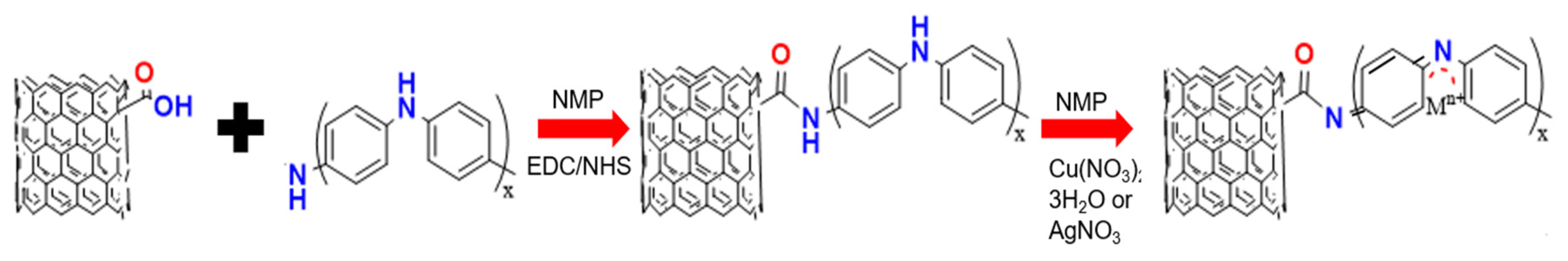
2.3. Preparation of MWCNT/PANI Conjugates
2.4. UV-Vis Spectroscopy
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. High-Resolution Scanning Electron Microscopy (HR-SEM)
2.7. Scanning Electron Cryomicroscopy (Cryo-SEM)
2.8. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
2.9. Thermogravimetric (TGA) Analysis
2.10. Design of the Chemoresistor Sensing Lab (CSL)
2.10.1. Source Measure Unit (SMU)
2.10.2. Diluter and Mass-Flow Controller
2.10.3. The Reservoir
2.10.4. The Chamber
2.10.5. The Sensing System
3. Results and Discussion
3.1. Synthesis of MWCNT/PANI Conjugates and Fabrication of the Sensing Device
3.2. UV-Vis Spectroscopy
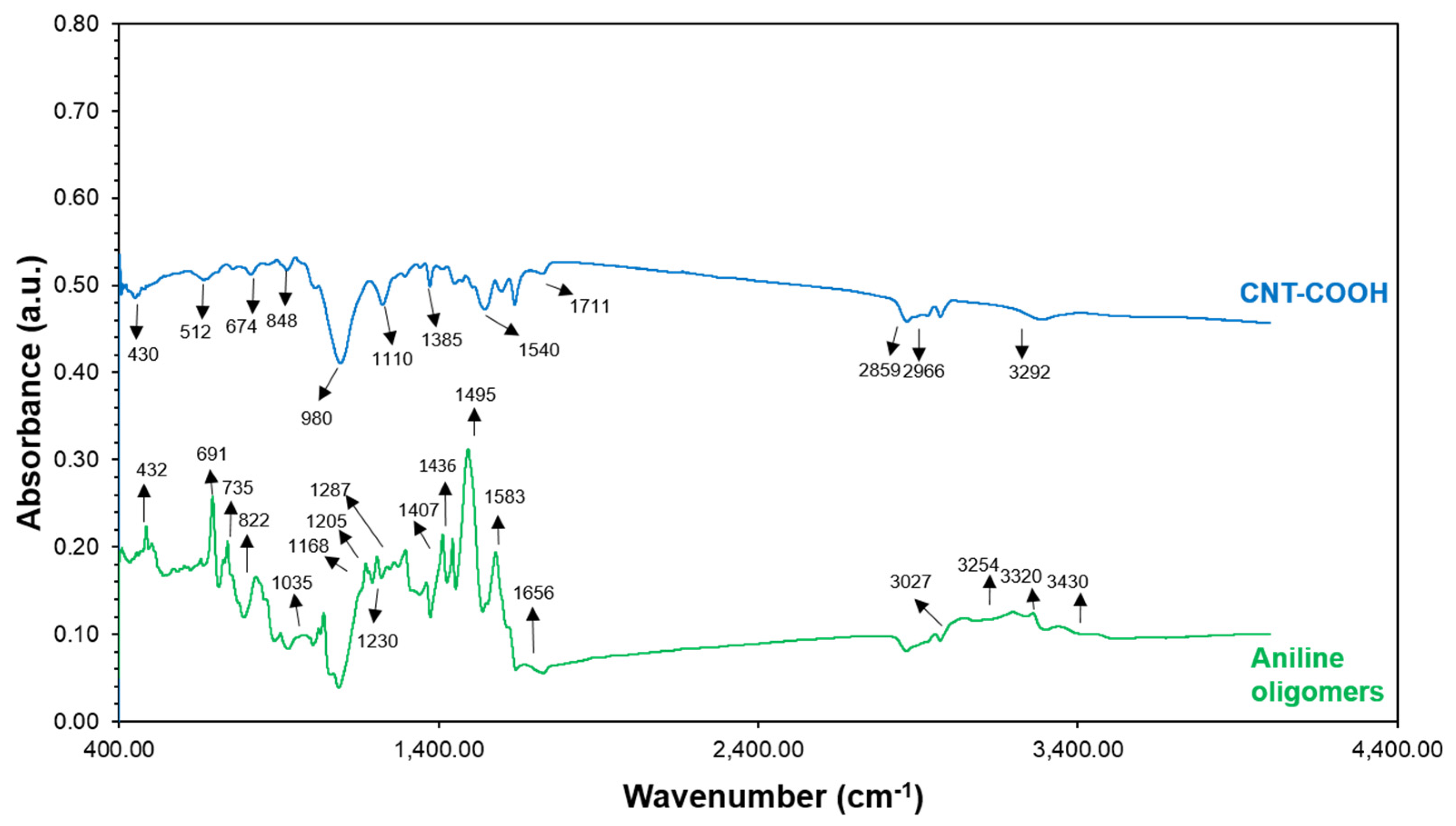
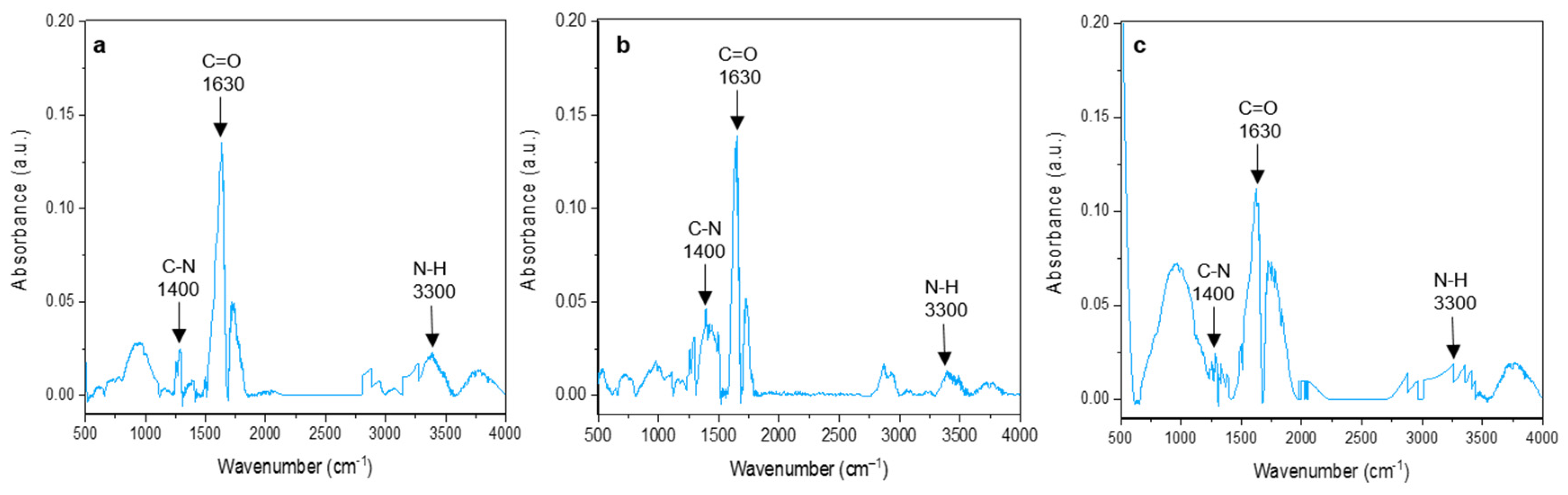
3.3. Fourier-Transform Infrared Spectroscopy (FTIR) Characterization
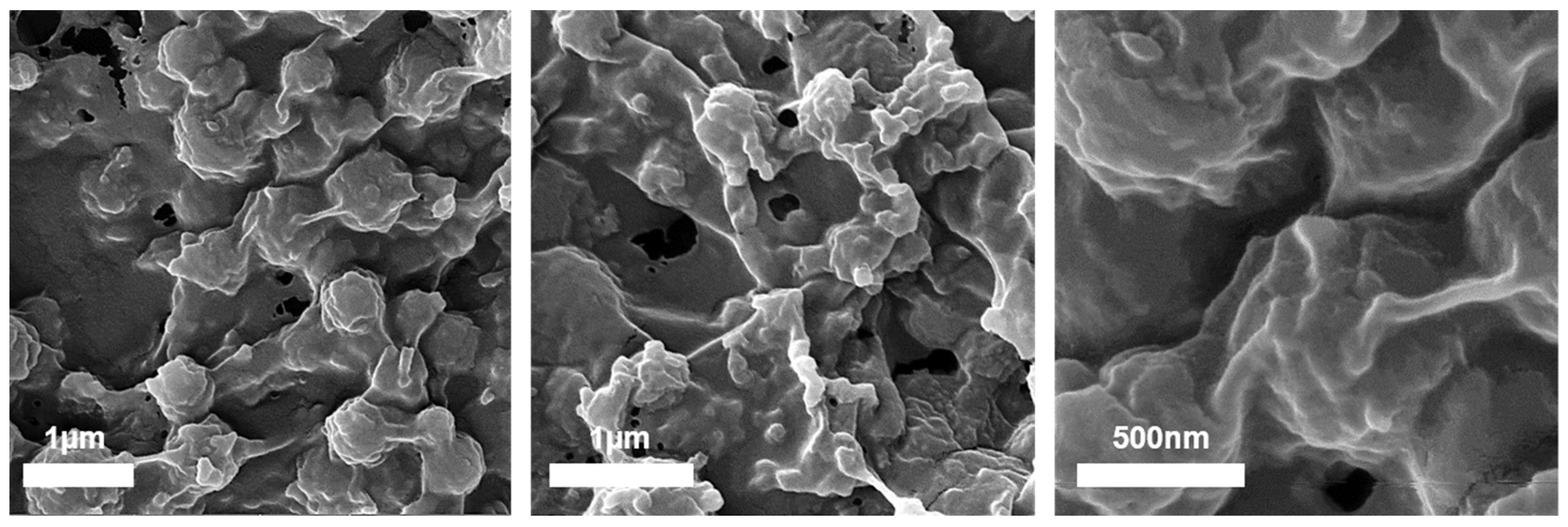
3.4. High-Resolution Scanning Electron Microscopy (HRSEM) and Energy-Dispersive X-ray Spectroscopy (EDX) Characterization
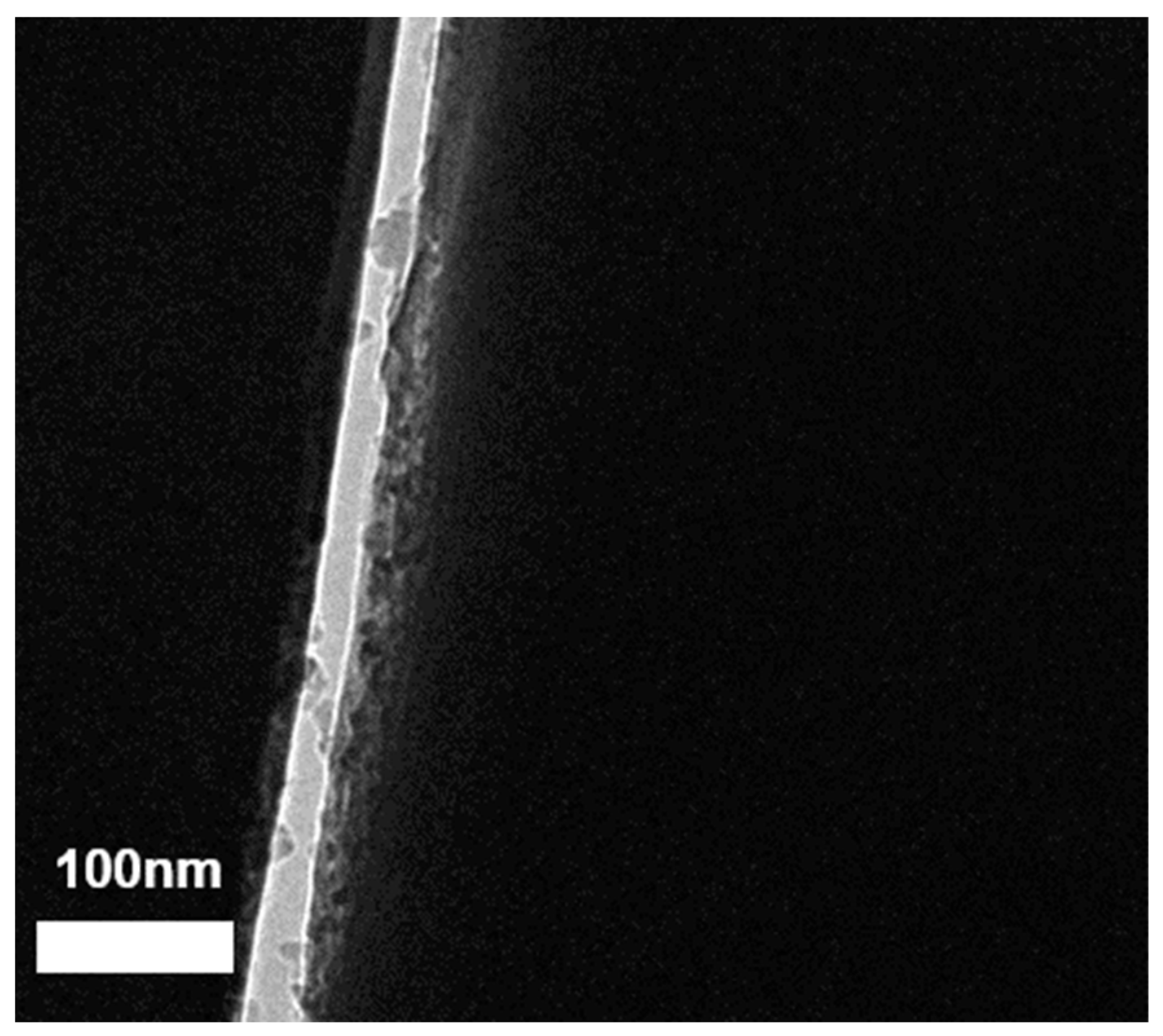
3.5. Scanning Electron Cryomicroscopy (Cryo-SEM) Characterization
3.6. Cryogenic Transmission Electron Microscopy (Cryo-TEM) Characterization
3.7. Thermogravimetric (TGA) Analysis
3.8. MWCNT/PANI Conjugates Incorporated into PVC/Polyacrylate Matrix
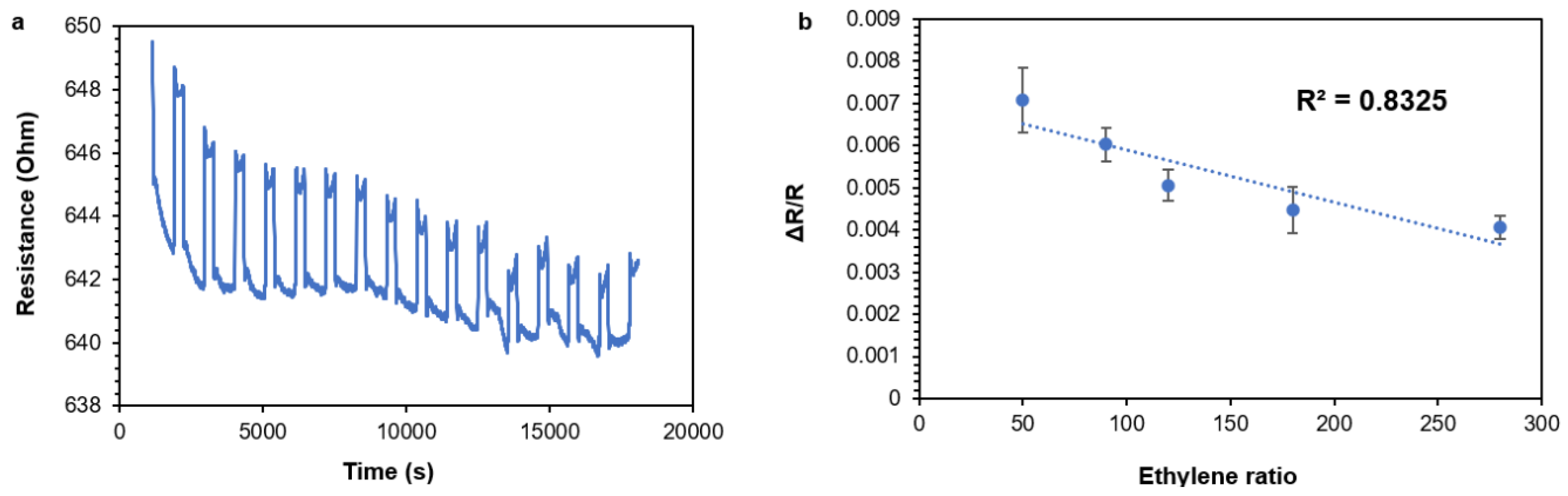
