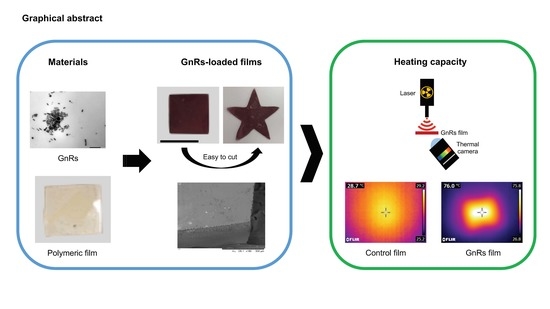
Potential of Polymeric Films Loaded with Gold Nanorods for Local Hyperthermia Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
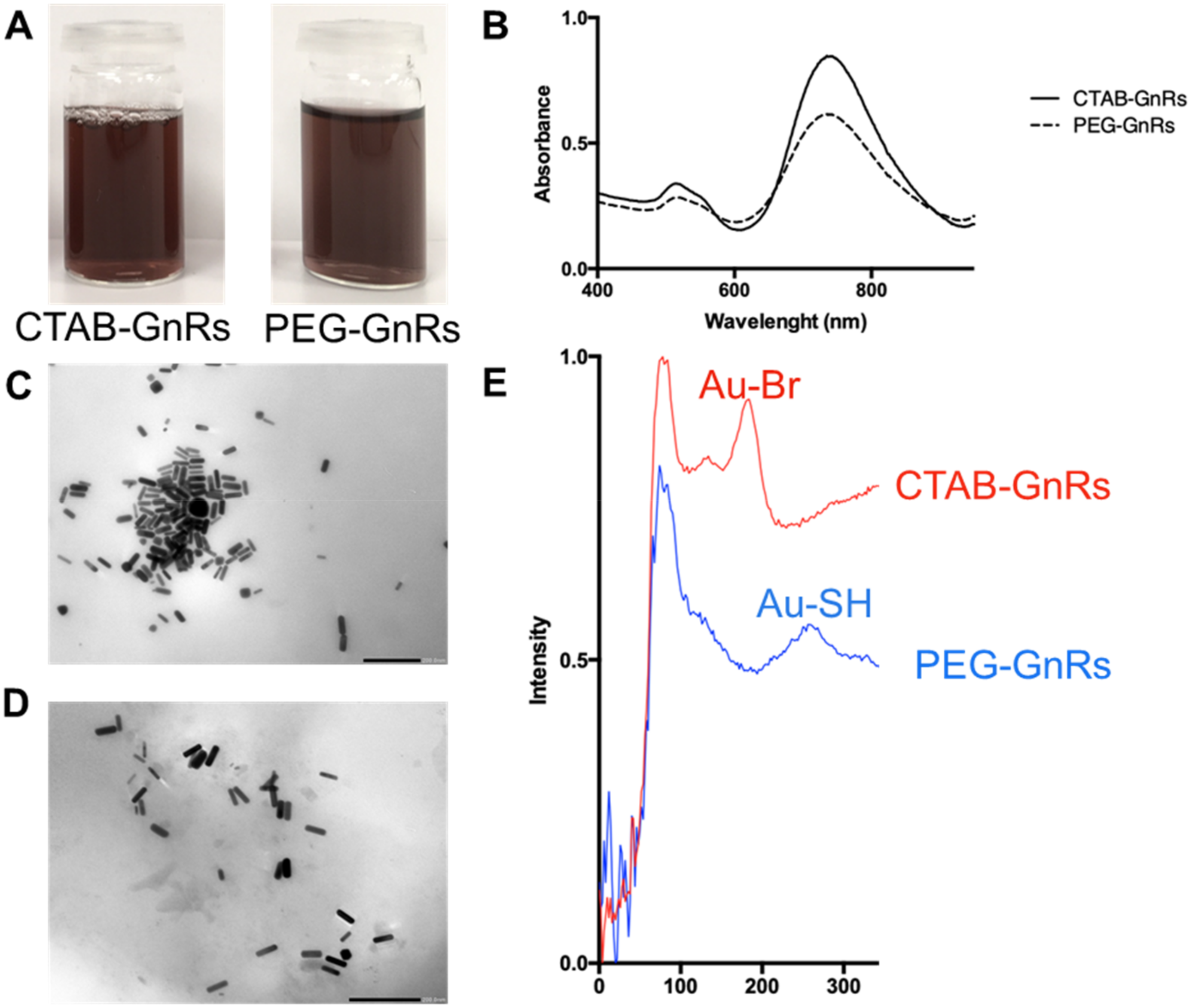
2.2. Synthesis and Characterization of Gold Nanorods (GnRs)
2.2.1. Functionalization of GnRs by Thiolated Poly(ethylene) Glycol (mPEG-SH) and Cetyltrimethylammonium Bromide (CTAB) Removal
2.2.2. UV Spectrum of GnRs
2.2.3. Transmission Electron Microscopy Images of GnRs
2.2.4. Z Potential of GnRs
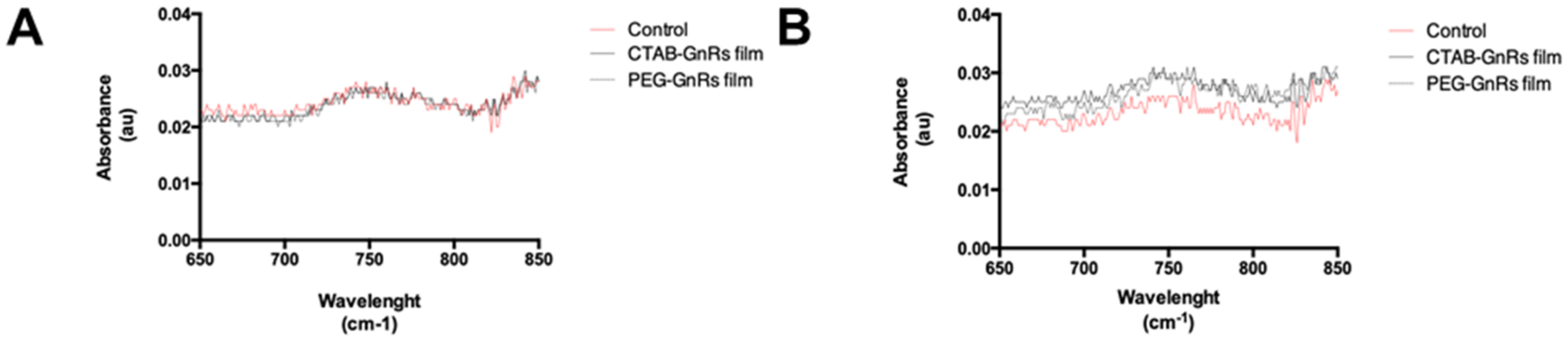
2.2.5. Raman Spectroscopy Characterization of CTAB and Pegylated GnRs (PEG-GnRs)
2.2.6. Gold Quantification in GnRs
