Capturing Amyloid-β Oligomers by Stirring with Microscaled Iron Oxide Stir Bars into Magnetic Plaques to Reduce Cytotoxicity toward Neuronal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
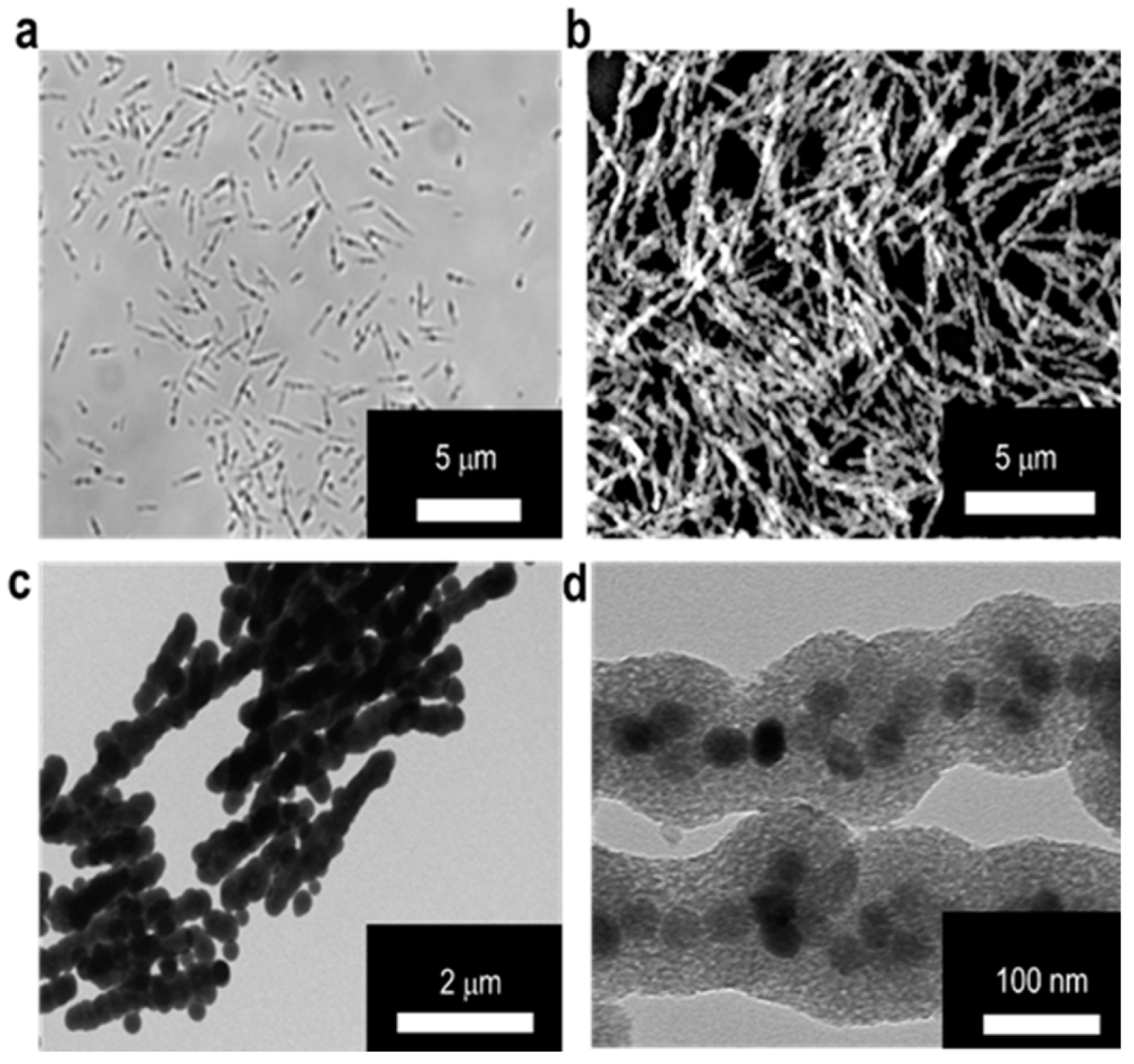
2.2. Preparation and Characterization of MSBs
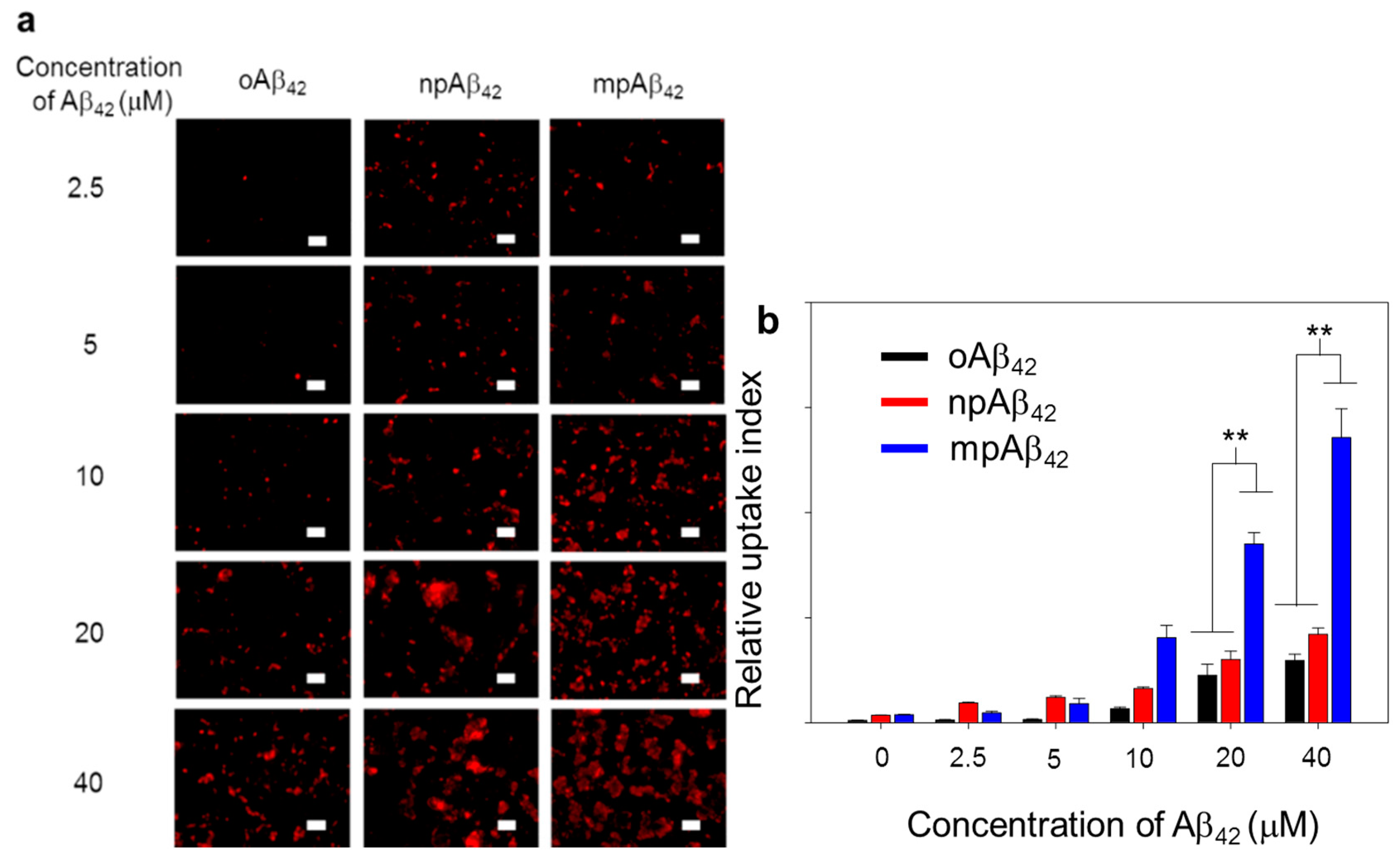
2.3. Preparation and Characterization of oAβ42 and Natural Aβ42 Plaques (npAβ42)
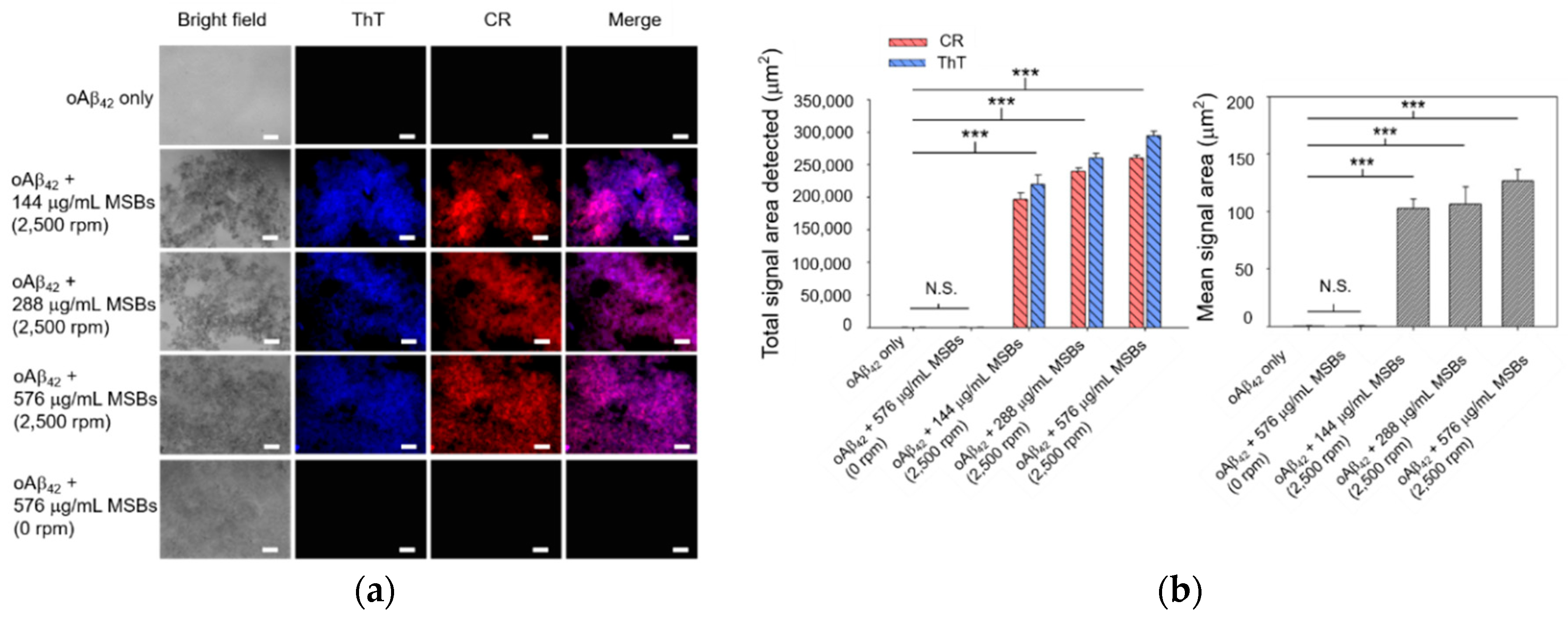
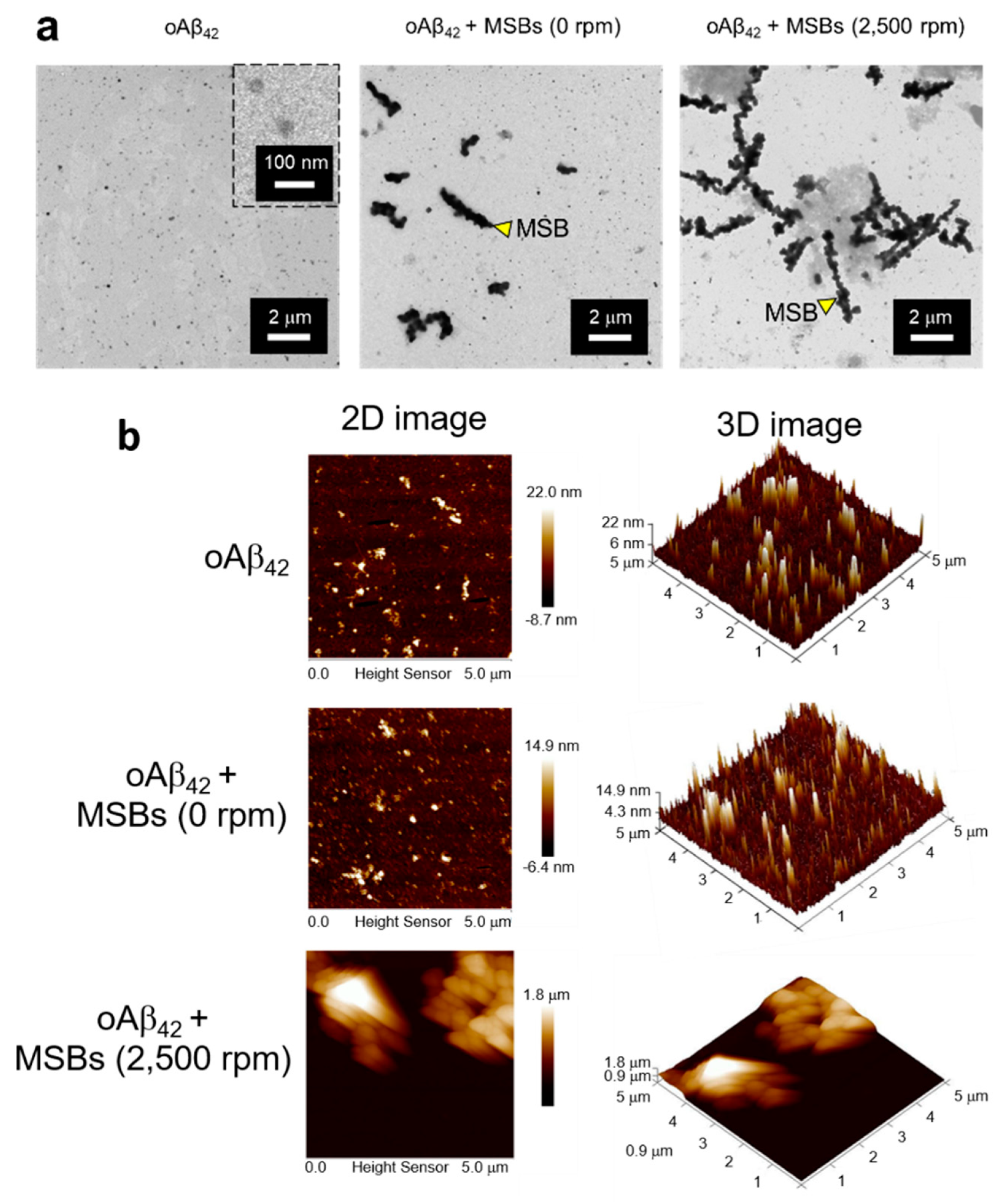
2.4. In Vitro Stirring-Induced oAβ42 Capture
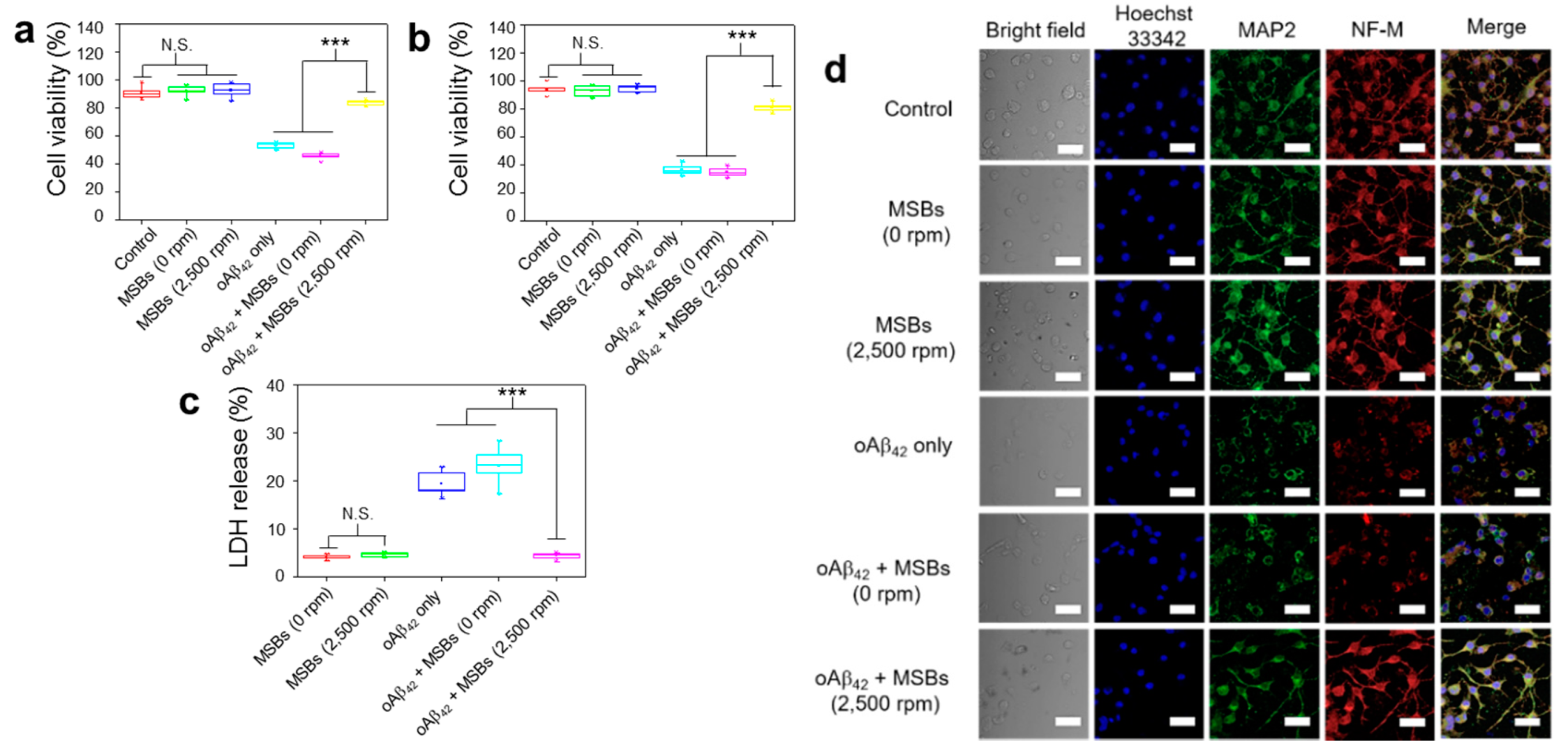
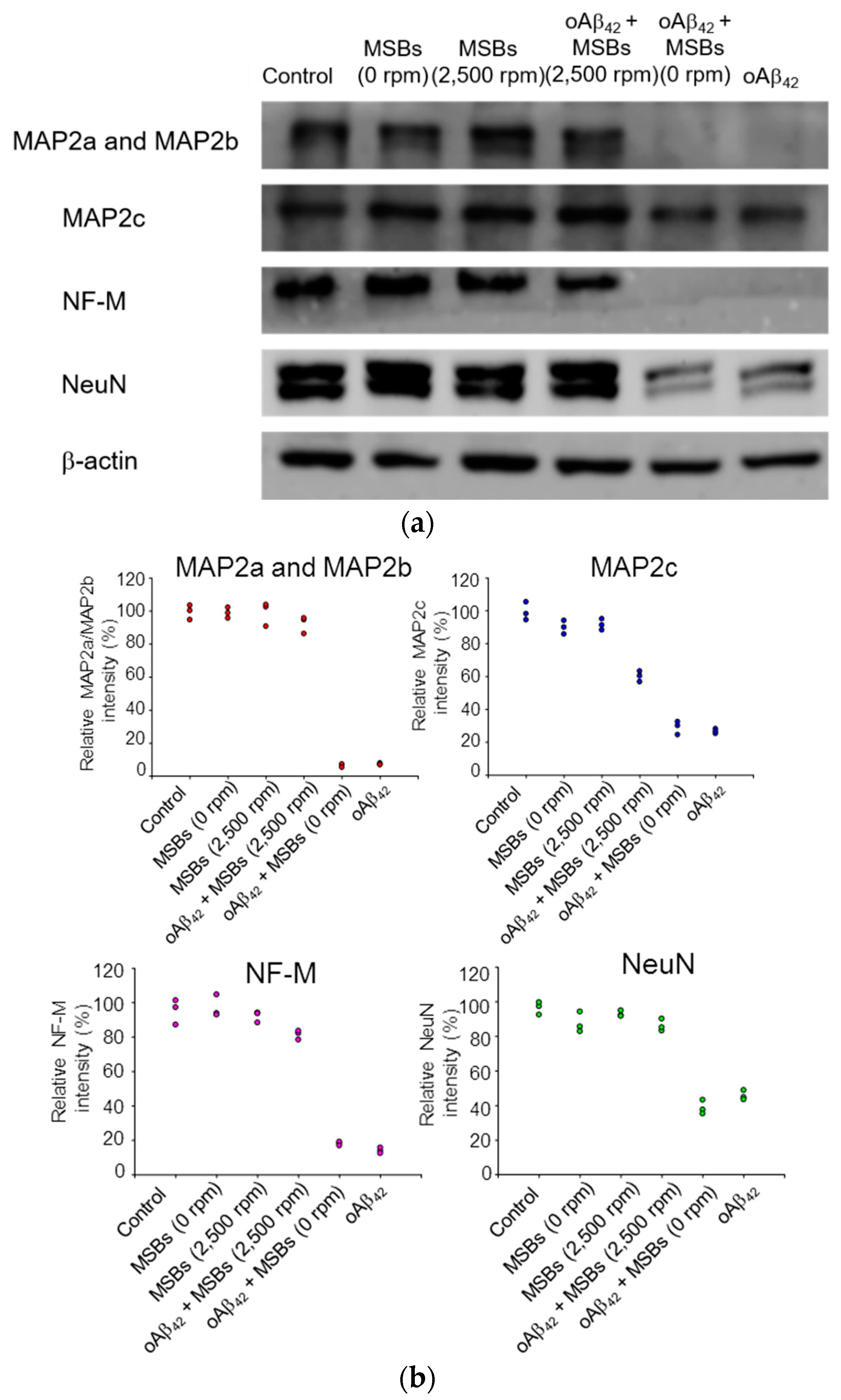
2.5. Cell Membrane Damage and Neurite Outgrowth Impairment
2.6. Cell Functions
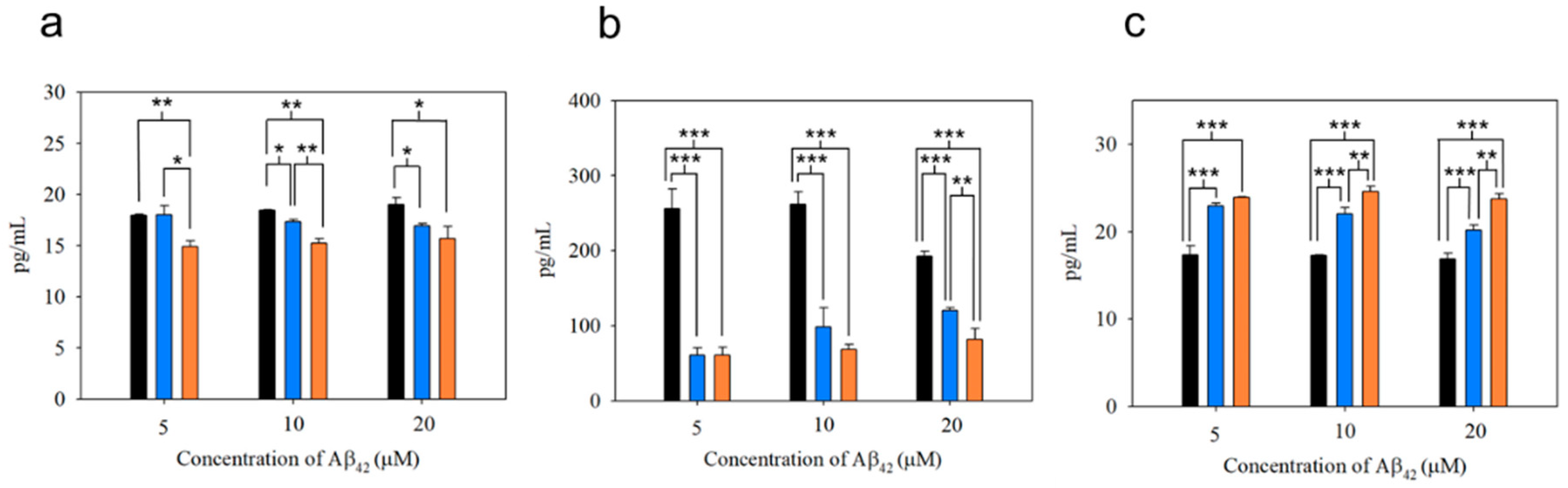
2.7. Phagocytic Activity of Microglial Cells
2.8. Cytokine Production
2.9. Brain Tissue Toxicity and Damage with MSB Stirring
2.10. Statistical Analysis
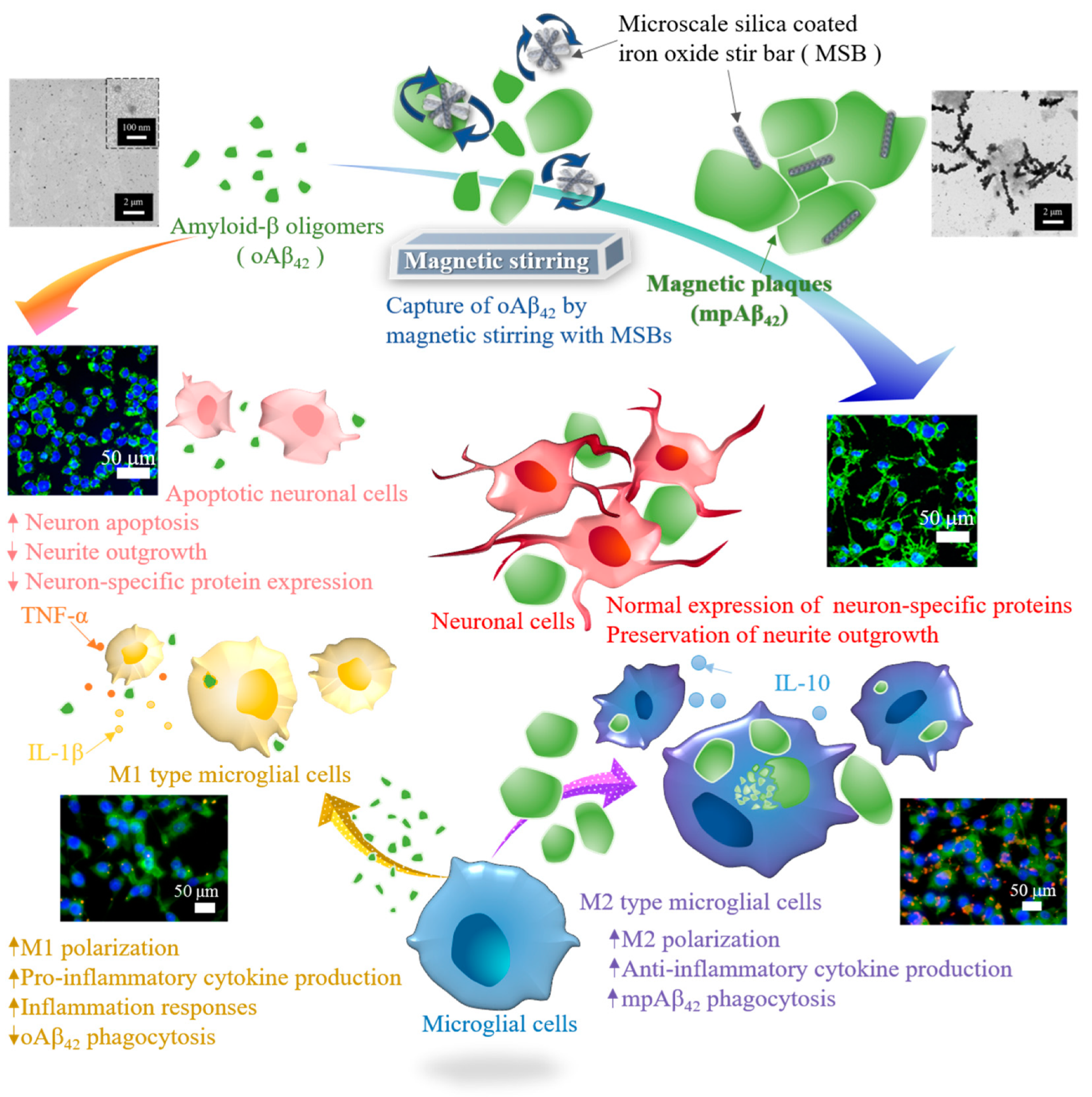
3. Results and Discussion
3.1. Preparation and Characterization of MSBs
3.2. Capture Efficiency of Aβ42 by Magnetic Stirring of MSBs and In Vitro Therapeutic Efficacy
3.3. Functionality of N2a Cells and Phagocytic Action of BV-2 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Klein, W.L.; Krafft, G.A.; Finch, C.E. Targeting small Abeta oligomers: The solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001, 24, 219–224. [Google Scholar] [CrossRef]
- Maia, M.A.; Sousa, E. BACE-1 and γ-secretase as therapeutic targets for Alzheimer’s disease. Pharmaceuticals 2019, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement. 2019, 5, 272–293. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Doody, R.S.; Thomas, R.G.