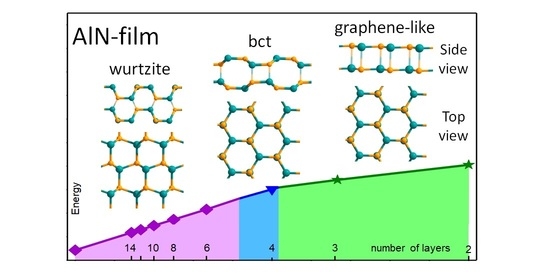
The Features of Phase Stability of GaN and AlN Films at Nanolevel
Abstract
1. Introduction
2. Methods
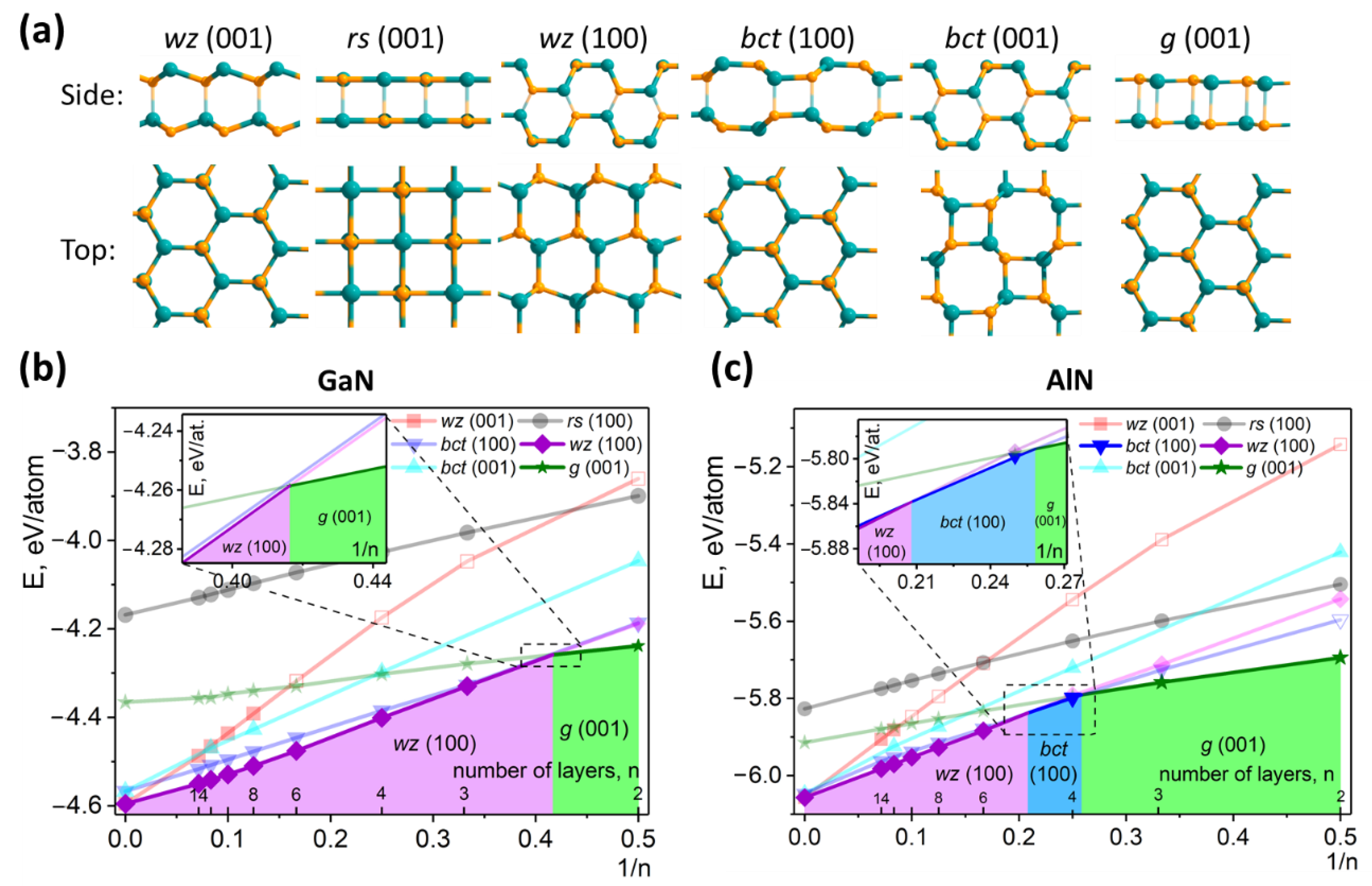
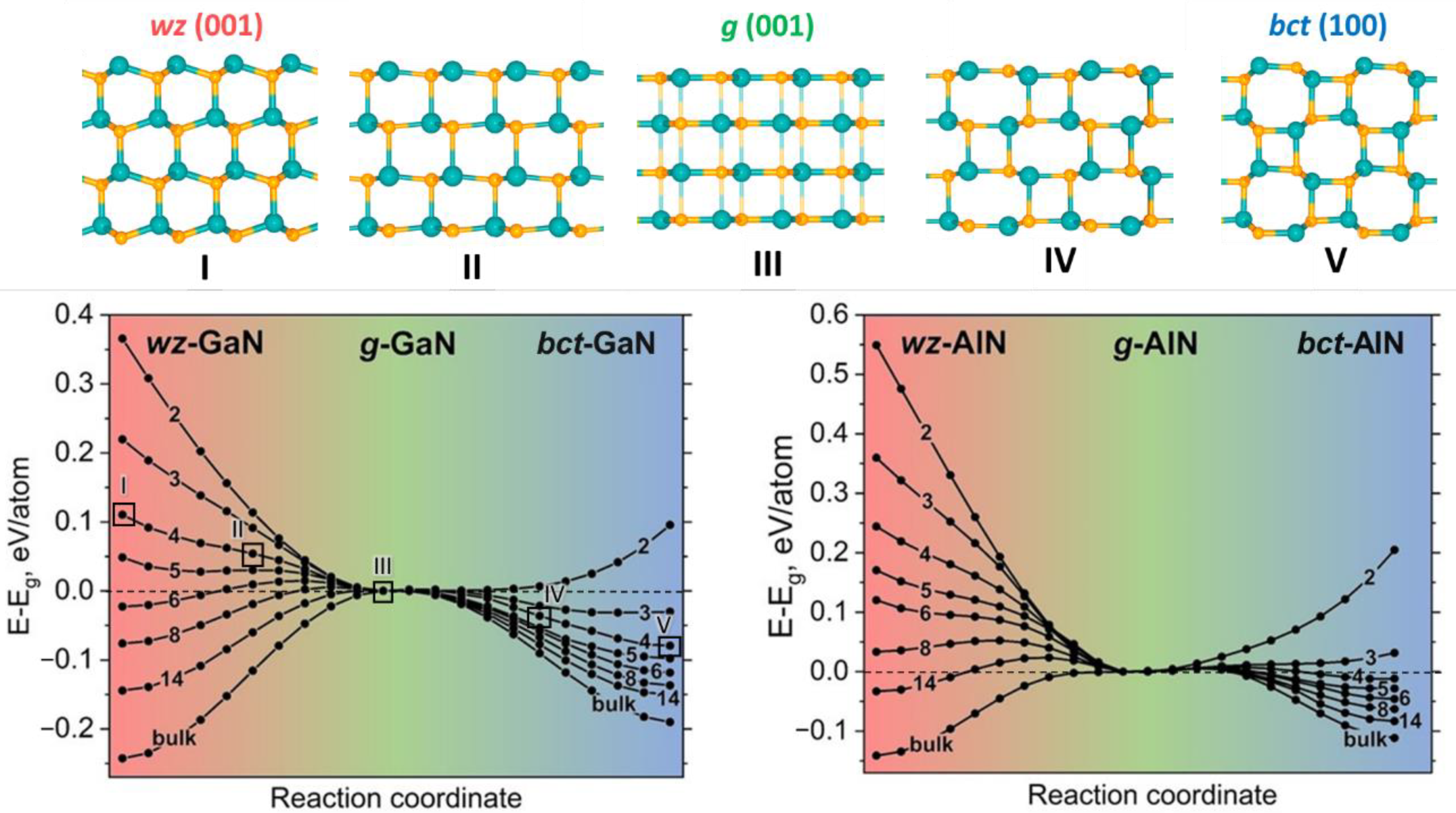
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S. Nobel Lecture: Graphene: Materials in the Flatland. Rev. Mod. Phys. 2011, 83, 837–849. [Google Scholar] [CrossRef]
- Kvashnin, A.G.; Chernozatonskii, L.A.; Yakobson, B.I.; Sorokin, P.B. Phase Diagram of Quasi-Two-Dimensional Carbon, From Graphene to Diamond. Nano Lett. 2014, 14, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Bakharev, P.V.; Huang, M.; Saxena, M.; Lee, S.W.; Joo, S.H.; Park, S.O.; Dong, J.; Camacho-Mojica, D.C.; Jin, S.; Kwon, Y.; et al. Chemically induced transformation of chemical vapour deposition grown bilayer graphene into fluorinated single-layer diamond. Nat. Nanotechnol. 2019, 15, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Erohin, S.V.; Ruan, Q.; Sorokin, P.B.; Yakobson, B.I. Nano-thermodynamics of chemically induced graphene-diamond transformation. Small 2020, 16, 2070256. [Google Scholar] [CrossRef]
- Sorokin, P.B.; Kvashnin, A.G.; Zhu, Z.; Tománek, D. Spontaneous Graphitization of Ultrathin Cubic Structures: A Computational Study. Nano Lett. 2014, 14, 7126–7130. [Google Scholar] [CrossRef]
- Kvashnin, A.G.; Pashkin, E.Y.; Yakobson, B.I.; Sorokin, P.B. Ionic Graphitization of Ultrathin Films of Ionic Compounds. J. Phys. Chem. Lett. 2016, 7, 2659–2663. [Google Scholar] [CrossRef]
- Freeman, C.L.; Claeyssens, F.; Allan, N.L.; Harding, J.H. Graphitic Nanofilms as Precursors to Wurtzite Films: Theory. Phys. Rev. Lett. 2006, 96, 066102. [Google Scholar] [CrossRef]
- Al Balushi, Z.Y.; Wang, K.; Ghosh, R.K.; Vilá, R.A.; Eichfeld, S.M.; Caldwell, J.D.; Qin, X.; Lin, Y.-C.; DeSario, P.A.; Stone, G.; et al. Two-dimensional gallium nitride realized via graphene encapsulation. Nat. Mater. 2016, 15, 1166–1171. [Google Scholar] [CrossRef]