2.3. Fabrication and Characterization of Polymeric Films Loaded with GnRs
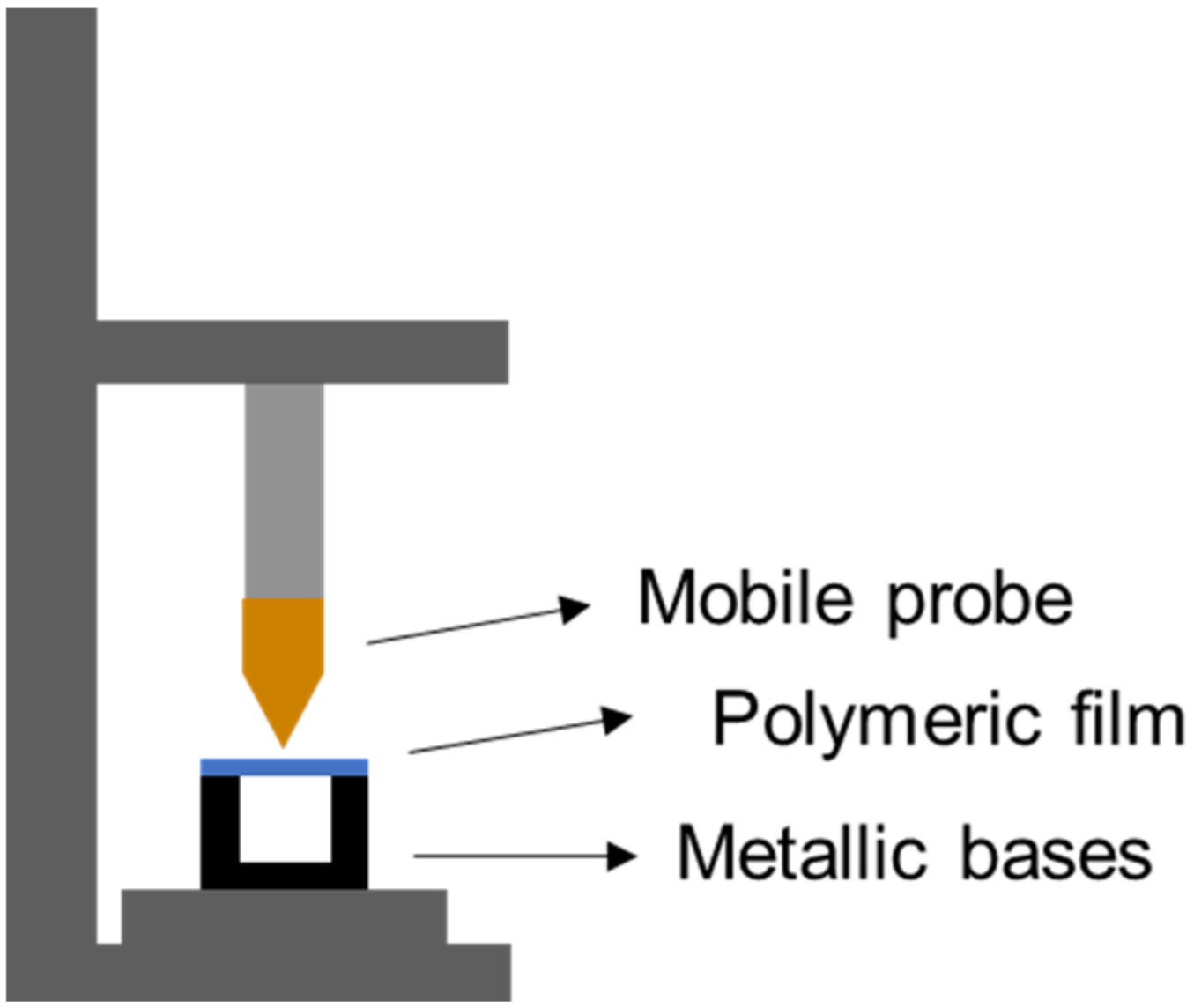
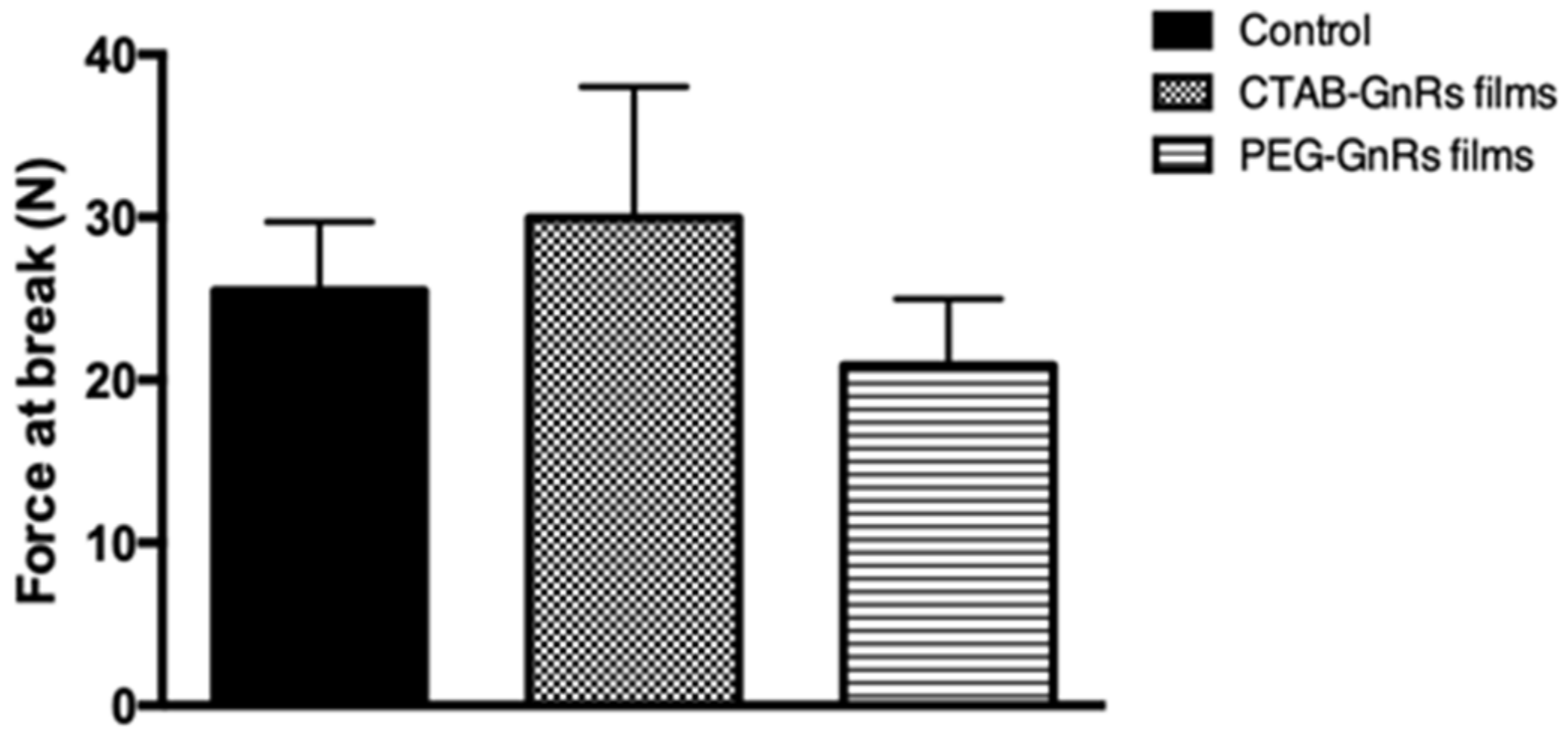
2.3.1. Mechanical Properties of Polymeric Films
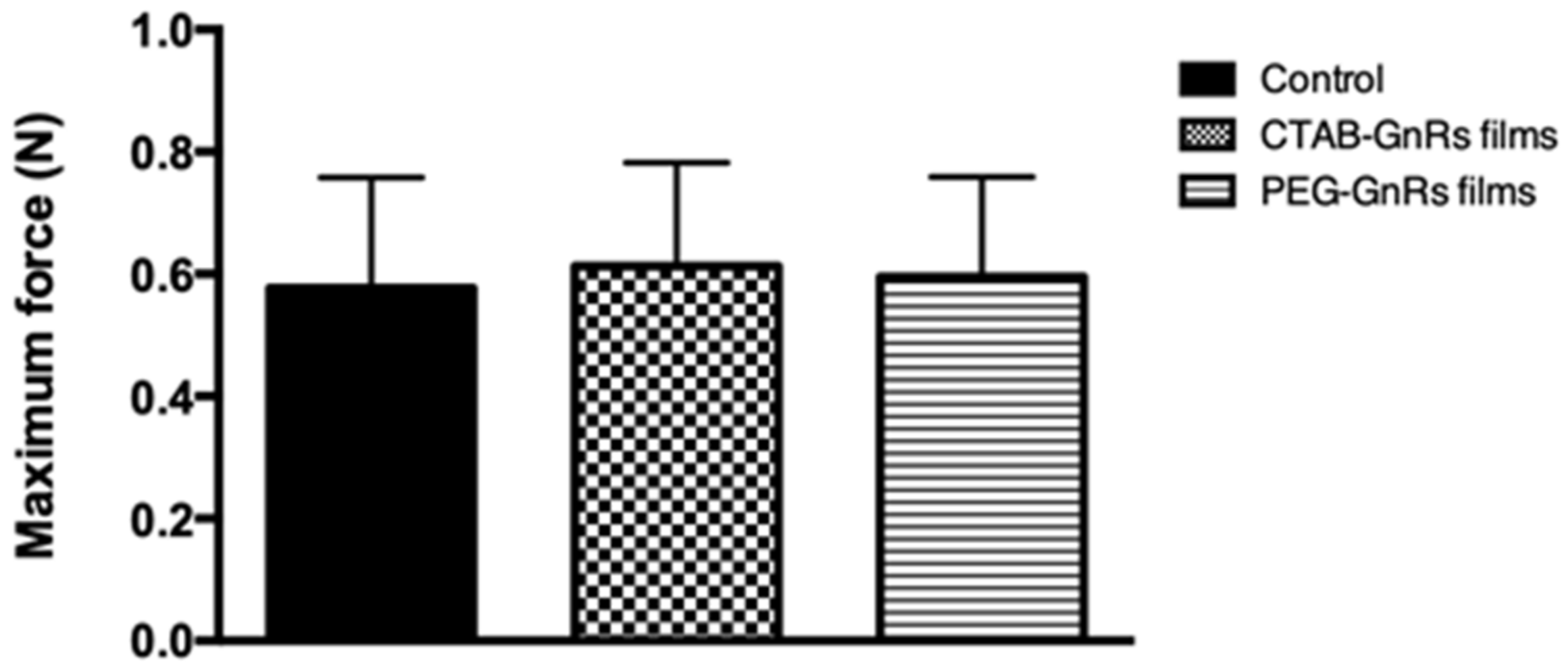
2.3.2. Adhesion of Polymeric Films to Neonate Porcine Skin
2.3.3. Swelling of Polymeric Films on Phosphate Buffered Saline (PBS)
