Toxicity, Bioaccumulation and Biotransformation of Glucose-Capped Silver Nanoparticles in Green Microalgae Chlorella vulgaris
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Glucose-Capped Silver Nanoparticles (AgNPs-G)
2.3. AgNPs-G Characterisation
2.4. Chlorella Vulgaris Culture
2.5. Growth-Inhibition Test
2.6. Chlorophyll Content
2.7. Biodistribution and Subcellular Localisation of AgNPs: Transmission Electron Microscope (TEM) Analysis
2.8. X-ray Diffraction (XRD) Analysis
2.9. Inductively Coupled Plasma–Optical Emission Spectrometry (ICP–OES) Analysis
2.10. Statistical Analysis
3. Results and Discussion
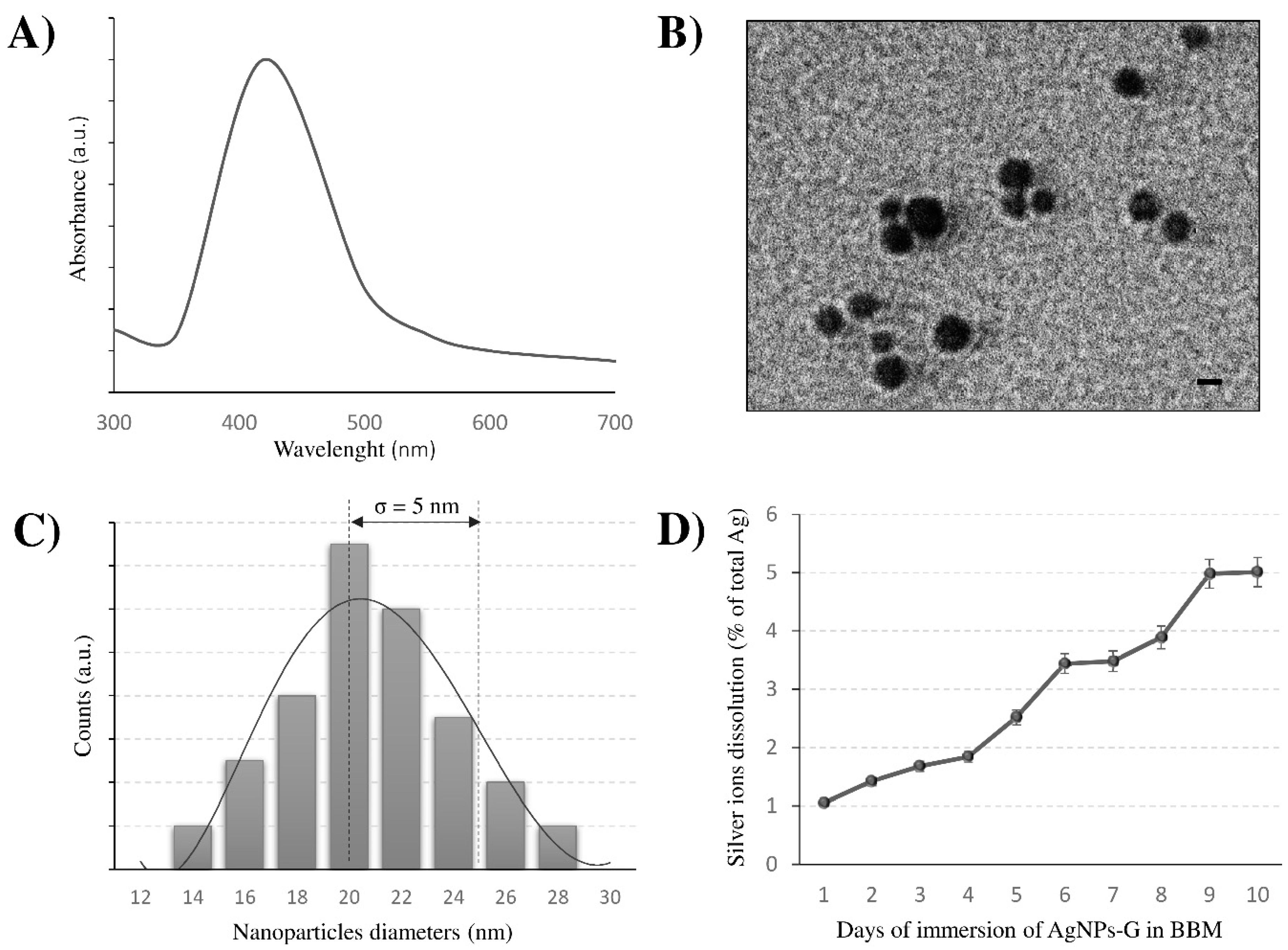
3.1. Characterisation of AgNPs-G: Shape, Size and Stability
3.2. AgNPs-G are Bio-Absorbed by C. vulgaris Maintaining Their Crystalline Structure
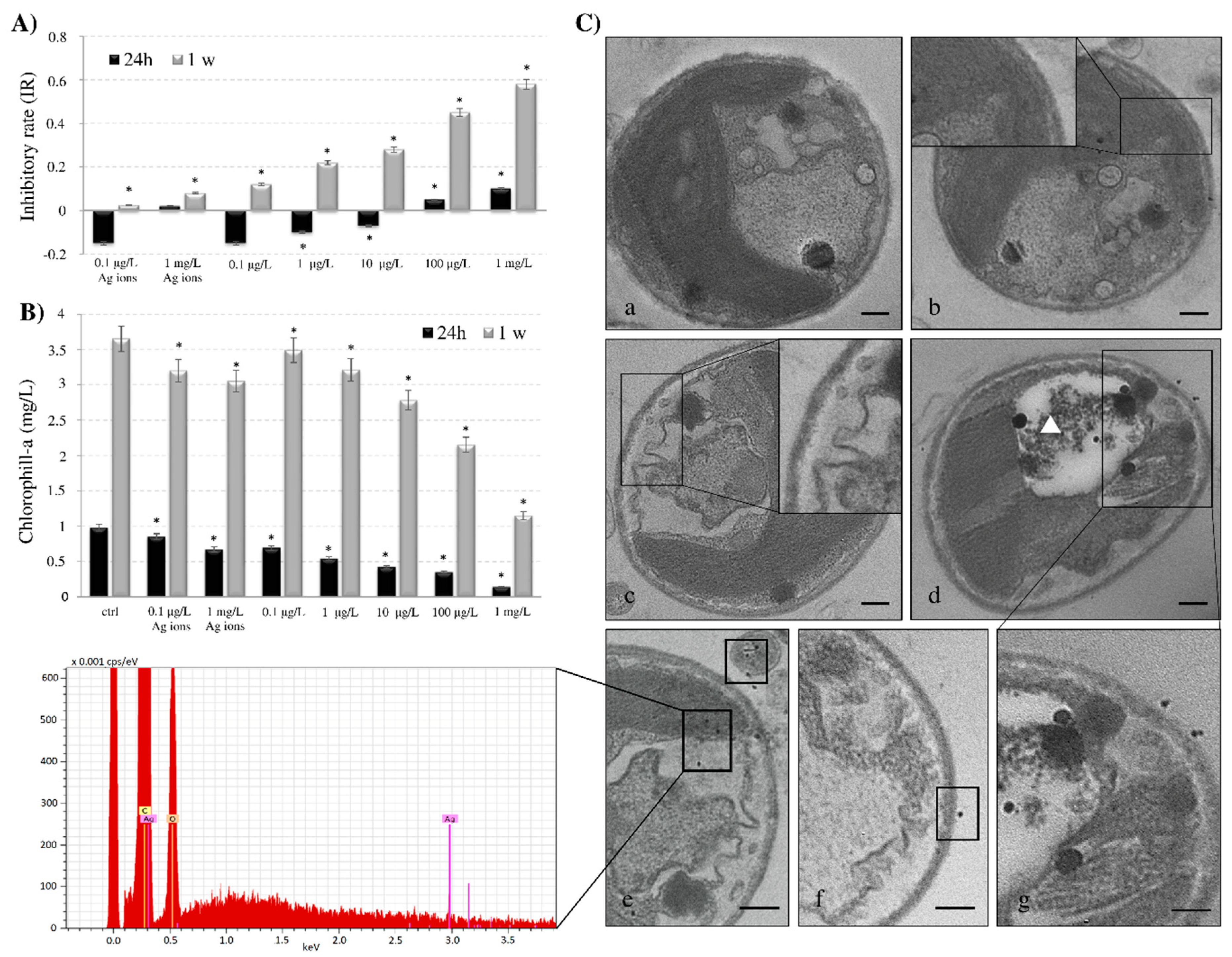
3.3. Cell Viability, Chlorophyll Content and Ultrastructure of AgNPs-G Treated C. vulgaris
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khan, I.; Khalid, S.; Idrees, K. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Stockmann-Juvala, H.; Lucaroni, F.; Savolainen, K. Nanomaterial exposure, toxicity, and impact on human health. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Semmler-Behnke, M.; Seitz, J.; Szymczak, W.; Wenk, A.; Mayer, P.; Oberdörster, G. Size dependence of the translocation of inhaled irid-ium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal. Toxicol. 2009, 21, 55–60. [Google Scholar] [CrossRef]
- Saber, A.T.; Lamson, J.S.; Jacobsen, N.R.; Ravn-Haren, G.; Hougaard, K.S.; Nyendi, A.N.; Wahlberg, P.; Madsen, A.M.; Jackson, P.; Wallin, H.; et al. Particle-induced pulmonary acute phase response correlates with neutrophil influx linking inhaled particles and cardiovascular risk. PLoS ONE 2013, 8, e69020. [Google Scholar] [CrossRef]
- Kinaret, P.; Ilves, M.; Fortino, V.; Rydman, E.; Karisola, P.; Lähde, A.; Koivisto, J.; Jokiniemi, J.; Wolff, H.; Savolainen, K.; et al. Inhalation and oropharyngeal aspiration exposure to rod-like carbon nanotubes induce similar airway inflammation and biological responses in mouse lungs. ACS Nano 2017, 11, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.R.; Scabilloni, J.F.; Hubbs, A.F.; Battelli, L.A.; McKinney, W.; Friend, S.; Wolfarth, M.G.; Andrew, M.; Castranova, V.; Porter, D.W. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol. 2013, 10, 33. [Google Scholar] [CrossRef]