3.9. MWCNT/PANI Conjugates in Ethylene Sensing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Itkis, M.E.; Borondics, F.; Yu, A.; Haddon, R.C. Thermal Conductivity Measurements of Semitransparent Single-Walled Carbon Nanotube Films by a Bolometric Technique. Nano Lett. 2007, 7, 900–904. [Google Scholar] [CrossRef]
- Choi, W.; Harada, D.; Oyaizu, K.; Nishide, H. Aqueous Electrochemistry of Poly(Vinylanthraquinone) for Anode-Active Materials in High-Density and Rechargeable Polymer/Air Batteries. J. Am. Chem. Soc. 2011, 133, 19839–19843. [Google Scholar] [CrossRef]
- Goldman, D.; Lellouche, J.-P. An Easy Method for the Production of Functional Polypyrrole/MWCNT and Polycarbazole/MWCNT Composites Using Nucleophilic Multi-Walled Carbon Nanotubes. Carbon 2010, 48, 4170–4177. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Star, A. Carbon Nanotube Gas and Vapor Sensors. Angew. Chem. Int. Ed. 2008, 47, 6550–6570. [Google Scholar] [CrossRef]
- Schnorr, J.M.; Swager, T.M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 2011, 23, 646–657. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Mubeen, S.; Myung, N.V.; Deshusses, M.A. Recent Progress in Carbon Nanotube-Based Gas Sensors. Nanotechnology 2008, 19, 332001. [Google Scholar] [CrossRef]
- Goldoni, A.; Petaccia, L.; Lizzit, S.; Larciprete, R. Sensing Gases with Carbon Nanotubes: A Review of the Actual Situation. J. Phys. Condens. Matter 2010, 22, 013001. [Google Scholar] [CrossRef]
- Wang, Y.; Yeow, J.T.W. A Review of Carbon Nanotubes-Based Gas Sensors. J. Sens. 2009, 2009, 1–24. [Google Scholar] [CrossRef]