- Tsipas, P.; Kassavetis, S.; Tsoutsou, D.; Xenogiannopoulou, E.; Golias, E.; Giamini, S.A.; Grazianetti, C.; Chiappe, D.; Molle, A.; Fanciulli, M.; et al. Evidence for graphite-like hexagonal AlN nanosheets epitaxially grown on single crystal Ag(111). Appl. Phys. Lett. 2013, 103, 251605. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K.; Liu, J.; Lv, T.; Wei, B.; Zhang, T.; Zeng, M.; Wang, Z.; Fu, L. Growth of 2D GaN Single Crystals on Liquid Metals. J. Am. Chem. Soc. 2018, 140, 16392–16395. [Google Scholar] [CrossRef]
- Sun, A.; Gao, S.-P.; Gu, G. Stability and electronic properties of GaN phases with inversion symmetry to inherently inhibit polarization. Phys. Rev. Mater. 2019, 3, 104604. [Google Scholar] [CrossRef]
- Kecik, D.; Onen, A.; Konuk, M.; Gürbüz, E.; Ersan, F.; Cahangirov, S.; Aktürk, E.; Durgun, E.; Ciraci, S. Fundamentals, progress, and future directions of nitride-based semiconductors and their composites in two-dimensional limit: A first-principles perspective to recent synthesis. Appl. Phys. Rev. 2018, 5, 011105. [Google Scholar] [CrossRef]
- Brus, L.E. Electron–electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, C.; Zhai, T.; Li, S.-L.; Wang, X.; Liu, J.; Jie, X.; Liu, D.; Liao, M.; Koide, Y.; et al. Flexible Ultraviolet Photodetectors with Broad Photoresponse Based on Branched ZnS-ZnO Heterostructure Nanofilms. Adv. Mater. 2014, 26, 3088–3093. [Google Scholar] [CrossRef]
- Shin, G.; Kim, H.-Y.; Kim, J. Deep-ultraviolet photodetector based on exfoliated n-type β-Ga2O3 nanobelt/p-Si substrate heterojunction. Korean J. Chem. Eng. 2018, 35, 574–578. [Google Scholar] [CrossRef]
- Taniyasu, Y.; Kasu, M. Polarization property of deep-ultraviolet light emission from C-plane AlN/GaN short-period superlattices. Appl. Phys. Lett. 2011, 99, 251112. [Google Scholar] [CrossRef]
- Verma, J.; Islam, S.M.; Protasenko, V.; Kumar Kandaswamy, P.; Xing, H.; Jena, D. Tunnel-injection quantum dot deep-ultraviolet light-emitting diodes with polarization-induced doping in III-nitride heterostructures. Appl. Phys. Lett. 2014, 104, 021105. [Google Scholar] [CrossRef]