- Rossi, E.M.; Pylkkänen, L.; Koivisto, A.J.; Nykäsenoja, H.; Wolff, H.; Savolainen, K.; Alenius, H. Inhalation exposure to nanosized and fine TiO2 particles inhibits features of allergic asthma in a murine model. Part. Fibre Toxicol. 2010, 7, 35. [Google Scholar] [CrossRef]
- Meesters, J.; Peijnenburg, W.; Hendriks, J.; van de Meent, D.; Quik, J. A Model Sensitivity Analysis to Determine the Most Important Physicochemical Properties Driving Environmental Fate and Exposure of Engineered Nanoparticles. Environ. Sci. Nano. 2019, 6, 2049–2060. [Google Scholar] [CrossRef]
- Xiaojia, H.; Peter, F.; Aker, W.G.; Hwang, H.M. Toxicity of engineered nanomaterials mediated by nano–bio–eco interactions. J. Environ. Sci. Health Part. C 2018, 36, 1–22. [Google Scholar]
- Panzarini, E.; Tenuzzo, B.; Vergallo, C.; Dini, L. Biological systems interact with Engineered NanoMaterials (ENMs): Possible environmental risks. Il Nuovo Cimento. C 2013, 36, 111–116. [Google Scholar]
- Quigg, A.; Chin, W.C.; Chen, C.S.; Zhang, S.; Jiang, Y.; Miao, A.J.; Schwehr, K.A.; Xu, C.; Santschi, P.H. Direct and indirect toxic effects of engineered nanoparticles on algae: Role of natural organic matter. ACS Sustain. Chem. Eng. 2013, 1, 686–702. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Benn, T.M.; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Egorova, E.M.; Kaba, S.I. The effect of surfactant micellization on the cytotoxicity of silver nanoparticles stabilized with aerosol-OT. Toxicol. Vitr. 2019, 57, 244–254. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Zaikova, T.; Dankovich, T.; Yun, J.; Ryan, M.; Hutchison, J.E.; Lowry, G.V. Effect of silver concentration and chemical transformations on release and antibacterial efficacy in silver-containing textiles. Nanoimpacticle 2018, 11, 51–57. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Surampalli, R.Y. Engineered nanoparticles in wastewater and wastewater sludge-evidence and impacts. Waste Manag. 2010, 30, 504–520. [Google Scholar] [CrossRef]
- Shafer, M.M.; Hoffmann, S.R.; Overdier, J.T.; Armstrong, D.E. Physical and kinetic speciation of copper and zinc in three geochemically contrasting marine estuaries. Environ. Sci. Technol. 2004, 38, 3810–3819. [Google Scholar] [CrossRef]
- Kaegi, R.; Voegelin, A.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Burkhardt, M.; Siegrist, H. Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ. Sci. Technol. 2011, 45, 3902–3908. [Google Scholar] [CrossRef]