- Meyyappan, M. Carbon Nanotube-Based Chemical Sensors. Small 2016, 12, 2118–2129. [Google Scholar] [CrossRef]
- Haick, H. Chemical Sensors Based on Molecularly Modified Metallic Nanoparticles. J. Phys. D Appl. Phys. 2007, 40, 7173–7186. [Google Scholar] [CrossRef]
- Caprioli, F.; Quercia, L. Ethylene Detection Methods in Post-Harvest Technology: A Review. Sens. Actuators B Chem. 2014, 203, 187–196. [Google Scholar] [CrossRef]
- Saini, P. Intrinsically Conducting Polymer-Based Blends and Composites for Electromagnetic Interference Shielding: Theoretical and Experimental Aspects. In Fundamentals of Conjugated Polymer Blends, Copolymers and Composites; Saini, P., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 449–518. ISBN 978-1-119-13716-0. [Google Scholar]
- Saini, P. Electrical Properties and Electromagnetic Interference Shielding Response of Electrically Conducting Thermosetting Nanocomposites. In Thermoset Nanocomposites; Mittal, V., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 211–237. ISBN 978-3-527-65964-7. [Google Scholar]
- Kara, S.; Arda, E.; Dolastir, F.; Pekcan, Ö. Electrical and Optical Percolations of Polystyrene Latex–Multiwalled Carbon Nanotube Composites. J. Colloid Interface Sci. 2010, 344, 395–401. [Google Scholar] [CrossRef]