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; Raman, R.; Sun, X.; Aisen, P.S. Phase 3 trials of Solanezumab for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Lauren, J.; Gimbel, D.A.; Nygaard, H.B.; Gilbert, J.W.; Strittmatter, S.M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 2009, 457, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Zhang, L.; Yu, W.; Wang, Y.; Chang, W. Cellular prion protein as a receptor of toxic amyloid-β42 oligomers is important for Alzheimer’s disease. Front. Cell Neurosci. 2019, 13, 339. [Google Scholar] [CrossRef]
- EI-Shimy, I.A.; Heikal, O.A.; Hamdi, N. Minocycline attenuates Aβ oligomers-induced pro-inflammatory phenotype in primary microglia while enhancing Aβ fibrils phagocytosis. Neurosci. Lett. 2015, 609, 36–41. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Tobinick, E.L.; Gross, H. Rapid cognitive improvement in Alzheimer’s disease following perispinal etanercept administration. J. Neuroinflamm. 2008, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.G.; Ferreira, S.T. Inflammation, defective insulin signalling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes 2014, 63, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Landreth, G.E. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 2010, 117, 949–960. [Google Scholar] [CrossRef]
- Yang, W.; Wu, Q.; Yuan, C.; Gao, J.; Xiao, M.; Gu, M.; Ding, J.; Hu, G. Aquaporin-4 mediates astrocyte response to β-amyloid. Cell Neruosci. 2012, 49, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, T.; Cirrito, J.R.; Liu, C.C.; Shinohara, M.; Li, J.; Schuler, D.R.; Shinohara, M.; Holtzman, D.M.; Bu, G. Neuronal clearance of amyloid-β by endocytic receptor LRP1. J. Neurosci. 