- Kleiven, M.; Macken, A.; Oughton, D.H. Growth inhibition in Raphidocelis subcapita-Evidence of nanospecific toxicity of silver nanoparticles. Chemosphere 2019, 221, 785–792. [Google Scholar] [CrossRef]
- Burchardt, A.D.; Carvalho, R.N.; Valente, A.; Nativo, P.; Gilliland, D.; Garcìa, C.P.; Passarella, R.; Pedroni, V.; Rossi, F.; Lettieri, T. Effects of silver nanoparticles in diatom Thalassiosira pseudonana and cyanobacterium Synechococcus sp. Environ. Sci. Technol. 2012, 46, 11336–11344. [Google Scholar] [CrossRef]
- Oukarroum, A.; Barhoumi, L.; Pirastru, L.; Dewez, D. Silver nanoparticle toxicity effect on growth and cellular viability of the aquatic plant Lemna gibba. Environ. Toxicol. Chem. 2013, 32, 902–907. [Google Scholar] [CrossRef]
- He, D.; Dorantes-Aranda, J.J.; Waite, T.D. Silver nanoparticle-algae interactions: Oxidative dissolution, reactive oxygen species generation and synergistic toxic effects. Environ. Sci Technol. 2012, 46, 8731–8738. [Google Scholar] [CrossRef]
- von Moos, N.; Maillard, L.; Slaveykova, V.I. Dynamics of sub-lethal effects of nano-CuO on the microalga Chlamydomonas reinhardtii during short-term exposure. Aquat. Toxicol. 2015, 161, 267–275. [Google Scholar] [CrossRef]
- Xiang, L.; Fang, J.; Cheng, H. Toxicity of silver nanoparticles to green algae M. aeruginosa and alleviation by organic matter. Environ. Monit. Assess. 2018, 190, 667. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Singh, A.P.; Chaudhary, B.R.; Singh, S.K.; Dash, D. Effect of silver nanoparticles on growth of eukaryotic green algae. Nano-Micro Lett. 2012, 4, 158–165. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, T.; Paliwal, C.; Maurya, R.; Ghosh, A.; Mishra, S. Green synthesis, characterization and antioxidant potential of silver nanoparticles biosynthesized from de-oiled biomass of thermotolerant oleaginous microalgae Acutodesmus dimorphus. RSC Adv. 2016, 6, 72269–72274. [Google Scholar] [CrossRef]
- Navarro, E.; Wagner, B.; Odzak, N.; Sigg, L.; Behra, R. Effects of differently coated silver nanoparticles on the photosynthesis of Chlamydomonas reinhardtii. Environ. Sci. Technol. 2015, 49, 8041–8047. [Google Scholar] [CrossRef]
- Lekamge, S.; Miranda, A.F.; Ball, A.S.; Shukla, R.; Nugegoda, D. The toxicity of coated silver nanoparticles to Daphnia carinata and trophic transfer from alga Raphidocelis subcapitata. PLoS ONE 2019, 14, e0214398. [Google Scholar] [CrossRef]