- Kyrylyuk, A.V.; Hermant, M.C.; Schilling, T.; Klumperman, B.; Koning, C.E.; van der Schoot, P. Controlling Electrical Percolation in Multicomponent Carbon Nanotube Dispersions. Nat. Nanotechnol. 2011, 6, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Bănică, F.-G. Chemical Sensors and Biosensors: Fundamentals and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2012; ISBN 978-1-118-35416-2. [Google Scholar]
- Cass, T. Nanosensors: Physical, Chemical, and Biological. Phys. Today 2012, 65, 55–56. [Google Scholar] [CrossRef] [Green Version]
- Esser, B.; Schnorr, J.M.; Swager, T.M. Selective Detection of Ethylene Gas Using Carbon Nanotube-Based Devices: Utility in Determination of Fruit Ripeness. Angew. Chem. Int. Ed. 2012, 51, 5752–5756. [Google Scholar] [CrossRef]
- Mechrez, G.; Krepker, M.A.; Harel, Y.; Lellouche, J.-P.; Segal, E. Biocatalytic Carbon Nanotube Paper: A ‘One-Pot’ Route for Fabrication of Enzyme-Immobilized Membranes for Organophosphate Bioremediation. J. Mater. Chem. B 2014, 2, 915–922. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon Properties and Their Role in Supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, J.; Liu, Y.; Xu, G.; Chen, Z.; Xu, Q. Controlled Growth of Whisker-like Polyaniline on Carbon Nanofibers and Their Long Cycle Life for Supercapacitors. RSC Adv. 2013, 3, 3957. [Google Scholar] [CrossRef]
- Ryu, K.S.; Kim, K.M.; Park, N.-G.; Park, Y.J.; Chang, S.H. Symmetric Redox Supercapacitor with Conducting Polyaniline Electrodes. J. Power Sources 2002, 103, 305–309. [Google Scholar] [CrossRef]