2013, 33, 19276–19283. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Winkler, E.; Julius, A.; Steiner, H.; Langosch, D. Homodimerization protects the amyloid precursor protein C99 fragment from cleavage by γ-secretase. Biochemistry 2015, 54, 6149–6152. [Google Scholar] [CrossRef]
- Parajuli, B.; Sonobe, Y.; Horiuchi, H.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Oligomeric amyloid β induces IL-1β processing via production of ROS: Implicationin Alzheimer’s disease. Cell Death Dis. 2013, 4, e975. [Google Scholar] [CrossRef]
- Prade, E.; Barucker, C.; Sarkar, R.; Althoff-Ospelt, G.; Lopez del Amo, J.M.; Hossain, S.; Zhong, Y.; Multhaup, G.; Reif, B. Sulindac sulfide induces the formation of large oligomeric aggregates of the Alzheimer’s disease amyloid-β peptide which exhibit reduced neurotoxicity. Biochemistry 2016, 55, 1839–1849. [Google Scholar] [CrossRef]
- Bieschke, J.; Herbst, M.; Wiglenda, T.; Friedrich, R.P.; Boeddrich, A.; Schiele, F.; Kleckers, D.; Lopez del Amo, J.M.; Gruning, B.A.; Wang, Q.; et al. Small-molecule conversion of toxic oligomers to nontoxic β-sheet-rich amyloid fibrils. Nat. Chem. Biol. 2011, 8, 93–101. [Google Scholar] [CrossRef]
- Keskin, A.D.; Kekus, M.; Adelsberger, H.; Neumann, U.; Shimshek, D.R.; Song, B.; Zott, B.; Peng, T.; Forstl, H.; Staufenbiel, M.; et al. BACE inhibition-dependent repair of Alzheimer’s pathophysiology. Proc. Natl. Acad. Sci. USA 2017, 114, 8631–8636. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.R.; Syvanen, S.; Hultgvist, G.; Fang, X.T.; Roshanbin, S.; Lannfelt, L.; Neumann, U.; Sehlin, D. Antibody-based in vivo PET imaging detects amyloid-β reduction in Alzheimer transgenic mice after BACE-1 inhibition. J. Nucl. Med. 2018, 59, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lu, I.L.; Chiang, W.H.; Lin, Y.W.; Tsai, Y.C.; Chen, H.H.; Chang, C.W.; Chiang, C.S.; Chiu, H.C. Tumortropic adipose-derived stem cells carrying smart nanotherapeutics for targeted delivery and dual-modality therapy of orthotopic glioblastoma. J. Control Release 2017, 254, 119–130. [Google Scholar] [CrossRef]
- Hu, Y.; He, L.; Yin, Y. Magnetically responsive photonic nanochains. Angew. Chem. Int. Ed. Engl. 2011, 50, 3747–3750. [Google Scholar] [CrossRef]
- Dahlgren, K.N.; Manelli, A.M.; Stine, W.B., Jr.; Baker, L.K.; Krafft, G.A.; LaDu, M.J. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J. Biol. Chem. 2002, 277, 32046–32053. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.P.; Viola, K.L.; Chromy, B.A.; Chang, L.; Morgan, T.E.; Yu, J.; Venton, D.L.; Krafft, G.A.; Finch, C.E.; Klein, W.L. Vaccination with soluble Abeta oligomers generates toxicity-neutralizing antibodies. J. Neurochem. 2001, 79, 595–605. [Google Scholar] [CrossRef]
- Kuznetsova, I.M.; Sulatskaya, A.I.; Uversky, V.N.; Turoverov, K.K. Analyzing thioflavin T binding to amyloid fibrils by an equilibrium microdialysis-based technique. PLoS ONE 2012, 7, e30724. [Google Scholar] [CrossRef]
- Hudson, S.A.; Ecroyd, H.; Kee, T.W.; Carver, J.A. The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J. 2009, 276, 5960–5972. [Google Scholar] [CrossRef]