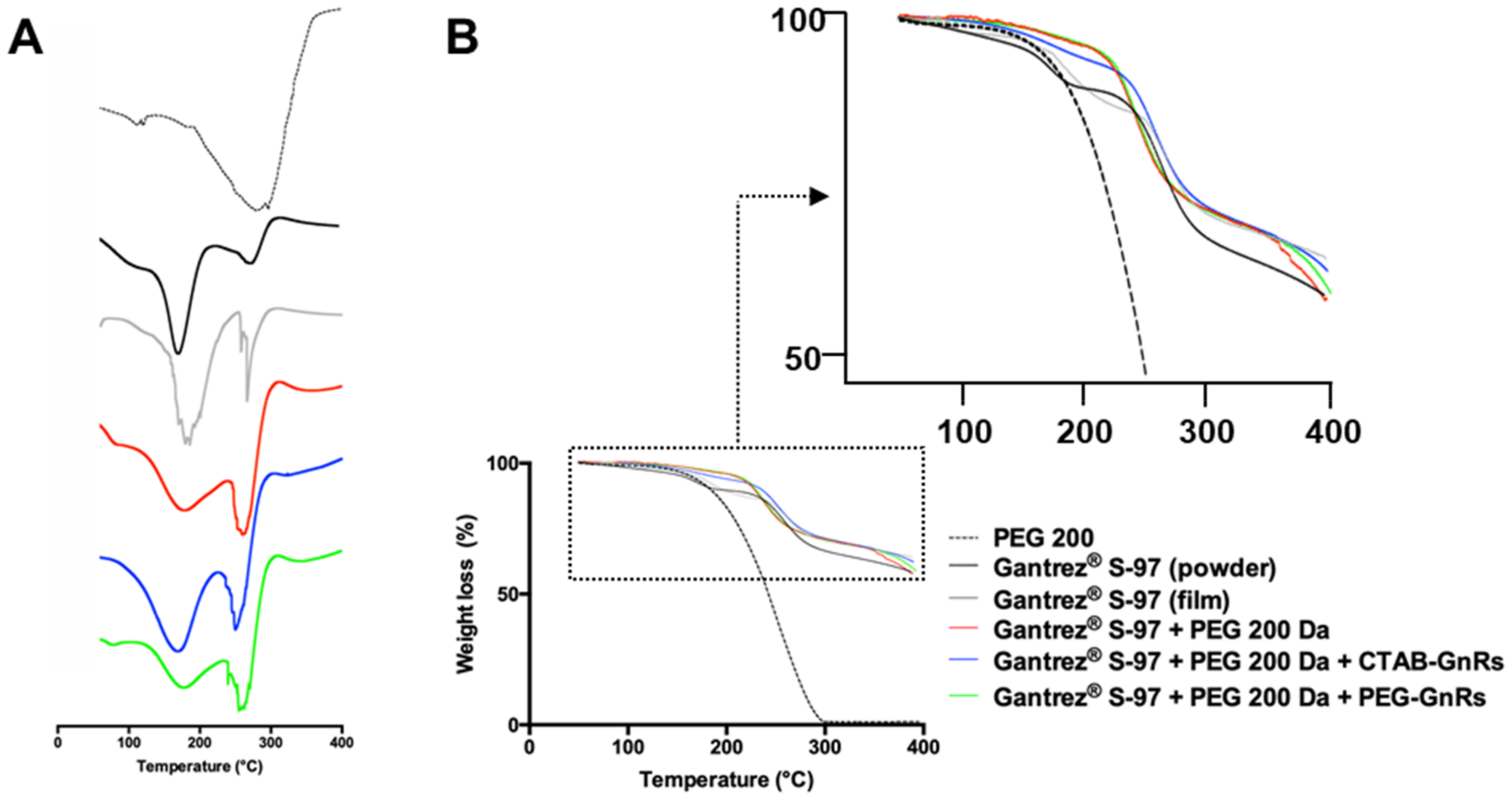
2.3.4. Thermal Analysis of Polymeric Films
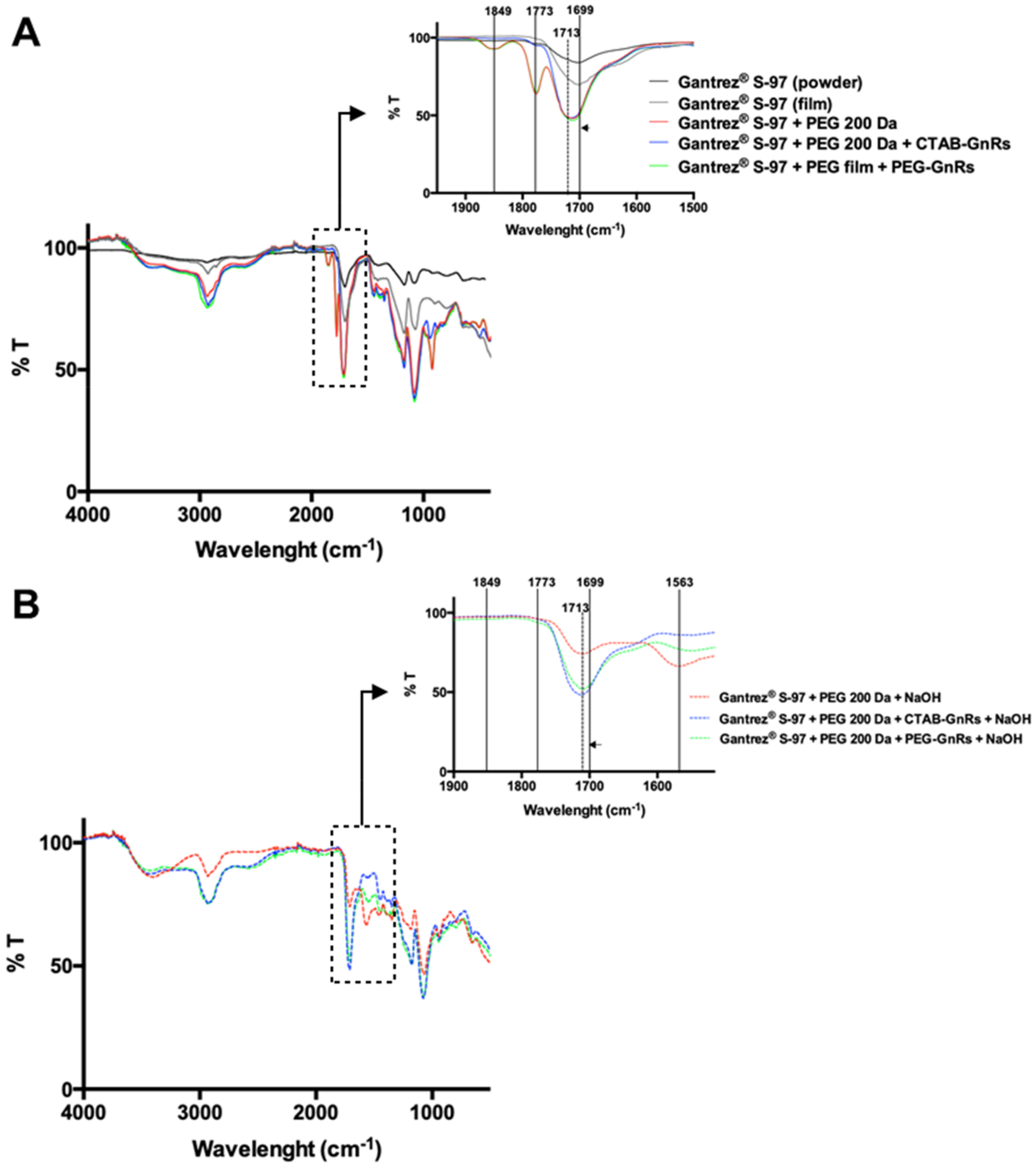
2.3.5. Fourier Transform Infrared Spectroscopy of Polymeric Films
2.3.6. GnRs Release from Polymeric Films by Immersion on PBS
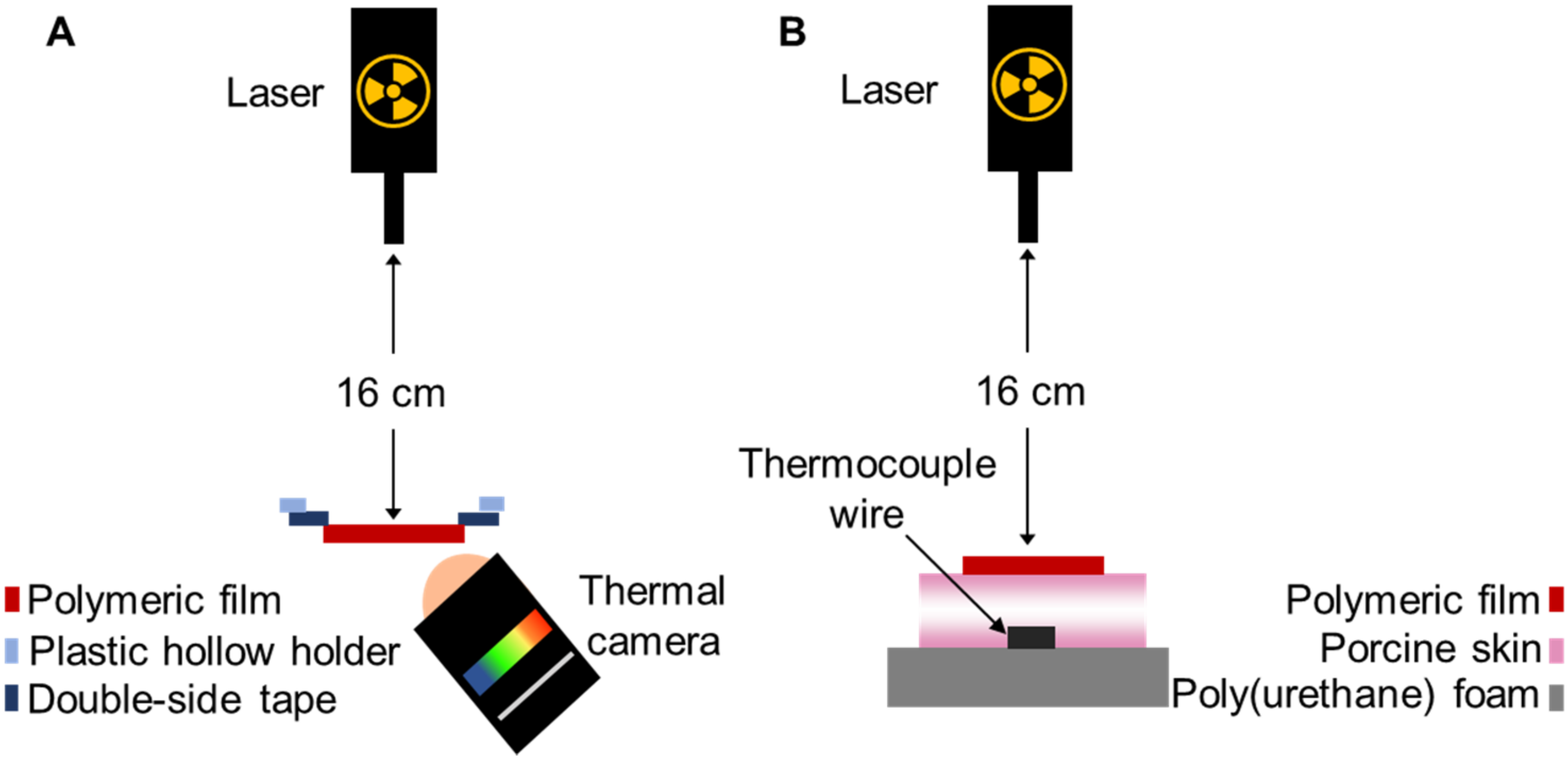
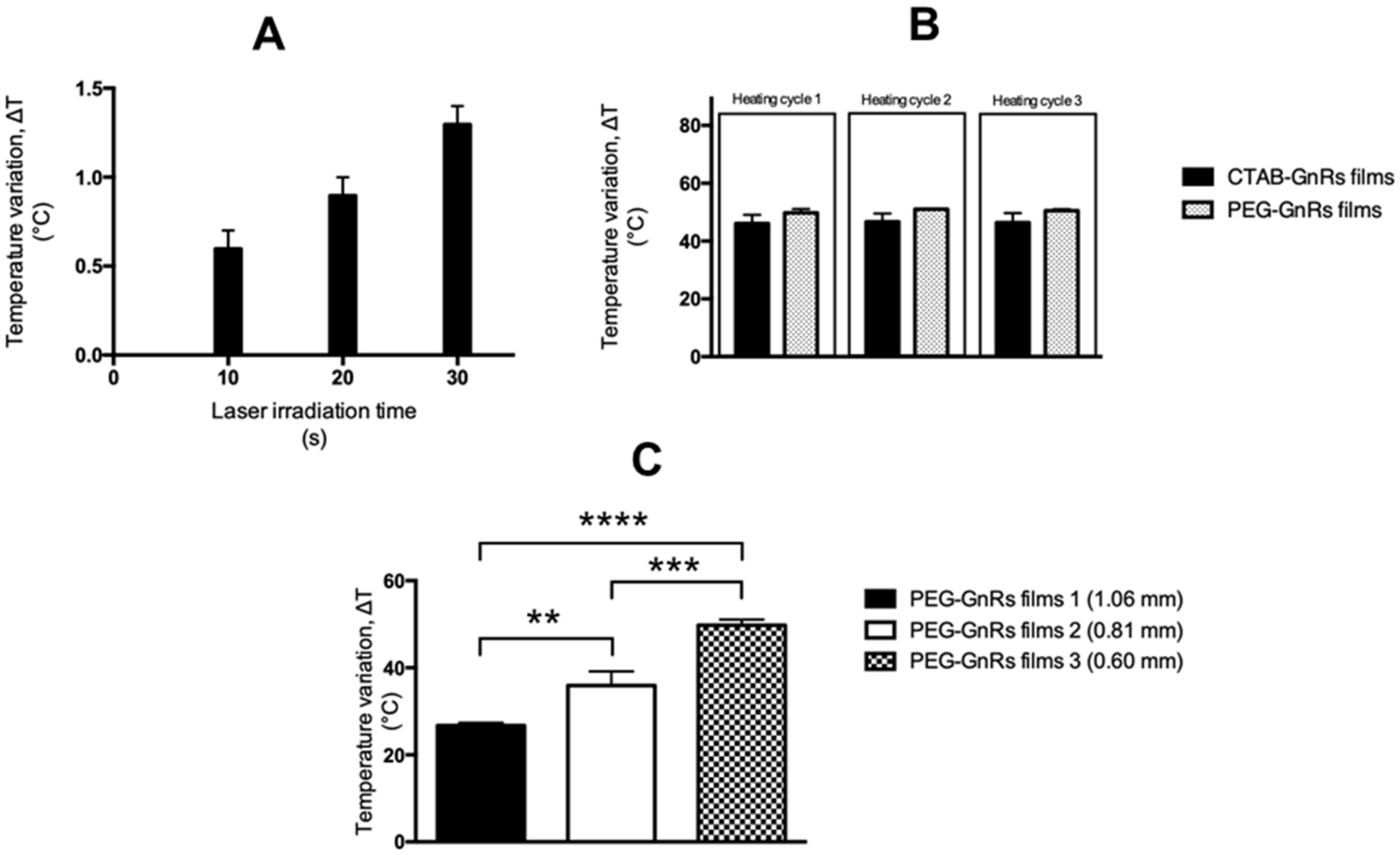