- Xing, S.; Zhao, C.; Jing, S.; Wang, Z. Morphology and Conductivity of Polyaniline Nanofibers Prepared by ‘Seeding’ Polymerization. Polymer 2006, 47, 2305–2313. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, H.; Wang, X.; Li, J.; Wang, F. Sandwich Nanocomposites of Polyaniline Embedded between Graphene Layers and Multi-Walled Carbon Nanotubes for Cycle-Stable Electrode Materials of Organic Supercapacitors. RSC Adv. 2013, 3, 1797–1807. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Charge Storage Mechanism of MnO2 Electrode Used in Aqueous Electrochemical Capacitor. Chem. Mater. 2004, 16, 3184–3190. [Google Scholar] [CrossRef]
- Wei, J.; Xing, G.; Gao, L.; Suo, H.; He, X.; Zhao, C.; Li, S.; Xing, S. Nickel Foam Based Polypyrrole–Ag Composite Film: A New Route toward Stable Electrodes for Supercapacitors. New J. Chem. 2013, 37, 337–341. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Saber, O.; Ahmed, F.; Aljaafari, A.; Kumar, S. Growth of Defect-Induced Carbon Nanotubes for Low-Temperature Fruit Monitoring Sensor. Chemosensors 2021, 9, 131. [Google Scholar] [CrossRef]
- Vong, K.; Eda, S.; Kadota, Y.; Nasibullin, I.; Wakatake, T.; Yokoshima, S.; Shirasu, K.; Tanaka, K. An Artificial Metalloenzyme Biosensor Can Detect Ethylene Gas in Fruits and Arabidopsis Leaves. Nat. Commun. 2019, 10, 5746. [Google Scholar] [CrossRef] [Green Version]