- Tsao, J.Y.; Chowdhury, S.; Hollis, M.A.; Jena, D.; Johnson, N.M.; Jones, K.A.; Kaplar, R.J.; Rajan, S.; de Walle, C.G.V.; Bellotti, E.; et al. Ultrawide-Bandgap Semiconductors: Research Opportunities and Challenges. Adv. Electron. Mater. 2018, 4, 1600501. [Google Scholar] [CrossRef]
- Wang, Y.; Song, N.; Song, X.; Zhang, T.; Yang, D.; Li, M. A first-principles study of gas adsorption on monolayer AlN sheet. Vacuum 2018, 147, 18–23. [Google Scholar] [CrossRef]
- Gürbüz, E.; Cahangirov, S.; Durgun, E.; Ciraci, S. Single layers and multilayers of GaN and AlN in square-octagon structure: Stability, electronic properties, and functionalization. Phys. Rev. B 2017, 96, 205427. [Google Scholar] [CrossRef]
- Camacho-Mojica, D.C.; López-Urías, F. GaN Haeckelite Single-Layered Nanostructures: Monolayer and Nanotubes. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.A.; Shuford, K.L. Archimedean (4,8)-tessellation of haeckelite ultrathin nanosheets composed of boron and aluminum-group V binary materials. Nanoscale 2016, 8, 19287–19301. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, F.-S.; Wu, Y.-B. Two single-layer porous gallium nitride nanosheets: A first-principles study. Solid State Commun. 2017, 250, 18–22. [Google Scholar] [CrossRef]
- Kolobov, A.V.; Fons, P.; Tominaga, J.; Hyot, B.; André, B. Instability and Spontaneous Reconstruction of Few-Monolayer Thick GaN Graphitic Structures. Nano Lett. 2016, 16, 4849–4856. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shu, H.; Liang, P.; Zhou, X.; Cao, D.; Chen, X. Intriguing electronic structures and carrier mobilities of two-dimensional GaN nanosheets: Thickness and surface effects. Comput. Mater. Sci. 2020, 172, 109337. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mat. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Schmerler, S.; Kortus, J. Ab initio study of AlN: Anisotropic thermal expansion, phase diagram, and high-temperature rocksalt to wurtzite phase transition. Phys. Rev. B 2014, 89, 064109. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Dion, M.; Rydberg, H.; Schröder, E.; Langreth, D.C.; Lundqvist, B.I. Van der Waals Density Functional for General Geometries. Phys. Rev. Lett. 2004, 92, 246401. [Google Scholar] [CrossRef] [PubMed]
