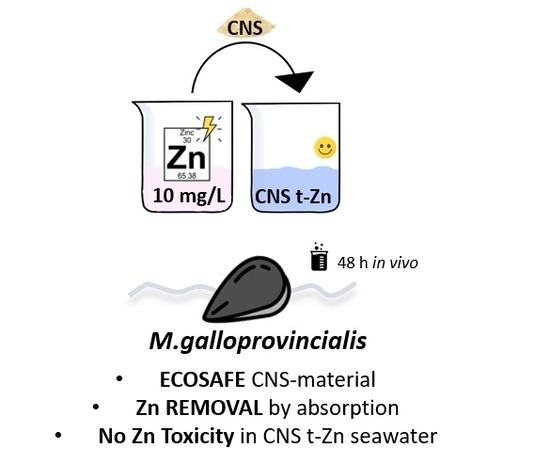
Effect-Based Approach to Assess Nanostructured Cellulose Sponge Removal Efficacy of Zinc Ions from Seawater to Prevent Ecological Risks
Abstract
1. Introduction
2. Materials and Methods
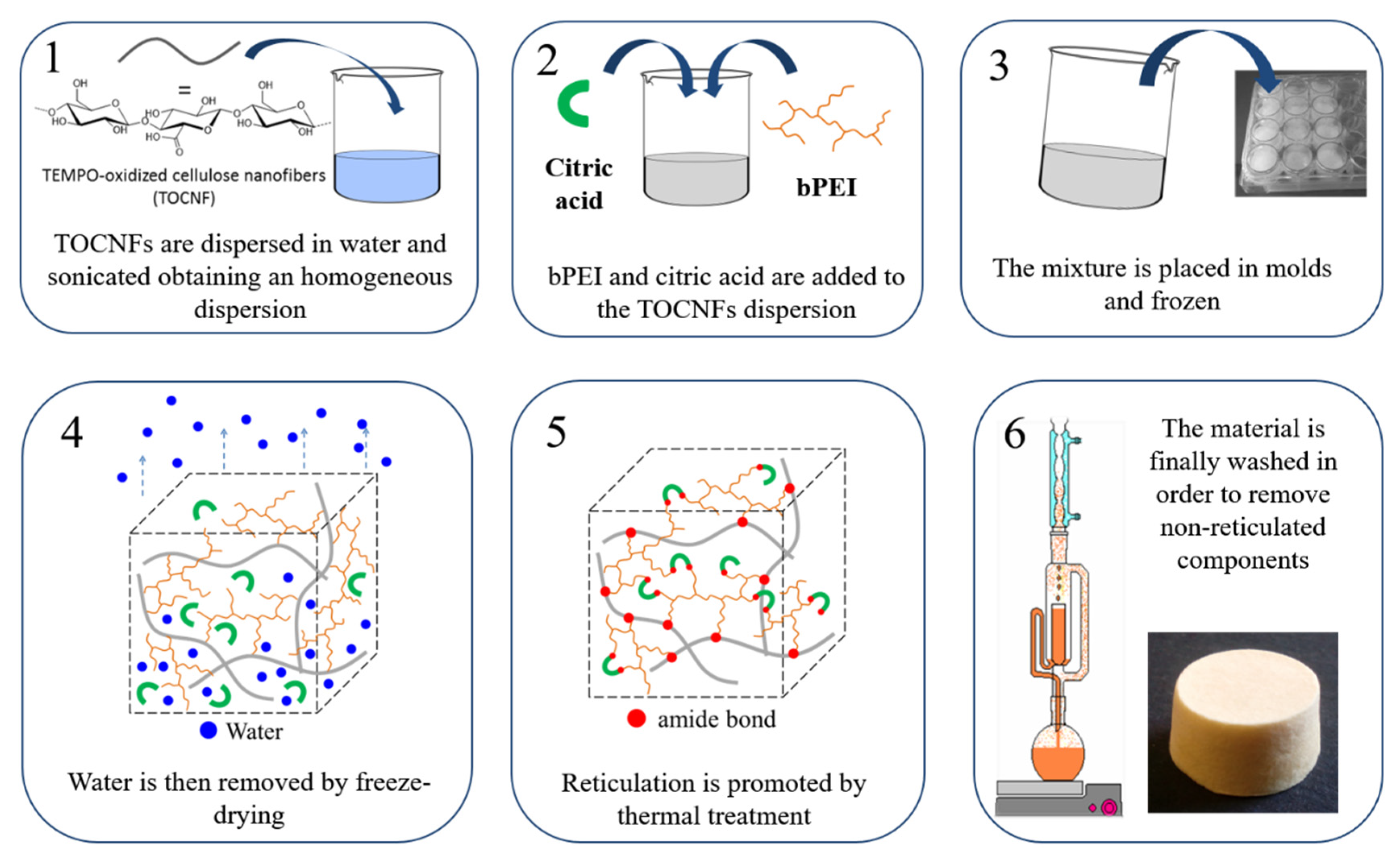
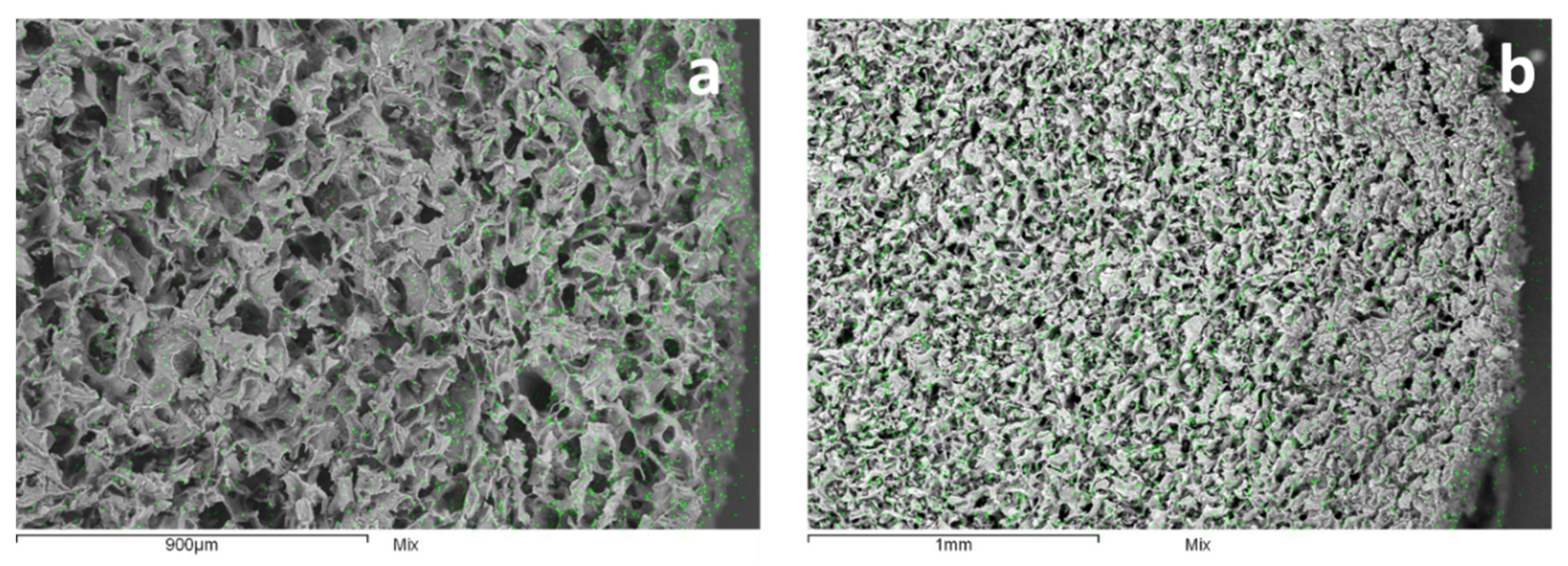
2.1. CNS Synthesis, Characterization and Zn(II) Adsorption
2.2. Experimental Design of Effect-Based Study
2.2.1. In Vivo Waterborne Exposure
2.2.2. Cellular Bioassays
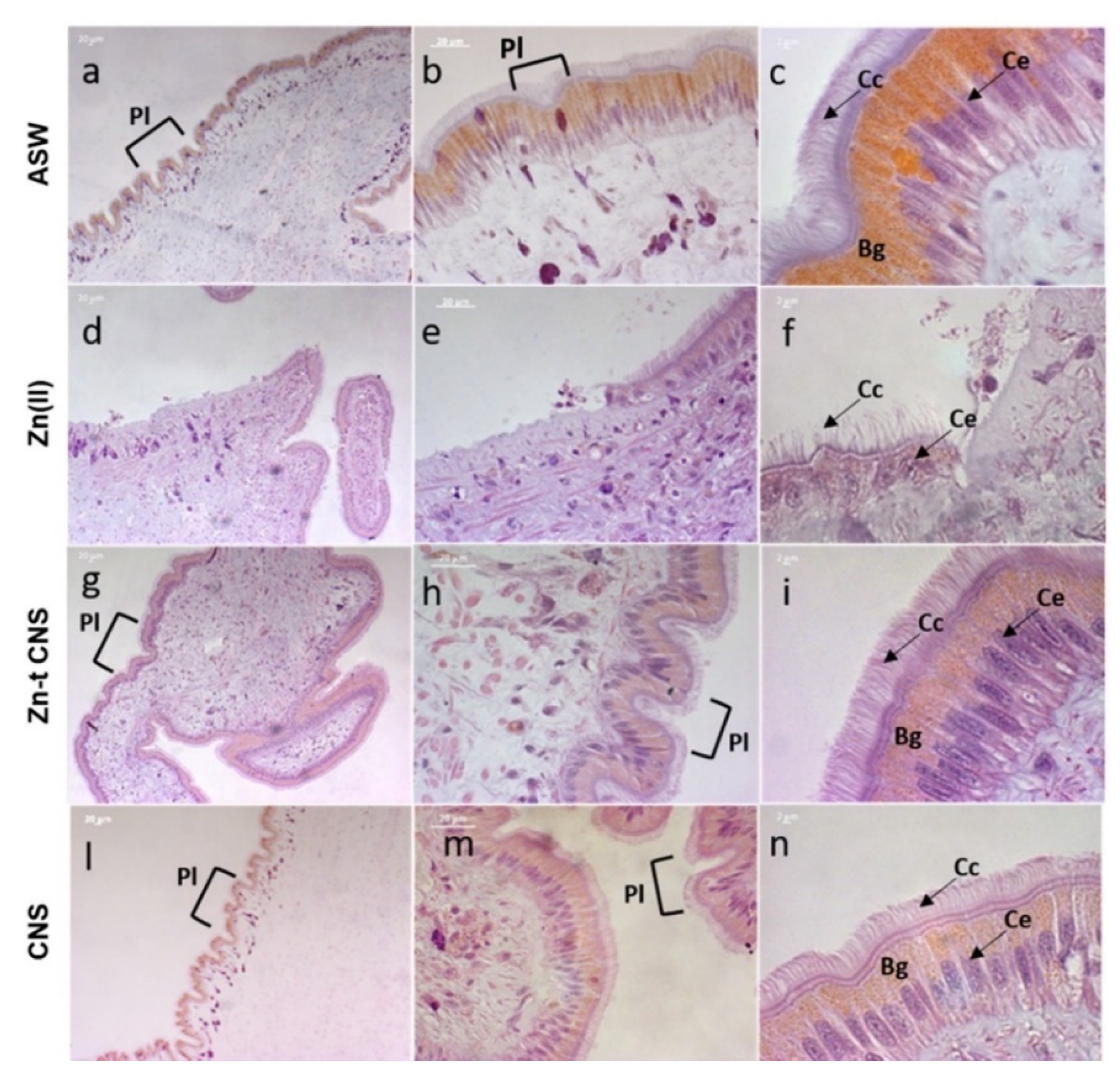