- Huang, C.; Xu, D.; Zhang, K.; Shentu, J.; Yan, S.; Wu, D.; Wang, Q.; Cui, W. A11-positive β-amyloid oligomer preparation and assessment using dot blotting analysis. J. Vis. Exp. 2018, 135, 57592. [Google Scholar]
- Xue, C.; Tran, J.; Wang, H.; Park, G.; Hsu, F.; Guo, Z. Aβ42 fibril formation from predominantly oligomeric samples suggests a link between oligomer heterogeneity and fibril polymorphism. R. Soc. Open Sci. 2019, 6, 190179. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Mondal, S.; Muthuraj, B.; Balaji, S.N.; Trivedi, V.; Iyer, P.K. Remarkably efficient blood-brain barrier crossing polyfluorene-chitosan nanoparticle selectively tweaks amyloid oligomer in cerebrospinal fluid and Aβ1–40. ACS Omega 2018, 3, 8059–8066. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhong, Y.; Gui, J.; Wang, X.; Zhuang, X.; Weng, J. A hydrogel biosensor for high selective and sensitive detection of amyloid-beta oligomers. Int. J. Nanomed. 2018, 13, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Wang, W.; Hou, T.T.; Jia, L.F.; Wu, Q.Q.; Quan, M.N.; Jia, J.P. Toxic amyloid-β oligomers induced self-replication in astrocytes triggering neuronal injury. EBioMedicine 2019, 42, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, P.; Tianbai, L.; Herring, A.; Ceballos-Diaz, C.; Das, P.; Golde, T.E. Hippocampal expression of murine IL-4 results in exacerbation of amyloid deposition. Mol. Neurodegener. 2012, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.Y.; Baleriola, J.; Fa, M.; Hengst, U.; Troy, C.M. Stereotaxic infusion of oligomeric amyloid-beta into the mouse hippocampus. J. Vis. Exp. 2015, 100, e52805. [Google Scholar] [CrossRef]