- Yang, X.Y.; Gondikas, A.P.; Marinakos, S.M.; Auffan, M.; Liu, J.; Hsu-Kim, H.; Meyer, J.N. Mechanism of Silver Nanoparticle Toxicity Is Dependent on Dissolved Silver and Surface Coating in Caenorhabditis elegans. Environ. Sci. Technol. 2012, 46, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Panacek, A.; Kvitek, L.; Prucek, R.; Kolar, M.; Vecerova, R.; Pizurova, N.; Sharma, V.K.; Nevecna, T.; Zboril, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Padil, V.V.T.; Wacławek, S.; Černík, M. Green synthesis: Nanoparticles and nanofibres based on tree gums for environmental applications. Ecol. Chem. Eng. S 2016, 23, 533–557. [Google Scholar] [CrossRef]
- Cinelli, M.; Coles, S.R.; Nadagouda, M.N.; Błaszczyński, J.; Słowiński, R.; Rajender, S.V.; Kirwan, K. A green chemistry-based classification model for the synthesis of silver nanoparticles. Green Chem. 2015, 17, 2825–2839. [Google Scholar] [CrossRef]
- Vinod, V.T.; Saravanan, P.; Sreedhar, B.; Devi, D.K.; Sashidhar, R.B. A facile synthesis and characterization of Ag, Au and Pt nanoparticles using a natural hydrocolloid gum kondagogu (Cochlospermum gossypium). Colloids Surf. B Biointerfaces 2011, 83, 291–298. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sharma, V. Biotic Strategies for Toxic Heavy Metal Decontamination. Recent Pat Biotechnol. 2017, 11, 218–228. [Google Scholar] [CrossRef]
- Rath, B. Microalgal bioremediation: Current practices and perspectives. J. Biochem. Technol. 2012, 3, 299–304. [Google Scholar]
- Gong, N.; Shao, K.; Feng, W.; Lin, Z.; Liang, C.; Sun, Y. Biotoxicity of nickel oxide nanoparticles and bio-remediation by microalgae Chlorella vulgaris. Chemosphere 2011, 83, 510–516. [Google Scholar] [CrossRef]
- Panzarini, E.; Mariano, S.; Dini, L. Glycans coated silver nanoparticles induces autophagy and necrosis in HeLa cells. In AIP Conference Proceedings; Rossi, M., Dini, L., Passeri, D., Terranova, M.L., Eds.; AIP Publishing LLC: Melville, NY, USA, 2015; Volume 1667, p. 020017. [Google Scholar] [CrossRef]
- Panzarini, E.; Mariano, S.; Vergallo, C.; Carata, E.; Fimia, G.M.; Mura, F.; Rossi, M.; Vergaro, V.; Ciccarella, G.; Corazzari, M.; et al. Glucose capped silver nanoparticles induce cell cycle arrest in HeLa cells. Toxicol. Vitr. 2017, 41, 64–74. [Google Scholar] [CrossRef]