- Bacaksiz, C.; Sahin, H.; Ozaydin, H.D.; Horzum, S.; Senger, R.T.; Peeters, F.M. Hexagonal AlN: Dimensional-crossover-driven band-gap transition. Phys. Rev. B 2015, 91, 085430. [Google Scholar] [CrossRef]
- Xu, D.; He, H.; Pandey, R.; Karna, S.P. Stacking and electric field effects in atomically thin layers of GaN. J. Phys. Condens. Matter 2013, 25, 345302. [Google Scholar] [CrossRef]
- Brandt, M.S.; Ager, J.W.; Götz, W.; Johnson, N.M.; Harris, J.S.; Molnar, R.J.; Moustakas, T.D. Local vibrational modes in Mg-doped gallium nitride. Phys. Rev. B 1994, 49, 14758–14761. [Google Scholar] [CrossRef]
- Schwarz, M.R.; Antlauf, M.; Schmerler, S.; Keller, K.; Schlothauer, T.; Kortus, J.; Heide, G.; Kroke, E. Formation and properties of rocksalt-type AlN and implications for high pressure phase relations in the system Si–Al–O–N. High. Press. Res. 2014, 34, 22–38. [Google Scholar] [CrossRef]
- Tikhomirova, N.A.; Tantardini, C.; Sukhanova, E.V.; Popov, Z.I.; Evlashin, V.P.; Martovitsky, V.P.; Tarkhov, M.A.; Dudin, A.A.; Oganov, A.R.; Kvashnin, D.G.; et al. Exotic Two-Dimensional Structure: The First Case of Hexagonal NaCl. J. Phys. Chem. Lett. 2020, 11, 3821–3827. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chepkasov, I.V.; Erohin, S.V.; Sorokin, P.B. The Features of Phase Stability of GaN and AlN Films at Nanolevel. Nanomaterials 2021, 11, 8. https://doi.org/10.3390/nano11010008
Chepkasov IV, Erohin SV, Sorokin PB. The Features of Phase Stability of GaN and AlN Films at Nanolevel. Nanomaterials. 2021; 11(1):8. https://doi.org/10.3390/nano11010008
Chicago/Turabian StyleChepkasov, Ilya V., Sergey V. Erohin, and Pavel B. Sorokin. 2021. "The Features of Phase Stability of GaN and AlN Films at Nanolevel" Nanomaterials 11, no. 1: 8. https://doi.org/10.3390/nano11010008
APA StyleChepkasov, I. V., Erohin, S. V., & Sorokin, P. B. (2021). The Features of Phase Stability of GaN and AlN Films at Nanolevel. Nanomaterials, 11(1), 8. https://doi.org/10.3390/nano11010008