2.4. Thermal Studies of GnR-Loaded Polymeric Films
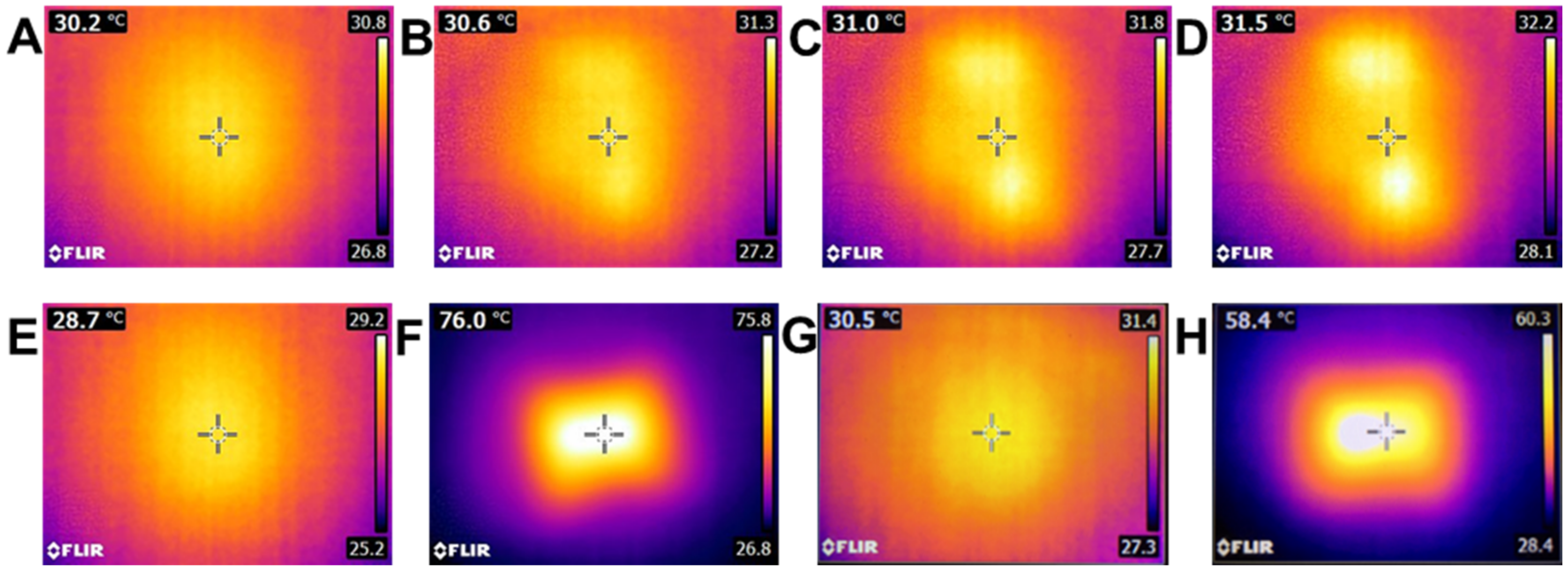
2.5. Heating Capacity of Polymeric Films on Neonate Porcine Skin
2.6. Statistical Analyses
3. Results and Discussion
3.1. Synthesis and Characterization of GnRs
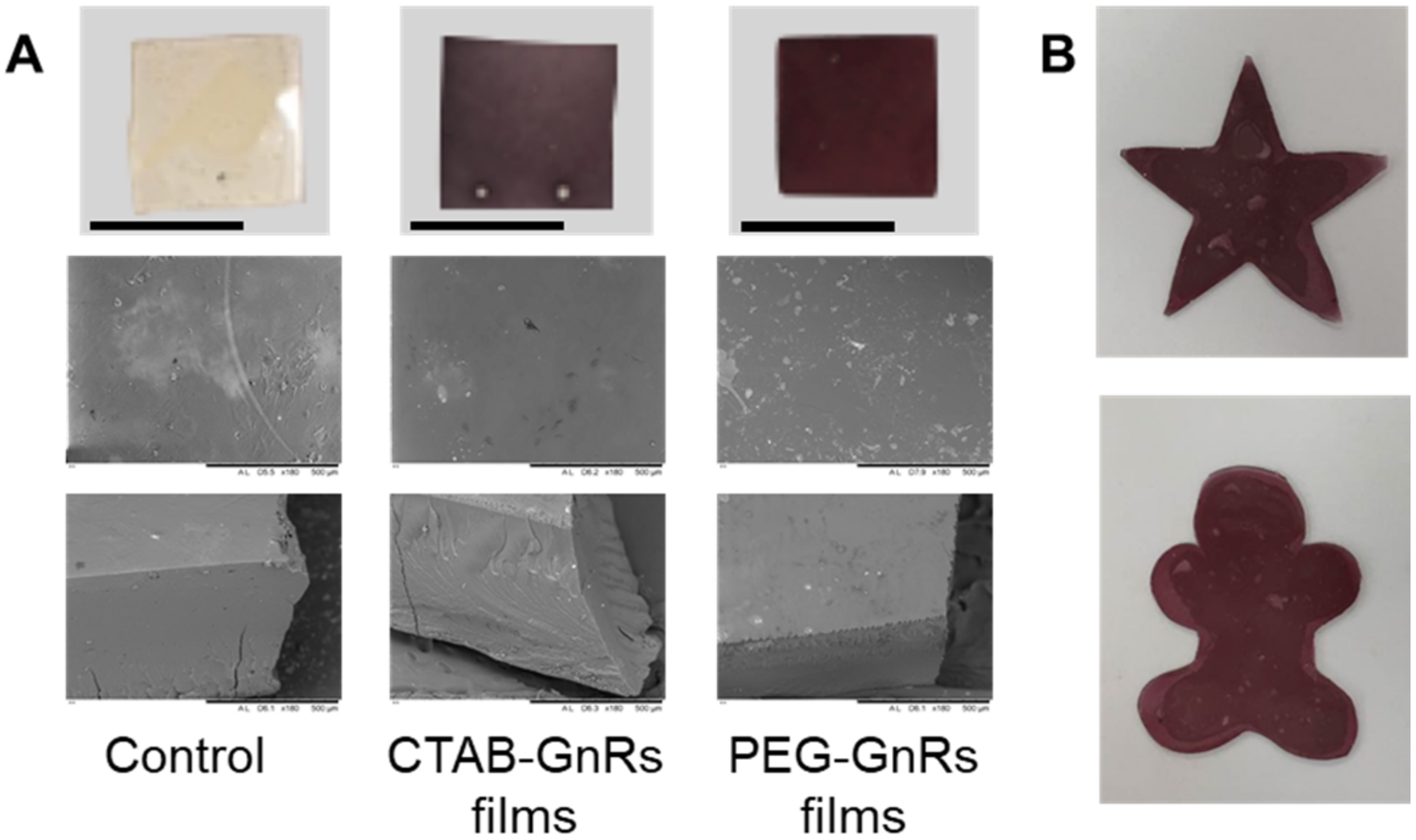
3.2. Preparation and Characterization of Polymeric Films Loaded with GnRs