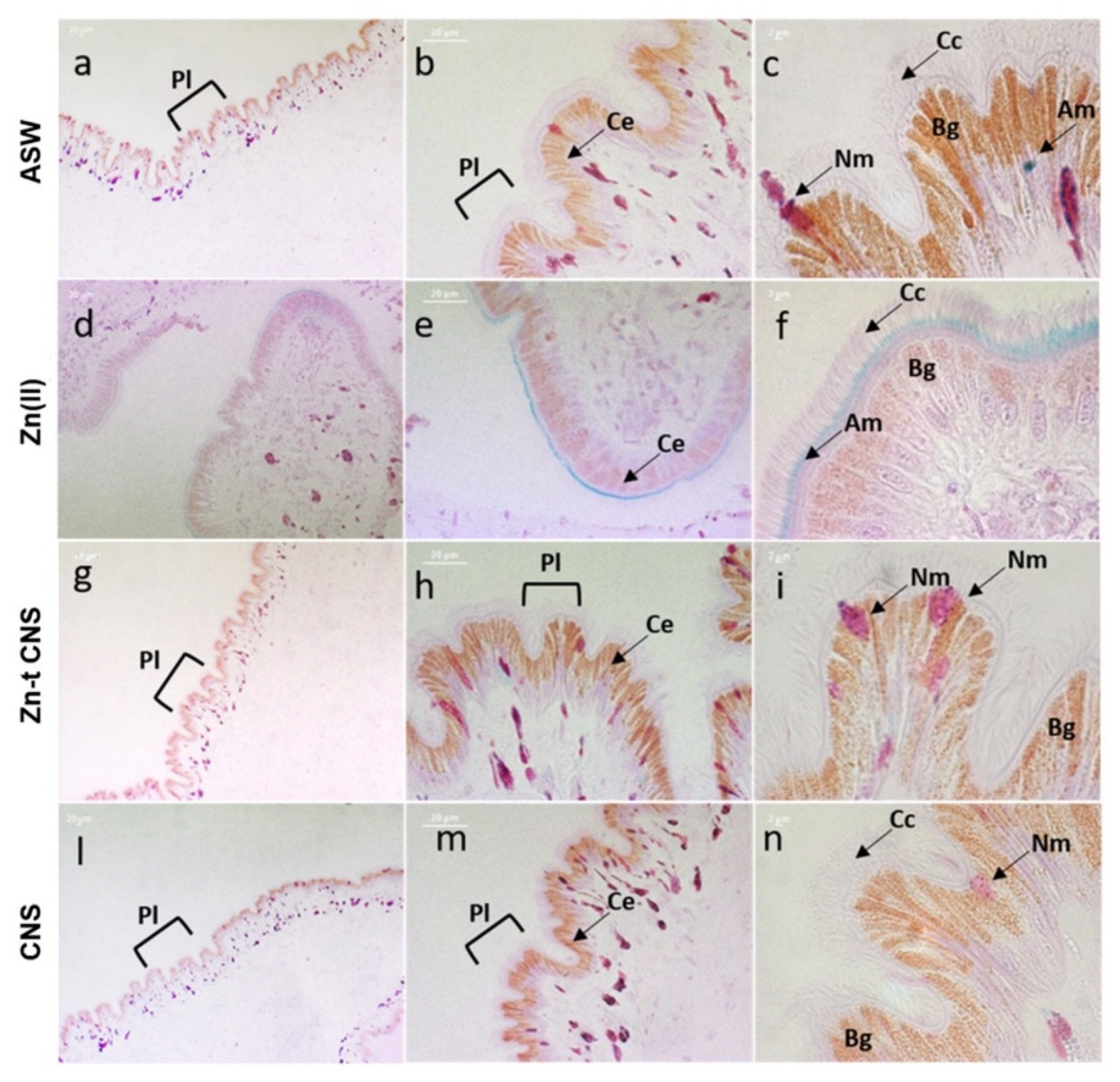
2.2.3. Mantle Histology and Histochemistry
2.3. Quantification of Total Zn Levels in Tested Solutions
2.4. Statistical Analysis
3. Results and Discussion
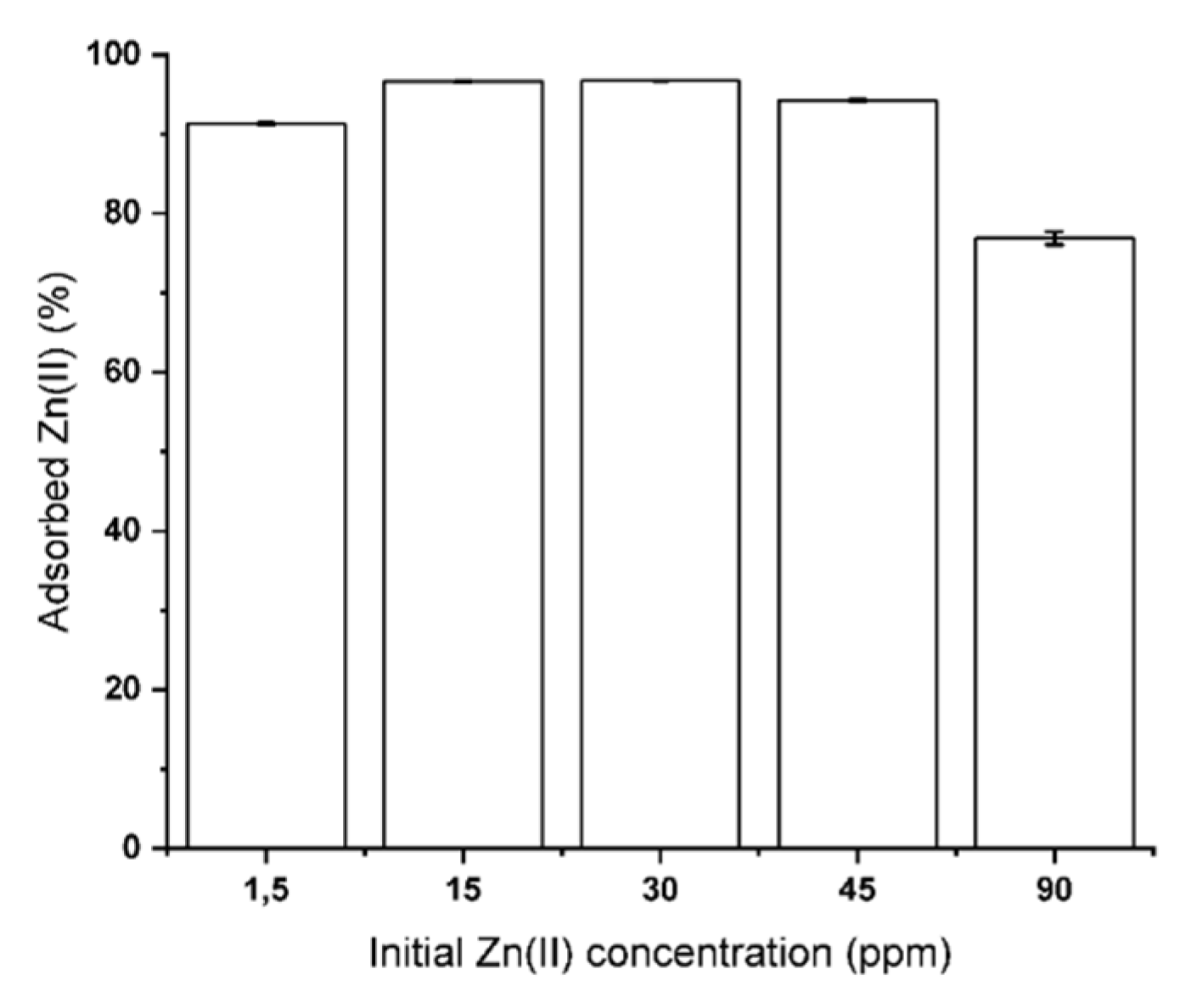
3.1. CNS Adsorption Performance of Zn(II) from ASW
3.2. Effect-Based Study
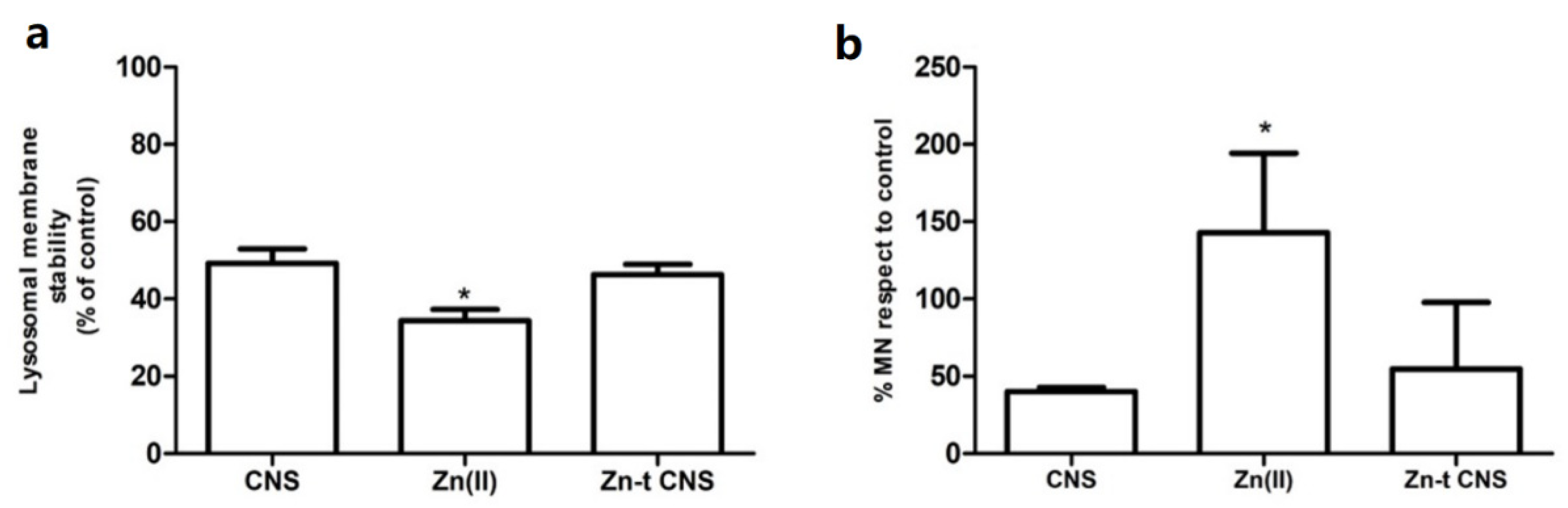
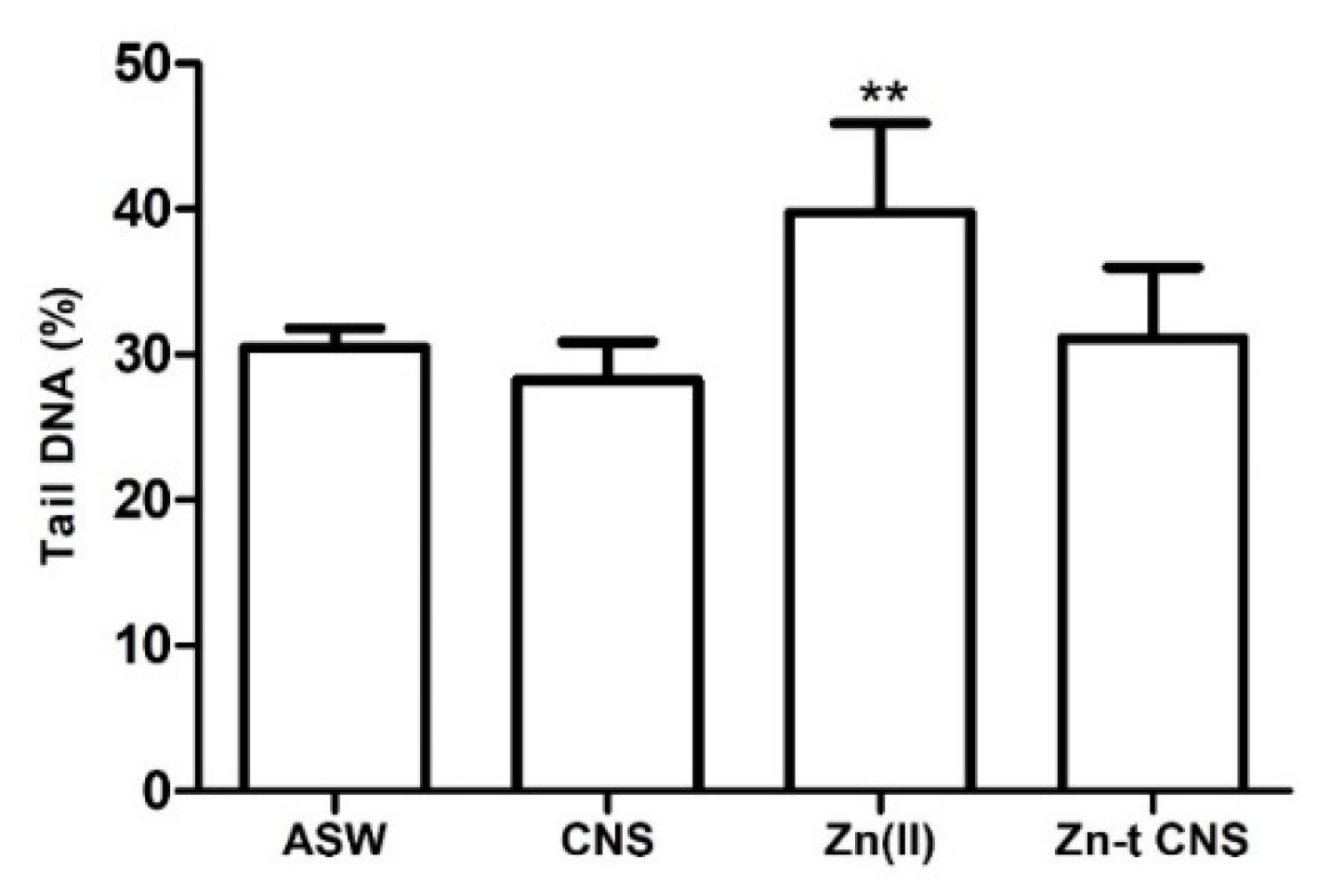
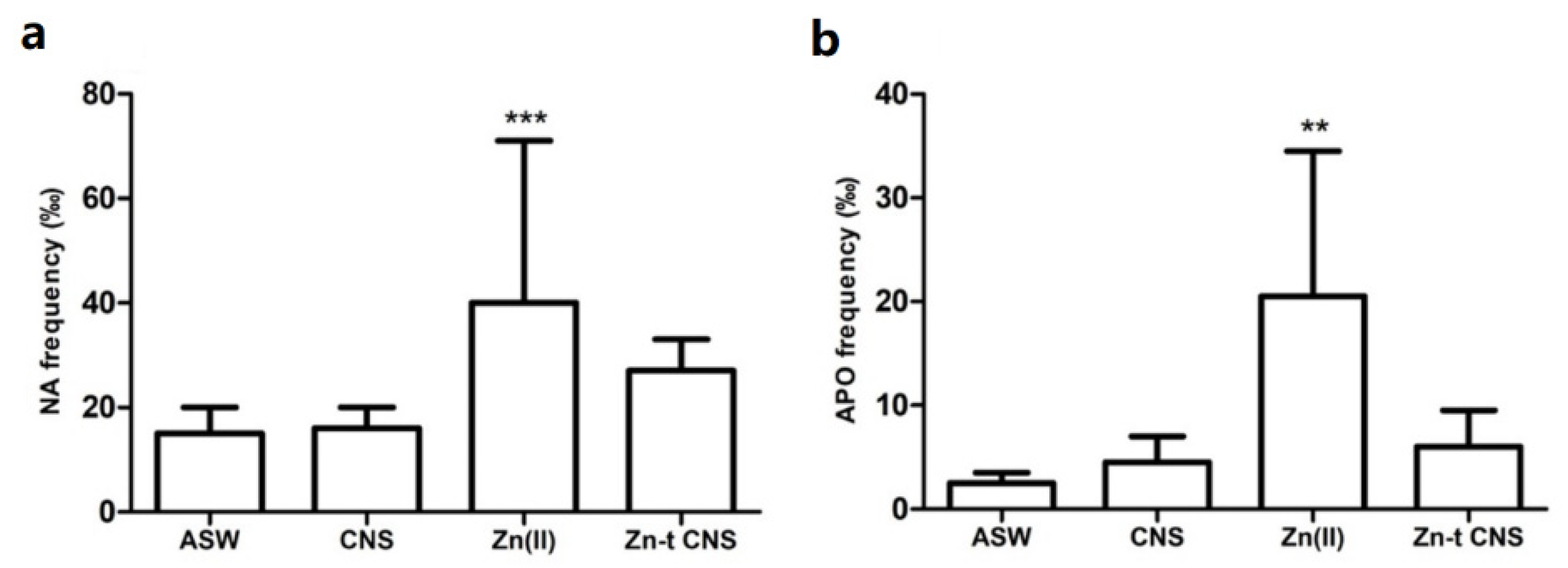
3.2.1. Cellular Bioassays
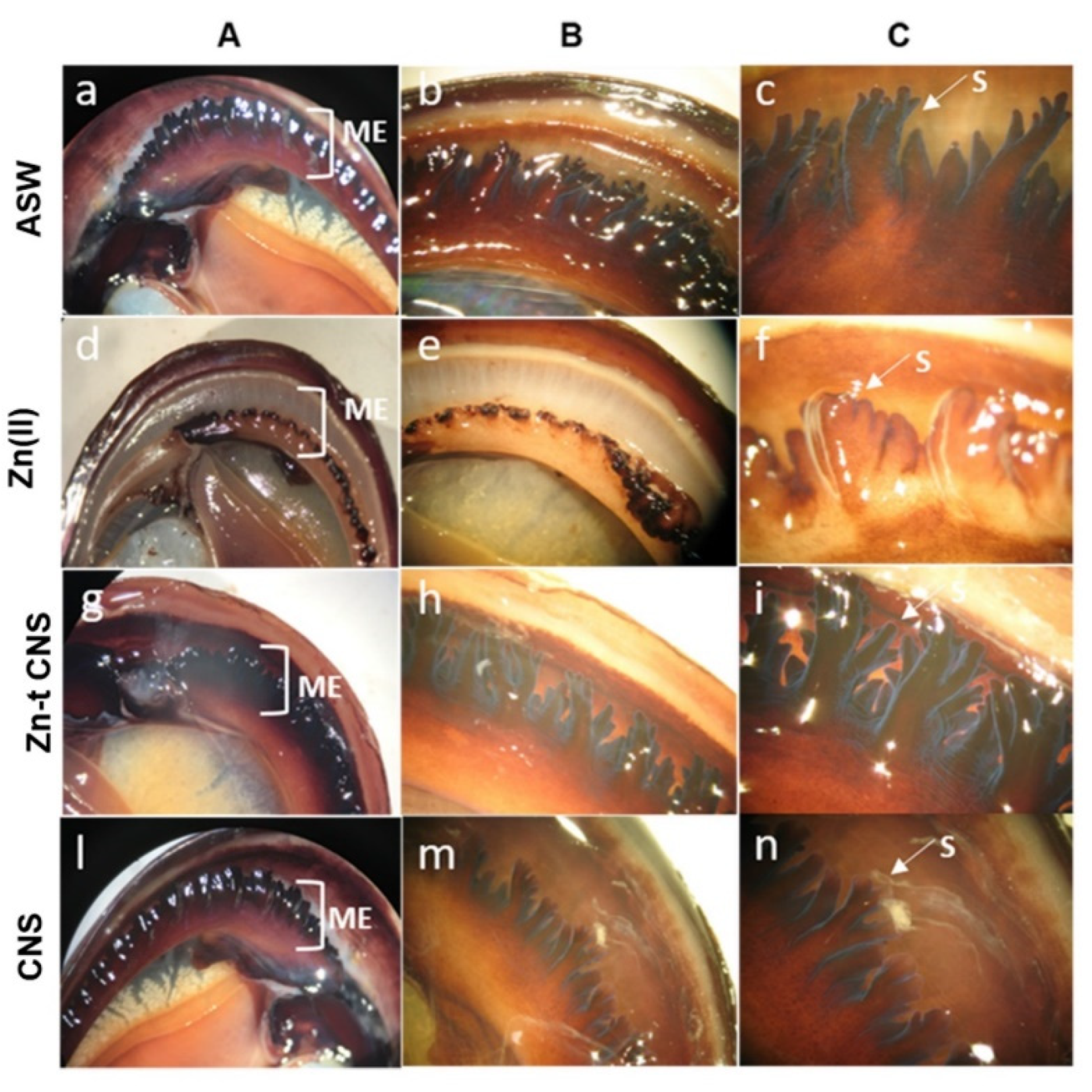
3.2.2. Mantle Histology and Histochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Furness, R.W.; Rainbow, P.S. Heavy Metals in Marine Environment. In Heavy Metals in the Marine Environment; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 1990. [Google Scholar]
- Bebianno, M.J.; Pereira, C.G.; Rey, F.; Cravo, A.; Duarte, D.; D’Errico, G.; Regoli, F. Integrated approach to assess ecosystem health in harbor areas. Sci. Tot. Environ. 2015, 514, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Sharifuzzaman, S.M.; Rahman, H.; Ashekuzzaman, S.M.; Islam, M.M.; Chowdhury, S.R.; Hossain, M.S. Heavy Metals Accumulation in Coastal Sediments. In Environmental Remediation Technologies for Metal-Contaminated Soils; Hasegawa, H., Rahman, M.M., Rahman, I., Azizur, M., Eds.; Springer: Tokyo, Japan, 2016; pp. 21–42. [Google Scholar]
- Marine Strategy Framework Directive. European Commission Directive 2008/56/EC. Available online: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/marine-strategy-framework-directive/index_en.htm (accessed on 1 June 2020).