- Chong, W.H.; Chin, L.K.; Tan, R.L.; Wang, H.; Liu, A.Q.; Chen, H. Stirring in suspension: Nanometer-sized magnetic stir bars. Angew. Chem. Int. Ed. Engl. 2013, 33, 8570–8573. [Google Scholar] [CrossRef]
- Malvindi, M.A.; De Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef]
- Younan, N.D.; Viles, J.H. A comparison of three fluorophores for the detection of amyloid fibers and prefibrillar oligomeric assemblies. ThT (Thioflavin T); ANS (1-Anilinonaphthalene-8-sulfonic Acid); and bisANS (4,4’-Dianilino-1,1’-binaphthyl-5,5’-disulfonic Acid). Biochemistry 2015, 54, 4297–4306. [Google Scholar] [CrossRef]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta 2010, 1804, 1405–1412. [Google Scholar] [CrossRef]
- Stsiapura, V.I.; Maskevich, A.A.; Kuzmitsky, V.A.; Turoverov, K.K.; Kuznetsova, I.M. Computational study of thioflavin T torsional relaxation in the excited state. J. Phys. Chem. A 2007, 111, 4829–4835. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Lin, T.Y.; Chang, D.; Guo, Z. Thioflavin T as an amyloid dye: Fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017, 4, 160696. [Google Scholar] [CrossRef] [PubMed]
- Frid, P.; Anisimov, S.V.; Popovic, N. Congo red and protein aggregation in neurodegenerative diseases. Brain Res. Rev. 2007, 53, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Meehan, S.; Berry, Y.; Luisi, B.; Dobson, C.M.; Carver, J.A.; MacPhee, C.E. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. J. Biol. Chem. 2004, 279, 3413–3419. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, U.; Nilson, A.N.; Kayed, R. The Role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine 2016, 6, 42–49. [Google Scholar] [CrossRef]