3.3. Thermal Studies of GnR-Loaded Polymeric Films
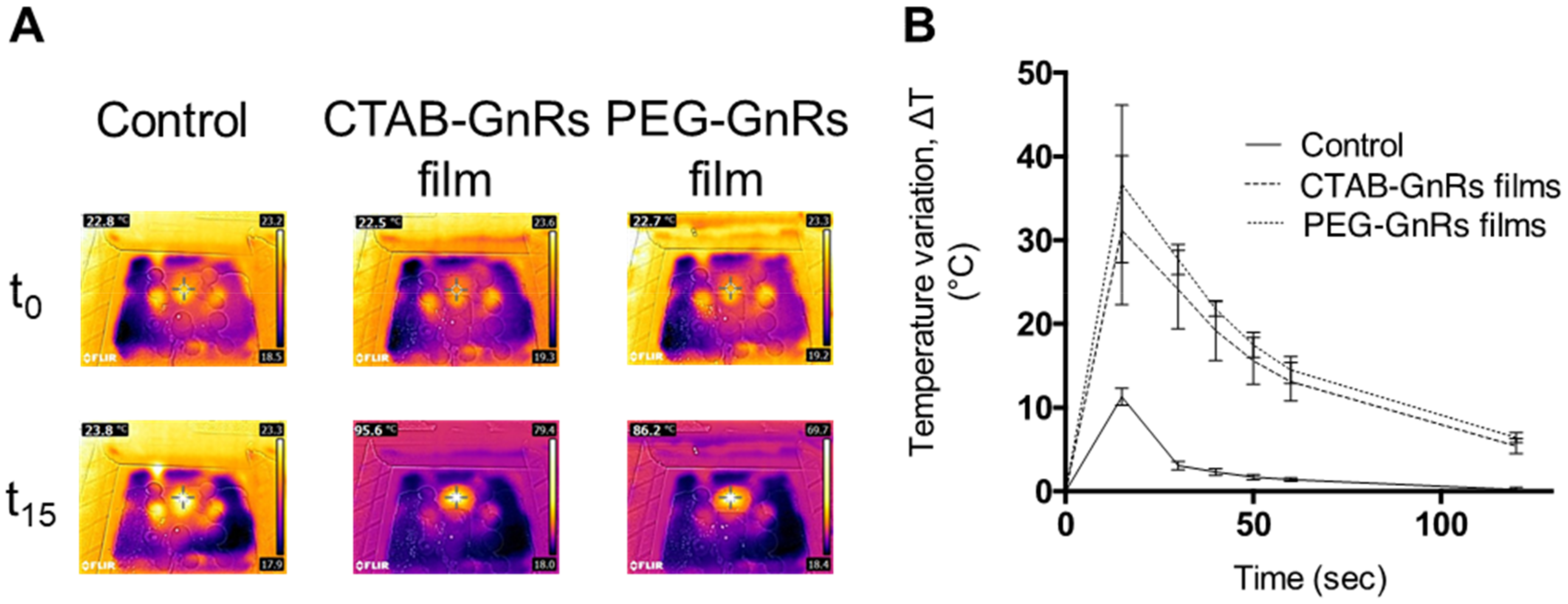
3.4. Heating Capacity of Polymeric Films on Neonate Porcine Skin
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Non-melanoma Skin Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/non-melanoma-skin-cancer. (accessed on 28 Novemeber 2019).