- Environmental Quality Standard Directive 2013/39/EU. Directive of the European Parliament and of the Council on Environmental Quality Standards in the Field of Water Policy Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=LEGISSUM:l28002b (accessed on 1 June 2020).
- USGS. Mineral Commodity Summaries. 2015. Available online: https://minerals.usgs.gov/minerals/pubs/mcs/2015/mcs2015.pdf (accessed on 14 January 2017).
- EPA (Environmental Protection Agency). Uniform National Discharge Standards for Vessels of the Armed Forces. Technical Development Document 821-R-99-001; 1999. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=20002KB0.TXT (accessed on 1 June 2020).
- Lu, P.J.; Huang, S.C.; Chen, Y.P.; Chiueh, L.C.; Shih, D.Y.C. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Bruland, K.W. Oceanographic distributions of cadmium, zinc, nickel, and copper in the North Pacific. Earth Planet. Sci. Lett. 1980, 47, 176–198. [Google Scholar] [CrossRef]
- Luoma, S.N. Can we determine the biological availability of sediment-bound trace elements? Hydrobiologia 1989, 176/177, 379–396. [Google Scholar] [CrossRef]
- Neff, J.M. Zinc in the Ocean. In Bioaccumulation in Marine Organisms; Elsevier: Amsterdam, The Netherlands, 2002; pp. 175–189. [Google Scholar]
- Millero, F.; Pierrot, D. Speciation of Metals in Natural Waters. In Chemistry of Marine Waters and Sediments; Gianguzza, A., Pelizzetti, E., Sammartano, S., Eds.; Springer: Berlin, Germany, 2002; pp. 193–220. [Google Scholar]
- Kalnejais, L.H.; Martin, W.R.; Bothner, M.H. The release of dissolved nutrients and metals from coastal sediments due to resuspension. Mar. Chem. 2010, 121, 224–235. [Google Scholar] [CrossRef]
- Sinoir, M.; Butler, E.C.V.; Bowie, A.R.; Mongin, M.; Nesterenko, P.N.; Hassler, C.S. Zinc marine biogeochemistry in seawater: A review. Mar. Fresh. Res. 2012, 63, 644–657. [Google Scholar] [CrossRef]
- Burton, J.D.; Statham, P.J. Trace Metals in Seawater. In Heavy Metals in the Marine Environment; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 1990. [Google Scholar]
- Li, L.; Zhen, X.; Wang, X.; Ren, Y.; Hu, L.; Bai, Y.; Liu, J.; Shi, X. Benthic trace metal fluxes in a heavily contaminated bay in China: Does the sediment become a source of metals to the water column? Environ. Pollut. 2020, 257, 113494. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Feedback interactions between zinc and phytoplankton in seawater. Limnol. Oceanogr. 1992, 37, 25–40. [Google Scholar] [CrossRef]
- Patil, S.S.; Shedbalkar, U.U.; Truskewycz, A.; Chopade, B.A.; Bal, A.S. Nanoparticles for environmental clean-up: A review of Potential risks and emerging solutions. Environ. Technol. Innov. 2016, 5, 10–21. [Google Scholar] [CrossRef]
- Corsi, I.; Winther-Nielsen, M.; Sethi, R.; Punta, C.; Della Torre, C.; Libralato, G.; Lofrano, G.; Sabatini, L.; Aiello, M.; Fiordi, L.; et al. Ecofriendly nanotechnologies and nanomaterials for environmental applications: Key issue and consensus recommendations for sustainable and ecosafe nanoremediation. Ecotox. Environ. Safe 2018, 154, 237–244. [Google Scholar] [CrossRef]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in Situ remediation: A Review of the Benefits and Potential Risks. Environ. Health Perspect. 2009, 117, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Corsi, I.; Cherr, G.N.; Lenihan, H.S.; Labille, J.; Hassellov, M.; Canesi, L.; Dondero, F.; Frenzilli, G.; Hristozov, D.; Puntes, V.; et al. Common Strategies and Technologies for the Ecosafety Assessment and Design of Nanomaterials Entering the Marine Environment. ACS Nano 2014, 8, 9694–9709. [Google Scholar] [CrossRef]
- Corsi, I.; Fiorati, A.; Grassi, G.; Bartolozzi, I.; Daddi, T.; Melone, L.; Punta, C. Environmentally Sustainable and Ecosafe Polysaccharide-Based Materials for Water Nano-Treatment: An Eco-Design Study. Materials 2018, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- Corsi, I.; Grassi, G. The role of ecotoxicology in the eco-design of nanomaterials for water remediation. In Ecotoxicology of Nanoparticles in Aquatic Systems; Blasco, J., Corsi, I., Eds.; CRC Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 219–229. [Google Scholar]
- Gao, Y.; Guo, S.; Wang, J.; Li, D.; Wang, H.; Zeng, D. Effects of different remediation treatments on crude oil contaminated saline soil. Chemosphere 2014, 117, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yong, Y.; Zhang, C. Toward Biocompatible Semiconductor Quantum Dots: From Biosynthesis and Bioconjugation to Biomedical Application. Chem. Rev. 2014, 115, 11669–11717. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, B.; Ghaffar, A.; Zhu, X. Nanocomposite Membrane with Polyethylenimine-Grafted Graphene Oxide as a Novel Additive to Enhance Pollutant Filtration Performance. Environ. Sci. Technol. 2018, 52, 5920–5930. [Google Scholar] [CrossRef]
- Pedrazzo, A.R.; Smarra, A.; Caldera, F.; Musso, G.; Kumar Dhakar, N.; Cecone, C.; Corsi, I.; Trotta, F. Eco-friendly β-cyclodextrin and linecaps polymers for the removal of heavy metals. Polymers 2019, 11, 1658. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Suopajärvi, T.; Liimatainen, H.; Niinimaa, J.; Sillanpää, M. Adsorption of Ni(II), Cu(II) and Cd(II) from aqueous solutions by amino modified nanostructured microfibrillated cellulose. Cellulose 2014, 21, 1471–1487. [Google Scholar] [CrossRef]
- Carpenter, A.W.; De Lannoy, C.; Wiesner, M.R. Cellulose Nanomaterials in Water Treatment Technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef]
- Suhas Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef]
- Bossa, N.; Carpenter, A.W.; Kumar, N.; De Lannoye, C.; Wiesner, M. Cellulose nanocrystal zero-valent iron nanocomposites for groundwater remediation. Environ. Sci. Nano. 2017, 4, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Chin, K.; Ting, S.; Ong, H.; Omar, M. Surface functionalized nanocellulose as a veritable inclusionary material in contemporary bioinspired applications: A review. J. Appl. Polym. 2018, 135, 46065. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Tam, K.C. Cellulose nanomaterials: Promising sustainable nanomaterials for application in water/wastewater treatment processes. Environ. Sci. Nano 2018, 5, 623–658. [Google Scholar] [CrossRef]
- Melone, L.; Rossi, B.; Pastori, N.; Panzeri, W.; Mele, A.; Punta, C. TEMPO-Oxidized Cellulose Cross-Linked with Branched Polyethyleneimine: Nanostructured Adsorbent Sponges for Water Remediation. ChemPlusChem 2015, 80, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Pierre, G.; Punta, C.; Delattre, C.; Melone, L.; Dubessay, P.; Fiorati, A.; Pastori, N.; Galante, Y.M.; Michaud, P. TEMPO-mediated oxidation of polysaccharides: An ongoing story. Carbohyd. Polym. 2017, 165, 71–85. [Google Scholar] [CrossRef]
- Paladini, G.; Venuti, V.; Almásy, L.; Melone, L.; Crupi, V.; Majolino, D.; Pastori, N.; Fiorati, A.; Punta, C. Cross-linked cellulose nano-sponges: A small angle neutron scattering (SANS) study. Cellulose 2019, 26, 9005–9019. [Google Scholar] [CrossRef]
- Fiorati, A.; Turco, G.; Travan, A.; Caneva, E.; Pastori, N.; Cametti, M.; Punta, C.; Melone, L. Mechanical and Drug Release Properties of Sponges from Cross-linked Cellulose Nanofibers. ChemPlusChem 2017, 82, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Bartolozzi, I.; Daddi, T.; Punta, C.; Fiorati, A.; Iraldo, F. Life cycle assessment of emerging environmental technologies in the early stage of development. A case study on nanostructured materials. J. Ind. Ecol. 2020, 24, 101–115. [Google Scholar] [CrossRef]
- Fiorati, A.; Grassi, G.; Pastori, N.; Graziano, A.; Liberatori, G.; Melone, L.; Punta, C.; Corsi, I. Eco-design of nanostructured cellulose sponges for sea-water decontamination from heavy metal ions. J. Clean. Prod. 2020, 246, 119009. [Google Scholar] [CrossRef]
- OSPARCOM. JAMP Guidelines for Monitoring Contaminants in Biota. OSPAR Commission, London, 2012, 122. Available online: https://mcc.jrc.ec.europa.eu/documents/OSPAR/Guidelines_forMonitoring_of_ContaminantsSeawater.pdf (accessed on 1 June 2020).