- Manno, D.; Serra, A.; Buccolieri, A.; Panzarini, E.; Carata, E.; Tenuzzo, B.; Izzo, D.; Vergallo, C.; Rossi, M.; Dini, L. Silver and carbon nanoparticles toxicity in sea urchin Paracentrotus lividus embryos. Bio. Nano. Mater. 2013, 14, 229–238. [Google Scholar]
- Amenorfenyo, D.K.; Huang, X.; Zhang, Y.; Zeng, Q.; Zhang, N.; Ren, J.; Huang, Q. Microalgae brewery wastewater treatment: Potentials, benefits and the challenges. Int. J. Environ. Res. Pub. He. 2019, 16, 1910. [Google Scholar] [CrossRef]
- Nichols, H.W. Growth media-freshwater. In Handbook of Phycological Methods. Culture Methods and Growth Measurements; Stein, J.R., Ed.; Cambridge University Press: New York, NY, USA, 1973; pp. 7–24. [Google Scholar]
- OECD. No. 201, freshwater algae and cyanobacteria, growth inhibition test. In OECD Guideline for Testing of Chemicals; OECD: Paris, France, 2011. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant. Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Niu, J.; Chen, Y. Photogeneration of reactive oxygen species on uncoated silver, gold, nickel, and silicon nanoparticles and their antibacterial effects. Langmuir 2013, 29, 4647–4651. [Google Scholar] [CrossRef]
- Tkalec, M.; Štefanić, P.P.; Balen, B. Phytotoxicity of silver nanoparticles and defence mechanisms. Analysis, Fate, and Toxicity of Engineered Nanomaterials. In Comprehensive Analytical Chemistry; Sandeep, K.V., Ashok, K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 84, pp. 145–148. [Google Scholar]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, S.; Wilkinson, K.J. Bioaccumulation of Nanosilver by Chlamydomonas reinhardtii-Nanoparticle or the Free Ion? Environ. Sci. Technol. 2014, 48, 358–364. [Google Scholar] [CrossRef]
- Turner, A.; Brice, D.; Brown, M.T. Interactions of silver nanoparticles with the marine macroalga, Ulva lactuca. Ecotoxicology 2012, 21, 148–154. [Google Scholar] [CrossRef]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Rybak-Smith, M.J. Effect of surface modification on toxicity of nanoparticles. Encycl. Nanotechnol. 2012, 645–652. [Google Scholar]
- Tiede, K.; Hassellöv, M.; Breitbarth, E.; Chaudhry, Q.; Boxall, A.B. Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles. J. Chromatogr. A 2009, 1216, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Bilberg, K.; Malte, H.; Wang, T.; Baatrup, E. Silver nanoparticles and silver nitrate cause respiratory stress in Eurasian perch (Perca fluviatilis). Aquat. Toxicol. 2010, 96, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Domingo, G.; Bracale, M.; Vannini, C. Phytotoxicity of silver nanoparticles to aquatic plants, algae, and microorganisms. In Nanomaterials in Plants, Algae and Microorganisms; Durgesh, K.T., Parvaiz, A., Shivesh, S., Devendra, K.C., Nawal, K.D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 143–168. [Google Scholar]