- Leangtanom, P.; Wisitsoraat, A.; Jaruwongrungsee, K.; Chanlek, N.; Phanichphant, S.; Kruefu, V. Highly Sensitive and Selective Ethylene Gas Sensors Based on CeOx-SnO2 Nanocomposites Prepared by a Co-Precipitation Method. Mater. Chem. Phys. 2020, 254, 123540. [Google Scholar] [CrossRef]
- Fong, D.; Luo, S.-X.; Andre, R.S.; Swager, T.M. Trace Ethylene Sensing via Wacker Oxidation. ACS Cent. Sci. 2020, 6, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, S.; Bahuguna, A.; Kumar, S.; Krishnan, V.; Labuta, J.; Nakanishi, T.; Tanaka, T.; Kataura, H.; Kon, Y.; Hong, D. Cascade Reaction-Based Chemiresistive Array for Ethylene Sensing. ACS Sens. 2020, 5, 1405–1410. [Google Scholar] [CrossRef]
- Higuchi, M.; Imoda, D.; Hirao, T. Redox Behavior of Polyaniline−Transition Metal Complexes in Solution. Macromolecules 1996, 29, 8277–8279. [Google Scholar] [CrossRef]
- Dias, H.V.R.; Wu, J. Structurally Characterized Coinage-Metal–Ethylene Complexes. Eur. J. Inorg. Chem. 2008, 2008, 499. [Google Scholar] [CrossRef]
- Albertin, G.; Antoniutti, S.; Botter, A.; Castro, J. Preparation of Hydride-Ethylene Complexes of Osmium: Preparation of Hydride-Ethylene Complexes of Osmium. Z. Anorg. Allg. Chem. 2016, 642, 250–254. [Google Scholar] [CrossRef]
- Nakano, R.; Chung, L.W.; Watanabe, Y.; Okuno, Y.; Okumura, Y.; Ito, S.; Morokuma, K.; Nozaki, K. Elucidating the Key Role of Phosphine−Sulfonate Ligands in Palladium-Catalyzed Ethylene Polymerization: Effect of Ligand Structure on the Molecular Weight and Linearity of Polyethylene. ACS Catal. 2016, 6, 6101–6113. [Google Scholar] [CrossRef]