- Farrington, J.W.; Tripp, B.W.; Tanabe, S.; Subramanian, A.; Sericano, J.L.; Wade, T.L.; Knap, A.H.; Edward, D. Goldberg’s proposal of “the mussel watch”: Reflections after 40 years. Mar. Pollut. Bull. 2016, 110, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Lobel, R.B. Short-Term and Long-Term Uptake of Zinc by the Mussel, Mytilus edulis: A Study in Individual Variability. Arch. Environ. Con. Toxicol. 1987, 16, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Regoli, F.; Orlando, E. Accumulation and subcellular distribution of metals (Cu, Fe, Mn, Pb and Zn) in the Mediterranean Mussel Mytilus galloprovincialis during a field transplant experiment. Mar. Pollut. Bull. 1994, 28, 592–600. [Google Scholar] [CrossRef]
- Vercauteren, K.; Blust, R. Bioavailability of dissolved zinc to the common mussel Mytilus edulis in complexing environments. Mar. Ecol. Prog. Ser. 1996, 137, 123–132. [Google Scholar] [CrossRef]
- Beyer, J.; Green, N.W.; Brooks, S.; Allan, I.J.; Ruus, A.; Gomes, T.; Bråte Schøyen, M. Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: A review. Mar. Environ. Res. 2017, 130, 338–365. [Google Scholar] [CrossRef]
- Lowe, D.M.; Moore, M.N. The cytochemical distributions of Zinc (Zn II) and Iron (Fe III) in the common mussel, Mytilus edulis, and their relationship with lysosomes. J. Mar. Biol. Assoc. UK 1979, 59, 851–858. [Google Scholar] [CrossRef]
- George, S.G.; Pirie, B.J.S. Metabolism of Zinc in the mussel, Mytilus edulis (L.): A combined ultrastructural and biochemical study. J. Mar. Biol. Assoc. UK 1980, 60, 575–590. [Google Scholar] [CrossRef]
- Soto, M.; Cajaraville, M.P.; Marigómez, I. Tissue and cell distribution of copper, zinc and cadmium in the mussel, Mytilus galloprovincialis, determined by autometallography. Tissue Cell 1996, 28, 557–568. [Google Scholar] [CrossRef]
- Marigómez, I.; Soto, M.; Cajaraville, M.P.; Angulo, E.; Giamberini, L. Cellular and Subcellular Distribution of Metals in Molluscs. Microsc. Res. Techniq. 2002, 56, 358–392. [Google Scholar] [CrossRef]
- Amiard-Triquet, C.; Berthet, B.; Metayer, C.; Amiard, J.C. Contribution to the ecotoxicological study of cadmium, copper and zinc in the mussel Mytilus edulis II. Experimental study. Mar. Biol. 1986, 92, 7. [Google Scholar] [CrossRef]
- Hietanen, B.; Sunila, I.; Kristoffersson, R. Toxic effects of zinc on the common mussel Mytilus edulis L. (Bivalvia) in brackish water. I. Physiological and histopathological studies. Ann. Zool. Fenn. 1988, 25, 341–347. [Google Scholar]
- Viarengo, A. Heavy metals in marine invertebrates: Mechanisms of regulation and toxicity at the cellular level. Aquat. Sci. Rev. 1989, 1, 295–317. [Google Scholar]
- Livingstone, D.R.; Pipe, R.K. Mussels and environmental contaminants: Molecular and cellular aspects. In The Mussel Mytilus: Ecology, Physiology, Genetics and Culture; Gosling, E.M., Ed.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1992; pp. 425–464. [Google Scholar]
- Burbidge, F.J.; Macey, D.J.; Webb, J.; Talbot, V. A Comparison Between Particulate (Elemental) Zinc and Soluble Zinc (ZnCl2) Uptake and Effects in the Mussel, Mytilus edulis. Arch. Environ. Con. Toxicol. 1994, 26, 466–472. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Chen, S.Y.; Qu, A.O.; Adeleye, A.O.; Di, Y.N. Utilization of isolated marine mussel cells as an in vitro model to assess xenobiotics induced genotoxicity. Toxicol. Vitro 2017, 44, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.B.; Dallas, L.J.; Comber, S.D.W.; Braungardt, C.B.; Worsfold, P.J.; Jha, A.N. Mixtures of tritiated water, zinc and dissolved organic carbon: Assessing interactive bioaccumulation and genotoxic effects in marine mussels, Mytilus galloprovincialis. J. Environ. Radioact. 2018, 187, 133–143. [Google Scholar] [CrossRef]
- ICES. Report of the ICES\OSPAR Workshop on Lysosomal Stability Data Quality and Interpretation (WKLYS), 13–17 September 2010, Alessandria, Italy. 2010. Available online: http://ices.dk/sites/pub/CM%20Doccuments/CM-2010/ACOM/ACOM6110.pdf (accessed on 1 June 2020).
- Bjärnmark, N.A.; Yarra, T.; Churcher, A.M.; Felix, R.C.; Clark, M.S.; Power, D.M. Transcriptomics provides insight into Mytilus galloprovincialis (Mollusca: Bivalvia) mantle function and its role in biomineralisation. Mar. Genomics. 2016, 27, 37–45. [Google Scholar] [CrossRef]
- Moëzzi, F.; Javanshir, A.; Eagderi, S.; Poorbagher, H. Histopathological effects of zinc (Zn) on mantle, digestive gland and foot in freshwater mussel, Anodonta cygnea (Linea, 1876). Int. J. Aquat. Biol. 2013, 1, 61–67. [Google Scholar]
- Pagano, M.; Porcino, C.; Briglia, M.; Fiorino, E.; Vazzana, M.; Silvestro, S.; Faggio, C. The Influence of Exposure of Cadmium Chloride and Zinc Chloride on Haemolymph and Digestive Gland Cells from Mytilus galloprovincialis. Int. J. Environ. Res. 2017, 11, 207–216. [Google Scholar] [CrossRef]
- Jha, A.N.; Cheung, V.V.; Foulkes, M.E.; Hill, S.J.; Depledge, M.H. Detection of genotoxins in the marine environment: Adoption and evaluation of an integrated approach using the embryo-larval stages of the marine mussel, Mytilus edulis. Mutat. Res. 2000, 464, 213–228. [Google Scholar] [CrossRef]
- Fernández, B.; Campillo, J.A.; Martínez-Gómez, C.; Benedicto, J. Micronuclei and other nuclear abnormalities in mussels (Mytilus galloprovincialis) as biomarkers of cyto-genotoxic pollution in mediterranean waters. Environ. Mol. Mutagen. 2011, 52, 479–491. [Google Scholar] [CrossRef]
- Ale, A.; Liberatori, G.; Vannuccini, M.L.; Bergami, E.; Ancora, S.; Mariotti, G.; Bianchi, N.; Galdoporpora, J.M.; Desimone, M.; Cazenave, J.; et al. Exposure to a nanosilver-enabled consumer product results in similar accumulation and toxicity of silver nanoparticles in the marine mussel Mytilus galloprovincialis. Aquat. Toxicol. 2019, 211, 46–56. [Google Scholar] [CrossRef]
- ASTM. International Standard Guide for Conducting Static Acute Toxicity Tests Starting with Embryos of Four Species of Salt Water Bivalve Molluscs. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/E724-98.htm (accessed on 1 June 2020).