- Rudolph, C.; Schnoor, M.; Eisemann, N.; Katalinic, A. Incidence trends of nonmelanoma skin cancer in Germany from 1998 to 2010. JDDG J. Dtsch. Dermatol. Ges. 2015, 13, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Birch-Johansen, F.; Jensen, A.; Mortensen, L.; Olesen, A.B.; Kjaer, S.K. Trends in the incidence of nonmelanoma skin cancer in Denmark 1978–2007: Rapid incidence increase among young Danish women. Int. J. Cancer 2010, 127, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.A.; Coronado, A.C.; Sarker, S.; Alvi, R. Estimating health care costs of non-melanoma skin cancer in saskatchewan using physician billing data. Curr. Oncol. 2019, 26, 114. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Harris, A.R.; Hinckley, M.R.; Feldman, S.R.; Fleischer, A.B.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch. Dermatol. 2010, 146, 283–287. [Google Scholar] [CrossRef]
- Perera, E.; Gnaneswaran, N.; Staines, C.; Win, A.K.; Sinclair, R. Incidence and prevalence of non-melanoma skin cancer in Australia: A systematic review. Australas. J. Dermatol. 2015, 56, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Mofidi, A.; Tompa, E.; Spencer, J.; Kalcevich, C.; Peters, C.E.; Kim, J.; Song, C.; Mortazavi, S.B.; Demers, P.A. The economic burden of occupational non-melanoma skin cancer due to solar radiation. J. Occup. Environ. Hyg. 2018, 15, 481–491. [Google Scholar] [CrossRef]
- Pondicherry, A.; Martin, R.; Meredith, I.; Rolfe, J.; Emanuel, P.; Elwood, M. The burden of non-melanoma skin cancers in Auckland, New Zealand. Australas. J. Dermatol. 2018, 59, 210–213. [Google Scholar] [CrossRef]
- Cakir, B.Ö.; Adamson, P.; Cingi, C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast. Surg. Clin. N. Am. 2012, 20, 419–422. [Google Scholar] [CrossRef]
- Bahner, J.D.; Bordeaux, J.S. Non-melanoma skin cancers: Photodynamic therapy, cryotherapy, 5-fluorouracil, imiquimod, diclofenac, or what? Facts and controversies. Clin. Dermatol. 2013, 31, 792–798. [Google Scholar] [CrossRef]
- Newlands, C.; Currie, R.; Memon, A.; Whitaker, S.; Woolford, T. Non-melanoma skin cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S125–S132. [Google Scholar] [CrossRef]
- Fahradyan, A.; Howell, A.; Wolfswinkel, E.; Tsuha, M.; Sheth, P.; Wong, A. Updates on the management of non-melanoma skin cancer (NMSC). Healthcare 2017, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Micali, G.; Lacarrubba, F.; Bhatt, K.; Nasca, M.R. Medical approaches to non-melanoma skin cancer. Expert Rev. Anticancer Ther. 2013, 13, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Moy, A.J.; Tunnell, J.W. Combinatorial immunotherapy and nanoparticle mediated hyperthermia. Adv. Drug Deliv. Rev. 2017, 114, 175–183. [Google Scholar] [CrossRef]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Rahman, M.A.; Wu, Y.; Han, T.; Peng, X.; Mackey, M.A.; Wang, D.; Shin, H.J.; Chen, Z.G.; Xiao, H.; et al. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3110–E3118. [Google Scholar] [CrossRef] [PubMed]
- Pattani, V.P.; Shah, J.; Atalis, A.; Sharma, A.; Tunnell, J.W. Role of apoptosis and necrosis in cell death induced by nanoparticle-mediated photothermal therapy. J. Nanopart. Res. 2015, 17, 20. [Google Scholar] [CrossRef]
- Seo, B.; Lim, K.; Kim, S.S.; Oh, K.T.; Lee, E.S.; Choi, H.-G.; Shin, B.S.; Youn, Y.S. Small gold nanorods-loaded hybrid albumin nanoparticles with high photothermal efficacy for tumor ablation. Colloids Surf. B Biointerfaces 2019, 179, 340–351. [Google Scholar] [CrossRef]
- Tiwari, P.; Vig, K.; Dennis, V.; Singh, S. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Liao, H.; Hafner, J.H. Gold nanorod bioconjugates. Chem. Mater. 2005, 17, 4636–4641. [Google Scholar] [CrossRef]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Maurer, E.I.; Park, K.; MacCuspie, R.I.; Afrooz, A.R.M.N.; Vaia, R.A.; Saleh, N.B.; Hussain, S.M. Does shape matter? Bioeffects of gold nanomaterials in a human skin cell model. Langmuir 2012, 28, 3248–3258. [Google Scholar] [CrossRef]
- Ye, X.; Zheng, C.; Chen, J.; Gao, Y.; Murray, C.B. Using binary surfactant mixtures to simultaneously improve the dimensional tunability and monodispersity in the seeded growth of gold nanorods. Nano Lett. 2013, 13, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wang, J.-H.; Liu, T.; Xie, Z.; Yu, X.-F.; Li, W. Surface chemistry but not aspect ratio mediates the biological toxicity of gold nanorods in vitro and in vivo. Sci. Rep. 2015, 5, 11398. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, I.; Issa, B.; Haik, Y. Magnetic properties of magnetic nanoparticles for efficient hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Nunes, Á.M.; da Silva, K.R.M.; Calado, C.M.S.; Saraiva, K.L.A.; Figueiredo, R.C.B.; Leite, A.C.R.; Meneghetti, M.R. Evaluation of gold nanorods toxicity on isolated mitochondria. Toxicology 2019, 413, 24–32. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Shatanawi, A.; Kurtz, T.; Caldwell, R.B.; Caldwell, R.W. Toxicity and cellular uptake of gold nanorods in vascular endothelium and smooth muscles of isolated rat blood vessel: Importance of surface modification. Small 2012, 8, 1270–1278. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Thompson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef]