- Wagner, S.; Gondikas, A.; Neubauer, E.; Hofmann, T.; von der Kammer, F. Spot the Difference: Engineered and Natural Nanoparticles in the Environment-Release, Behavior and Fate. Angew. Chem. Int. Ed. 2014, 53, 12398–12419. [Google Scholar] [CrossRef]
- Lekamge, S.; Miranda, A.F.; Abraham, A.; Li, V.; Shukla, R.; Bansal, V.; Nugegoda, D. The toxicity of Silver Nanoparticles (AgNPs) to three freshwater invertebrates with different life strategies: Hydra vulgaris, Daphnia carinata, and Paratya australiensis. Front. Environ. Sci. 2018, 6, 152. [Google Scholar] [CrossRef]
- Angel, B.M.; Batley, G.E.; Jarolimek, C.V.; Rogers, N.J. The impact of size on the fate and toxicity of nanoparticulate silver in aquatic systems. Chemosphere 2013, 93, 359–365. [Google Scholar] [CrossRef]
- Sendra, M.; Yeste, M.P.; Gatica, J.M.; Moreno-Garrido, I.; Blasco, J. Direct and indirect effects of silver nanoparticles on freshwater and marine microalgae (Chlamydomonas reinhardtii and Phaeodactylum tricornutum). Chemosphere 2017, 179, 279–289. [Google Scholar] [CrossRef]
- Wang, F.; Guan, W.; Xu, L.; Ding, Z.; Ma, H.; Ma, A.; Terry, N. Effects of Nanoparticles on Algae: Adsorption, Distribution, Ecotoxicity and Fate. Appl. Sci. 2019, 9, 1534. [Google Scholar] [CrossRef]
- Hazeem, L.J.; Kuku, G.; Dewailly, E.; Slomianny, C.; Barras, A.; Hamdi, A.; Boukherroub, R.; Culha, M.; Bououdina, M. Toxicity Effect of Silver Nanoparticles on Photosynthetic Pigment Content, Growth, ROS Production and Ultrastructural Changes of Microalgae Chlorella vulgaris. Nanomaterials 2019, 9, 914. [Google Scholar] [CrossRef]
- Zemke-White, W.L.; Clements, K.D.; Harris, P.J. Acid lysis of macroalgae by marine herbivorous fishes: Effects of acid pH on cell wall porosity. J. Exp. Mar. Biol. Ecol. 2000, 245, 57. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhang, M.Y.; Xia, I.; Zhang, J.; Xing, G.M. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, R.; Liu, Z.; Liang, X.; Feng, W. Cytotoxicity of NiO nanoparticles and its conversion inside Chlorella vulgaris. Chem. Res. Chin. Univ. 2017, 33, 107–111. [Google Scholar] [CrossRef]