- D’Silva, C.; Kureishy, T.W. Experimental studies on the accumulation of copper and zinc in the green mussel. Mar. Pollut. Bull. 1978, 9, 187–190. [Google Scholar] [CrossRef]
- Akberali, H.B.; Wong, T.; Trueman, E. Behavioural and siphonal tissue responses of Scrobicularia plana (bivalvia) to zinc. Mar. Environ. Res. 1981, 5, 251–264. [Google Scholar] [CrossRef]
- Neuberger-Cywiak, L.; Achituv, Y.; Garcia, E.M. Effects of Zinc and Cadmium on the Burrowing Behavior, LC 50, and LT 50 on Donax trunculus Linnaeus (Bivalvia-Donacidae). Environ. Contam. Toxicol. 2003, 70, 713–722. [Google Scholar] [CrossRef]
- Vlahogianni, T.H.; Valavanidis, A. Heavy-metal effects on lipid peroxidation and antioxidant defence enzymes in mussels Mytilus galloprovincialis. J. Chem. Ecol. 2007, 23, 361–371. [Google Scholar] [CrossRef]
- Ramakritinan, C.M.; Chandurvelan, R.; Kumaraguru, A.K. Acute Toxicity of Metals: Cu, Pb, Cd, Hg and Zn on Marine Molluscs, Cerithedia cingulata G., and Modiolus philippinarum H. Indian J. Geo-Mar. Sci. 2012, 41, 141–145. [Google Scholar]
- Howard, D.W.; Lewis, E.J.; Keller, B.J.; Smith, C.S. Histological Techniques for Marine Bivalve Mollusks and Crustaceans, 2nd ed.; NOAA/National Centers for Coastal Ocean Science: Oxford, UK, 2004. [Google Scholar]
- Lowe, D.M.; Soverchia, C.; Moore, M.N. Lysosomal membrane responses in the blood and digestive cells of mussels experimentally exposed to fluoranthene. Aquat. Toxicol. 1995, 33, 105–112. [Google Scholar] [CrossRef]
- Davies, I.M.; Gubbins, M.; Hylland, K.; Maes, T.; Martínez-Gómez, C.; Giltrap, M.; Burgeot, T.; Wosniok, W.; Lang, T.; Vethaak, A.D. Technical annex: Assessment criteria for biological effects measurements. In Integrated Monitoring of Chemicals and Their Effects; Davies, I.M., Vethaak, A.D., Eds.; ICES Cooperative Research Report; ICES: Copenhagen, Denmark, 2012; p. 315. [Google Scholar]
- Venier, P.; Maron, S.; Canova, S. Detection of micronuclei in gill cells and haemocytes of mussels exposed to benzo(a)pyrene. Mutat. Res. 1997, 390, 33–34. [Google Scholar] [CrossRef]
- Guidi, P.; Lyons, B.P.; Frenzilli, G. The Comet Assay in Marine Animals. In Genotoxicity Assessment. Methods in Molecular Biology; Dhawan, A., Bajpayee, M., Eds.; Humana: New York, NY, USA, 2019; Volume 2031. [Google Scholar]
- Frenzilli, G.; Nigro, M.; Scarcelli, V.; Gorbi, S.; Regoli, F. DNA integrity and total oxyradical scavenging capacity in the Mediterranean mussel, Mytilus galloprovincialis: A field study in a highly eutrophicated coastal lagoon. Aquat. Toxicol. 2001, 53, 19–32. [Google Scholar] [CrossRef]
- Rocco, L.; Santonastaso, M.; Nigro, M.; Mottola, F.; Costagliola, D.; Bernardeschi, M.; Guidi, P.; Lucchesi, P.; Scarcelli, V.; Corsi, I.; et al. Genomic and chromosomal damage in the marine mussel Mytilus galloprovincialis: Effects of the combined exposure to titanium dioxide nanoparticles and cadmium chloride. Mar. Environ. Res. 2015, 111, 144–148. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Lowe, D.M.; Fossato, V.U. The influence of environmental contaminants on lysosomal activity in the digestive cells of mussels (Mytilus galloprovincialis) from the Venice Lagoon. Aquat. Toxicol. 2000, 48, 75–85. [Google Scholar] [CrossRef]
- EEA (European Environment Agency). Hazardous Substances in Europe’s Fresh and Marine Waters. An Overview; EEA Technical Report No 8; European Environment Agency: Copenhagen, Denmark, July 2011. [Google Scholar]
- Varotto, L.; Domeneghetti, S.; Rosani, U.; Manfrin, C.; Cajaraville, M.P.; Raccanelli, S.; Pallavicini, A.; Venier, P. DNA Damage and Transcriptional Changes in the Gills of Mytilus galloprovincialis Exposed to Nanomolar Doses of Combined Metal Salts (Cd, Cu, Hg). PLoS ONE 2013, 8, e54602. [Google Scholar] [CrossRef]
- Gherras Touahri, H.; Boutiba, Z.; Benguedda, W.; Shaposhnikov, S. Active biomonitoring of mussels Mytilus galloprovincialis with integrated use of micronucleus assay and physiological indices to assess harbour pollution. Mar. Pollut. Bull. 2016, 110, 52–64. [Google Scholar] [CrossRef]
- Majone, F.; Beltrame, C.; Brunetti, R. Frequencies of micronuclei detected on Mytilus galloprovincialis by different staining techniques after treatment with zinc chloride. Mutat. Res. 1988, 209, 131–134. [Google Scholar] [CrossRef]
- Fowler, B.A.; Wolte, D.A.; Hettler, W.F. Mercury and iron uptake by cytosomes in mantle epithelial cells of quahog clams (Mercenaria mercenaria) exposed to mercury. J. Fish. Res. Board. Can. 1975, 32, 1767–1775. [Google Scholar] [CrossRef]
- Al-Subiai, S.N.; Moody, A.J.; Mustafa, S.A.; Jha, A.N. A multiple biomarker approach to investigate the effects of copper on the marine bivalve mollusc, Mytilus edulis. Ecotoxicol. Environ. Saf. 2011, 74, 1913–1920. [Google Scholar] [CrossRef]
- Larramendy, M.L. Ecotoxicology and Genotoxicology: Non-traditional Aquatic Models (Issues in Toxicology); The RSC: Cambridge, UK, 2017. [Google Scholar]
- Calvo-Iglesias, J.; Pérez-Estévez, D.; Lorenzo-Abalde, S.; Sánchez-Correa, B.; Quiroga, M.I.; Fuentes, J.M.; González-Fernández, A. Characterization of a Monoclonal Antibody Directed against Mytilus spp Larvae Reveals an Antigen Involved in Shell Biomineralization. PLoS ONE 2015, 11, e0152210. [Google Scholar] [CrossRef]
- Beninger, P.G.; St-Jean, S.D. The role of mucus in particle processing by suspension-feeding marine bivalves: Unifying principles. Mar. Biol. 1997, 129, 389–397. [Google Scholar] [CrossRef]
- Sze, P.W.C.; Lee, S.Y. The potential role of mucus in the depuration of copper from the mussels Perna viridis (L.) and Septifer virgatus (Wiegmann). Mar. Poll. Bull. 1995, 31, 390–393. [Google Scholar] [CrossRef]
- Moraes, R.B.; Silva, M.L.P. Toxicity of zinc in the mussel Perna perna (Linne, 1758). Arq. Biol. Tecnol. 1995, 38, 541–547. [Google Scholar]
- Davies, M.S.; Hawkins, S.J. Mucus from Marine Molluscs. Adv. Mar. Biol. 1998, 34, 1–71. [Google Scholar]
- Gerdol, M.; Gomez-Chiarri, M.; Castillo, M.G.; Figueras, A.; Fiorito, G.; Moreira, R.; Novoa, B.; Pallavicini, A.; Ponte, G.; Roumbedakis, K.; et al. Immunity in Molluscs: Recognition and Effector Mechanisms, with a Focus on Bivalvia. In Advances in Comparative Immunology; Cooper, E.L., Ed.; Springer International Publishing AG, part of Springer Nature: Cham, Switzerland, 2018; pp. 225–341. [Google Scholar]
- Loker, E.S.; Bayne, C.J. Molluscan immunobiology: Challenges in the Anthropocene epoch. In Advances in Comparative Immunology; Cooper, E.L., Ed.; Springer International Publishing AG, Springer Nature: Cham, Switzerland, 2018; pp. 343–407. [Google Scholar]
- Fasulo, S.; Mauceri, A.; Giannetto, A.; Maisano, M.; Bianchi, N.; Parrino, V. Expression of metallothionein mRNAs by in situ hybridization in the gills of Mytilus galloprovincialis, from natural polluted environments. Aquat. Toxicol. 2008, 88, 62–68. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, A.; Fasulo, S.; Dallas, L.J.; Fisher, A.S.; Maisano, M.; Readman, J.W.; Jha, A.N. Enhanced toxicity of ‘bulk’ titanium dioxide compared to ‘fresh’ and ‘aged’ nano-TiO2 in marine mussels (Mytilus galloprovincialis). Nanotoxicology 2013, 1–10, 1743–5404. [Google Scholar]
- Rickerby, D.; Morrison, M. Report from the Workshop on Nanotechnologies for Environmental Remediation. Presented at the Joint Research Centre, Ispra, Italy, 16–17 April 2007; Available online: http://www.nanowerk.com/nanotechnology/reports/reportpdf/report101.pdf (accessed on 1 June 2020).