- Jin, N.; Zhang, Q.; Yang, M.; Yang, M. Detoxification and functionalization of gold nanorods with organic polymers and their applications in cancer photothermal therapy. Microsc. Res. Tech. 2019, 82, 670–679. [Google Scholar] [CrossRef]
- Borgheti-Cardoso, L.N.; Viegas, J.S.R.; Silvestrini, A.V.P.; Caron, A.L.; Praça, F.G.; Kravicz, M.; Bentley, M.V.L.B. Nanotechnology approaches in the current therapy of skin cancer. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, M. Fast loading of PEG–SH on CTAB-protected gold nanorods. RSC Adv. 2014, 4, 17760. [Google Scholar] [CrossRef]
- González-Vázquez, P.; Larrañeta, E.; McCrudden, M.T.C.; Jarrahian, C.; Rein-Weston, A.; Quintanar-Solares, M.; Zehrung, D.; McCarthy, H.; Courtenay, A.J.; Donnelly, R.F. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J. Control. Release 2017, 265, 30–40. [Google Scholar] [CrossRef]
- Leng, Y.; Yin, X.; Hu, F.; Zou, Y.; Xing, X.; Li, B.; Guo, Y.; Ye, L.; Lu, Z. High-yield synthesis and fine-tuning aspect ratio of (200) faceted gold nanorods by the pH-adjusting method. RSC Adv. 2017, 7, 25469–25474. [Google Scholar] [CrossRef]
- Wawra, S.E.; Pflug, L.; Thajudeen, T.; Kryschi, C.; Stingl, M.; Peukert, W. Determination of the two-dimensional distributions of gold nanorods by multiwavelength analytical ultracentrifugation. Nat. Commun. 2018, 9, 4898. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold nanorods: From synthesis and properties to biological and biomedical applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef] [PubMed]
- Orendorff, C.J.; Murphy, C.J. Quantitation of metal content in the silver-assisted growth of gold nanorods. J. Phys. Chem. B 2006, 110, 3990–3994. [Google Scholar] [CrossRef] [PubMed]
- Canonico-May, S.A.; Beavers, K.R.; Melvin, M.J.; Alkilany, A.M.; Duvall, C.L.; Stone, J.W. High conversion of HAuCl 4 into gold nanorods: A re-seeding approach. J. Colloid Interface Sci. 2016, 463, 229–232. [Google Scholar] [CrossRef]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef]
- Chung, K.H.; Wu, C.S.; Malawer, E.G. Glass transition temperatures of poly (methyl vinyl ether-co-maleic anhydride) (PMVEMA) and poly (methyl vinyl ether-co-maleic acid) (PMVEMAC) and the kinetics of dehydration of PMVEMAC by thermal analysis. J. Appl. Polym. Sci. 1990, 41, 793–803. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Peresin, M.S.; Tamminen, T.; Rodríguez, A.; Larrañeta, E.; Jääskeläinen, A.-S. Lignin-based hydrogels with “super-swelling” capacities for dye removal. Int. J. Biol. Macromol. 2018, 115, 1249–1259. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.E.M.; Brady, A.J.; Vicente-Pérez, E.M.; Woolfson, A.D.; Thakur, R.R.S.; Donnelly, R.F. Microwave-assisted preparation of hydrogel-forming microneedle arrays for transdermal drug delivery applications. Macromol. Mater. Eng. 2015, 300, 586–595. [Google Scholar] [CrossRef]
- Lu, C.-H.; Chen, W.-T.; Hsieh, C.-H.; Kuo, Y.-Y.; Chao, C.-Y. Thermal cycling-hyperthermia in combination with polyphenols, epigallocatechin gallate and chlorogenic acid, exerts synergistic anticancer effect against human pancreatic cancer PANC-1 cells. PLoS ONE 2019, 14, e0217676. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sun, Y.; Lu, C.; Chao, C. Thermal cycling as a novel thermal therapy to synergistically enhance the anticancer effect of propolis on PANC-1 cells. Int. J. Oncol. 2019, 55, 617–628. [Google Scholar] [CrossRef] [PubMed]

| Sample | Length (nm) | Width (nm) | Aspect Ratio |
|---|---|---|---|
| CATB-GnRs | 53.91 ± 7.45 | 17.70 ± 2.69 | 3.11 ± 0.73 |
| PEG-GnRs | 53.00 ± 7.50 | 15.02 ± 2.32 | 3.60 ± 0.78 |
| Sample | Thickness (mm) | Area (cm2) | Weight (g) |
|---|---|---|---|
| CTAB-GnRs | 0.61 ± 0.12 | 1.40 ± 0.18 | 0.10 ± 0.03 |
| PEG-GnRs | 0.60 ± 0.08 | 1.80 ± 0.17 | 0.12 ± 0.01 |
| Control | 0.64 ± 0.05 | 1.26 ± 0.08 | 0.11 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárcamo-Martínez, Á.; Domínguez-Robles, J.; Mallon, B.; Raman, M.T.; Cordeiro, A.S.; Bell, S.E.J.; Larrañeta, E.; Donnelly, R.F. Potential of Polymeric Films Loaded with Gold Nanorods for Local Hyperthermia Applications. Nanomaterials 2020, 10, 582. https://doi.org/10.3390/nano10030582
Cárcamo-Martínez Á, Domínguez-Robles J, Mallon B, Raman MT, Cordeiro AS, Bell SEJ, Larrañeta E, Donnelly RF. Potential of Polymeric Films Loaded with Gold Nanorods for Local Hyperthermia Applications. Nanomaterials. 2020; 10(3):582. https://doi.org/10.3390/nano10030582
Chicago/Turabian StyleCárcamo-Martínez, Álvaro, Juan Domínguez-Robles, Brónach Mallon, Md. Taifur Raman, Ana Sara Cordeiro, Steven E. J. Bell, Eneko Larrañeta, and Ryan F. Donnelly. 2020. "Potential of Polymeric Films Loaded with Gold Nanorods for Local Hyperthermia Applications" Nanomaterials 10, no. 3: 582. https://doi.org/10.3390/nano10030582
APA StyleCárcamo-Martínez, Á., Domínguez-Robles, J., Mallon, B., Raman, M. T., Cordeiro, A. S., Bell, S. E. J., Larrañeta, E., & Donnelly, R. F. (2020). Potential of Polymeric Films Loaded with Gold Nanorods for Local Hyperthermia Applications. Nanomaterials, 10(3), 582. https://doi.org/10.3390/nano10030582