- Kayed, R.; Lasagna-Reeves, C.A. Molecular mechanisms of amyloid oligomers toxicity. J. Alzheimers Dis. 2013, 33, 67–78. [Google Scholar] [CrossRef]
- Kumar, S.; Wirths, O.; Stuber, K.; Wunderlich, P.; Koch, P.; Theil, S.; Rezaei-Ghaleh, N.; Zweckstetter, M.; Bayer, T.A.; Brustle, O.; et al. Walter, Phosphorylation of the amyloid β-peptide at Ser26 stabilizes oligomeric assembly and increases neurotoxicity. Acta Neuropathol. 2016, 131, 525–537. [Google Scholar] [CrossRef]
- Benilova, I.; Karran, E.; De Strooper, B. The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef]
- Doi, Y.; Mizuno, T.; Maki, Y.; Jin, S.; Mizoguchi, H.; Ikeyama, M.; Doi, M.; Michikawa, M.; Takeuchi, H.; Suzumura, A. Microglia activated with the toll-like receptor 9 ligand CpG attenuate oligomeric amyloid {beta} neurotoxicity in in vitro and in vivo models of Alzheimer’s disease. Am. J. Pathol. 2009, 5, 2121–2132. [Google Scholar] [CrossRef]
- Suzumura, A. Neurotoxicity by microglia: The mechanisms and potential therapeutic strategy. Fukuoka Igaku Zasshi 2009, 1, 243–247. [Google Scholar]
- Richard, K.L.; Filali, M.; Prefontaine, P.; Rivest, S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J. Neurosci. 2008, 28, 5784–5793. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Kim, H.D.; Jin, J.J.; Maxwell, J.A.; Li, L.; Fukuchi, K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain 2006, 129, 3006–3019. [Google Scholar] [CrossRef] [PubMed]
- Fujikura, M.; Iwahara, N.; Hisahara, S.; Kawamata, J.; Matsumura, A.; Yokokawa, K.; Saito, T.; Manabe, T.; Matsushita, T.; Suzuki, S.; et al. CD14 and toll-like receptor 4 promote fibrillar Aβ42 uptake by microglia through a clathrin-mediated