- Grieger, K.D.; Wickson, F.; Andersen, H.B.; Renn, O. Improving risk governance of emerging technologies through public engagement: The neglected case of nano-remediation? Int. J. Emerging Technol. Soc. 2012, 10, 61–78. [Google Scholar]
- Trujillo-Reyes, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Supported and unsupported nanomaterials for water and soil remediation: Are they a useful solution for worldwide pollution? J. Hazard. Mater. 2014, 280, 487–503. [Google Scholar] [CrossRef]
- Pedersen, K.B.; Lejon, T.; Ottosen, L.M.; Jensen, P.E. Screening of variable importance for optimizing electrodialytic remediation of heavy metals from polluted harbour sediments. Environ. Toxicol. 2015, 36, 2364–2373. [Google Scholar] [CrossRef]
- Elliott, J.; Rösslein, M.; Song, N.; Toman, B.; Kinsner-Ovaskainen, A.; Maniratanachote, R.; Salit, M.; Petersen, E.; Sequeira, F.; Romsos, E.; et al. Toward achieving harmonization in a nanocytotoxicity assay measurement through an interlaboratory comparison study. ALTEX—Altern. Anim. Exp. 2017, 34, 201–218. [Google Scholar] [CrossRef]
- Holden, P.A.; Gardea-Torresdey, J.L.; Klaessig, F.; Turco, R.F.; Mortimer, M.; Hund-Rinke, K.; Cohen Hubal, E.A.; Avery, D.; Barceló, D.; Behra, R.; et al. Considerations of environmentally relevant test conditions for improved evaluation of ecological hazards of engineered nanomaterials. Environ. Sci. Technol. 2016, 50, 6124–6145. [Google Scholar] [CrossRef]
- Selck, H.; Handy, R.D.; Fernandes, T.F.; Klaine, S.J.; Petersen, E.J. Nanomaterials in the aquatic environment: A European Union–United States perspective on the status of ecotoxicity testing, research priorities, and challenges ahead. Environ. Toxicol. Chem. 2016, 35, 1055–1067. [Google Scholar] [CrossRef]
- Oomen, A.G.; Steinhäuser, K.G.; Bleeker, E.A.J.; van Broekhuizen, F.; Sips, A.; Dekkers, S.; Wijnhoven, W.P.S.; Sayre, P.G. Risk assessment frameworks for nanomaterials: Scope, link to regulations, applicability, and outline for future directions in view of needed increase in efficiency. Nanoimpact 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Hu, Y.; Wang, R.; Chen, M.; Wu, J.; Wang, Y.; Kang, S.; Sun, Y.; Zhu, M. Behavior, remediation effect and toxicity of nanomaterials in water environments. Environ. Res. 2019, 174, 54–60. [Google Scholar] [CrossRef]
- Sánchez, A.; Recillas, S.; Font, X.; Casals, E.; González, E.; Puntes, V. Ecotoxicity of, and remediation with, engineered inorganic nanoparticles in the environment. TrAC-Trend. Anal. Chem. 2011, 30, 507–516. [Google Scholar] [CrossRef]
- Garner, K.L.; Keller, A.A. Emerging patterns for engineered nanomaterials in the environment: A review of fate and toxicity studies. J. Nanopart. Res. 2014, 16, 2503. [Google Scholar] [CrossRef]
- Hjorth, R.; van Hove, L.; Wickson, F. What can nanosafety learn from drug development? The feasibility of “safety by design”. Nanotoxicology 2017, 11, 305–312. [Google Scholar] [CrossRef]
- Singh, A.V.; Laux, P.; Luch, A.; Sudrik, C.; Wiehr, S.; Wild, A.M.; Santomauro, G.; Bill, J.; Sitti, M. Review of emerging concepts in nanotoxicology: Opportunities and challenges for safer nanomaterial design. Toxicol. Mech. Methods 2019, 29, 378–387. [Google Scholar] [CrossRef]
- Seed, R.; Suchanek, T.H. The mussel Mytilus: Ecology, Physiology, Genetics and Culture. In Development in Aquaculture and Fisheries Science; Gosling, E., Ed.; Elsevier: Amsterdam, The Netherlands, 1992; p. 25. [Google Scholar]
| Exposure Groups | Zn(II) T0 | Zn(II) T24h |
|---|---|---|
| Zn(II) | 8.567 ± 0.185 ** | 6.006 ± 0.604 ** |
| Zn-t CNS | 0.710 ± 0.0568 * | 0.510 ± 0.0258 * |
| CNS | 0.00251 ± 0.0001 * | 0.0245 ± 0.0008 * |
| ASW | 0.0047 ± 0.0006 | 0.0005 ± 0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liberatori, G.; Grassi, G.; Guidi, P.; Bernardeschi, M.; Fiorati, A.; Scarcelli, V.; Genovese, M.; Faleri, C.; Protano, G.; Frenzilli, G.; et al. Effect-Based Approach to Assess Nanostructured Cellulose Sponge Removal Efficacy of Zinc Ions from Seawater to Prevent Ecological Risks. Nanomaterials 2020, 10, 1283. https://doi.org/10.3390/nano10071283
Liberatori G, Grassi G, Guidi P, Bernardeschi M, Fiorati A, Scarcelli V, Genovese M, Faleri C, Protano G, Frenzilli G, et al. Effect-Based Approach to Assess Nanostructured Cellulose Sponge Removal Efficacy of Zinc Ions from Seawater to Prevent Ecological Risks. Nanomaterials. 2020; 10(7):1283. https://doi.org/10.3390/nano10071283
Chicago/Turabian StyleLiberatori, Giulia, Giacomo Grassi, Patrizia Guidi, Margherita Bernardeschi, Andrea Fiorati, Vittoria Scarcelli, Massimo Genovese, Claudia Faleri, Giuseppe Protano, Giada Frenzilli, and et al. 2020. "Effect-Based Approach to Assess Nanostructured Cellulose Sponge Removal Efficacy of Zinc Ions from Seawater to Prevent Ecological Risks" Nanomaterials 10, no. 7: 1283. https://doi.org/10.3390/nano10071283
APA StyleLiberatori, G., Grassi, G., Guidi, P., Bernardeschi, M., Fiorati, A., Scarcelli, V., Genovese, M., Faleri, C., Protano, G., Frenzilli, G., Punta, C., & Corsi, I. (2020). Effect-Based Approach to Assess Nanostructured Cellulose Sponge Removal Efficacy of Zinc Ions from Seawater to Prevent Ecological Risks. Nanomaterials, 10(7), 1283. https://doi.org/10.3390/nano10071283