- Ribeiro, F.; Gallego-Urrea, J.A.; Jurkschat, K.; Crossley, A.; Hassellöv, M.; Taylor, C.; Soares, A.M.; Loureiro, S. Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci. Total Environ. 2014, 466–467, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; Bras, S.; Perreault, F.; Popovic, R. Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicol. Environ. Saf. 2012, 78, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Andrade, J.M.; Avalos-Borja, M.; Vilchis-Nestor, A.R.; Sanchez-Vargas, L.O.; Castro-Longoria, E. Dual function of EDTA with silver nanoparticles for root canal treatment-A novel modification. PLoS ONE 2018, 13, e0190866. [Google Scholar] [CrossRef]
- Chappell, M.A.; Miller, L.F.; George, A.J.; Pettway, B.A.; Price, C.L.; Porter, B.E.; Bednar, A.J.; Seiter, J.M.; Kennedy, A.J.; Steevens, J.A. Simultaneous dispersion-dissolution behavior of concentrated silver nanoparticle suspensions in the presence of model organic solutes. Chemosphere 2011, 84, 1108–1116. [Google Scholar] [CrossRef]
- Ahmad, A.; Bhat, A.H.; Buang, A. Enhanced biosorption of transition metals by living Chlorella vulgaris immobilized in Ca-alginate beads. Environ. Technol. 2019, 40, 1793–1809. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariano, S.; Panzarini, E.; Inverno, M.D.; Voulvoulis, N.; Dini, L. Toxicity, Bioaccumulation and Biotransformation of Glucose-Capped Silver Nanoparticles in Green Microalgae Chlorella vulgaris. Nanomaterials 2020, 10, 1377. https://doi.org/10.3390/nano10071377
Mariano S, Panzarini E, Inverno MD, Voulvoulis N, Dini L. Toxicity, Bioaccumulation and Biotransformation of Glucose-Capped Silver Nanoparticles in Green Microalgae Chlorella vulgaris. Nanomaterials. 2020; 10(7):1377. https://doi.org/10.3390/nano10071377
Chicago/Turabian StyleMariano, Stefania, Elisa Panzarini, Maria D. Inverno, Nick Voulvoulis, and Luciana Dini. 2020. "Toxicity, Bioaccumulation and Biotransformation of Glucose-Capped Silver Nanoparticles in Green Microalgae Chlorella vulgaris" Nanomaterials 10, no. 7: 1377. https://doi.org/10.3390/nano10071377
APA StyleMariano, S., Panzarini, E., Inverno, M. D., Voulvoulis, N., & Dini, L. (2020). Toxicity, Bioaccumulation and Biotransformation of Glucose-Capped Silver Nanoparticles in Green Microalgae Chlorella vulgaris. Nanomaterials, 10(7), 1377. https://doi.org/10.3390/nano10071377