- Swarts, A.J.; Zheng, F.; Smith, V.J.; Nordlander, E.; Mapolie, S.F. Reactivity of Bis(Pyridyl)-N-Alkylaminato Methylpalladium Complexes toward Ethylene: Insights from Experiment and Theory. Organometallics 2014, 33, 2247–2256. [Google Scholar] [CrossRef]
- Chatt, J.; Duncanson, L.A. 586. Olefin Co-Ordination Compounds. Part III. Infra-Red Spectra and Structure: Attempted Preparation of Acetylene Complexes. J. Chem. Soc. 1953, 2939. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M. Aniline Oligomers versus Polyaniline. Polym. Int. 2012, 61, 240–251. [Google Scholar] [CrossRef]
- Dimitriev, O.P. Doping of Polyaniline by Transition-Metal Salts. Macromolecules 2004, 37, 3388–3395. [Google Scholar] [CrossRef]
- Butenko, A.V.; Shalaev, V.M.; Stockman, M.I. Fractals: Giant Impurity Nonlinearities in Optics of Fractal Clusters. Z. Phys. D At. Mol. Clust. 1988, 10, 81–92. [Google Scholar] [CrossRef]
- Mechrez, G.; Suckeveriene, R.Y.; Zelikman, E.; Rosen, J.; Ariel-Sternberg, N.; Cohen, R.; Narkis, M.; Segal, E. Highly-Tunable Polymer/Carbon Nanotubes Systems: Preserving Dispersion Architecture in Solid Composites via Rapid Microfiltration. ACS Macro Lett. 2012, 1, 848–852. [Google Scholar] [CrossRef]