pathway. J. Alzheimers Dis. 2019, 68, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Geloso, M.C.; Corvino, V.; Marchese, E.; Serrano, A.; Michetti, F.; D’Ambrosi, N. The dual role of microglia in ALS: Mechanisms and therapeutic approaches. Front. Aging Neurosci. 2017, 9, 242. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-C.; Luo, J.-C.; Liu, T.-I.; Lu, I.-L.; Shen, M.-Y.; Chuang, C.-Y.; Chern, C.-S.; Chiu, H.-C. Capturing Amyloid-β Oligomers by Stirring with Microscaled Iron Oxide Stir Bars into Magnetic Plaques to Reduce Cytotoxicity toward Neuronal Cells. Nanomaterials 2020, 10, 1284. https://doi.org/10.3390/nano10071284
Tsai Y-C, Luo J-C, Liu T-I, Lu I-L, Shen M-Y, Chuang C-Y, Chern C-S, Chiu H-C. Capturing Amyloid-β Oligomers by Stirring with Microscaled Iron Oxide Stir Bars into Magnetic Plaques to Reduce Cytotoxicity toward Neuronal Cells. Nanomaterials. 2020; 10(7):1284. https://doi.org/10.3390/nano10071284
Chicago/Turabian StyleTsai, Yuan-Chung, Jing-Chian Luo, Te-I Liu, I-Lin Lu, Ming-Yin Shen, Chun-Yu Chuang, Chorng-Shyan Chern, and Hsin-Cheng Chiu. 2020. "Capturing Amyloid-β Oligomers by Stirring with Microscaled Iron Oxide Stir Bars into Magnetic Plaques to Reduce Cytotoxicity toward Neuronal Cells" Nanomaterials 10, no. 7: 1284. https://doi.org/10.3390/nano10071284
APA StyleTsai, Y.-C., Luo, J.-C., Liu, T.-I., Lu, I.-L., Shen, M.-Y., Chuang, C.-Y., Chern, C.-S., & Chiu, H.-C. (2020). Capturing Amyloid-β Oligomers by Stirring with Microscaled Iron Oxide Stir Bars into Magnetic Plaques to Reduce Cytotoxicity toward Neuronal Cells. Nanomaterials, 10(7), 1284. https://doi.org/10.3390/nano10071284