- Wang, W.; Ruderer, M.A.; Metwalli, E.; Guo, S.; Herzig, E.M.; Perlich, J.; Müller-Buschbaum, P. Effect of Methanol Addition on the Resistivity and Morphology of PEDOT:PSS Layers on Top of Carbon Nanotubes for Use as Flexible Electrodes. ACS Appl. Mater. Interfaces 2015, 7, 8789–8797. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, H.; Mani, K.A.; Chauhan, V.; Yaakov, N.; Grzegorzewski, F.; Domb, A.J.; Mechrez, G. Covalent Immobilization of Polyaniline Doped with Ag+ or Cu2+ on Carbon Nanotubes for Ethylene Chemical Sensing. Nanomaterials 2021, 11, 1993. https://doi.org/10.3390/nano11081993
Klein H, Mani KA, Chauhan V, Yaakov N, Grzegorzewski F, Domb AJ, Mechrez G. Covalent Immobilization of Polyaniline Doped with Ag+ or Cu2+ on Carbon Nanotubes for Ethylene Chemical Sensing. Nanomaterials. 2021; 11(8):1993. https://doi.org/10.3390/nano11081993
Chicago/Turabian StyleKlein, Hagai, Karthik Ananth Mani, Vinay Chauhan, Noga Yaakov, Franziska Grzegorzewski, Abraham J. Domb, and Guy Mechrez. 2021. "Covalent Immobilization of Polyaniline Doped with Ag+ or Cu2+ on Carbon Nanotubes for Ethylene Chemical Sensing" Nanomaterials 11, no. 8: 1993. https://doi.org/10.3390/nano11081993
APA StyleKlein, H., Mani, K. A., Chauhan, V., Yaakov, N., Grzegorzewski, F., Domb, A. J., & Mechrez, G. (2021). Covalent Immobilization of Polyaniline Doped with Ag+ or Cu2+ on Carbon Nanotubes for Ethylene Chemical Sensing. Nanomaterials, 11(8), 1993. https://doi.org/10.3390/nano11081993







