High Density Display of an Anti-Angiogenic Peptide on Micelle Surfaces Enhances Their Inhibition of αvβ3 Integrin-Mediated Neovascularization In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PEG-b-PPS Copolymer and Self-Assembly of Micelles
2.3. Synthesis of aANGP Peptide Constructs
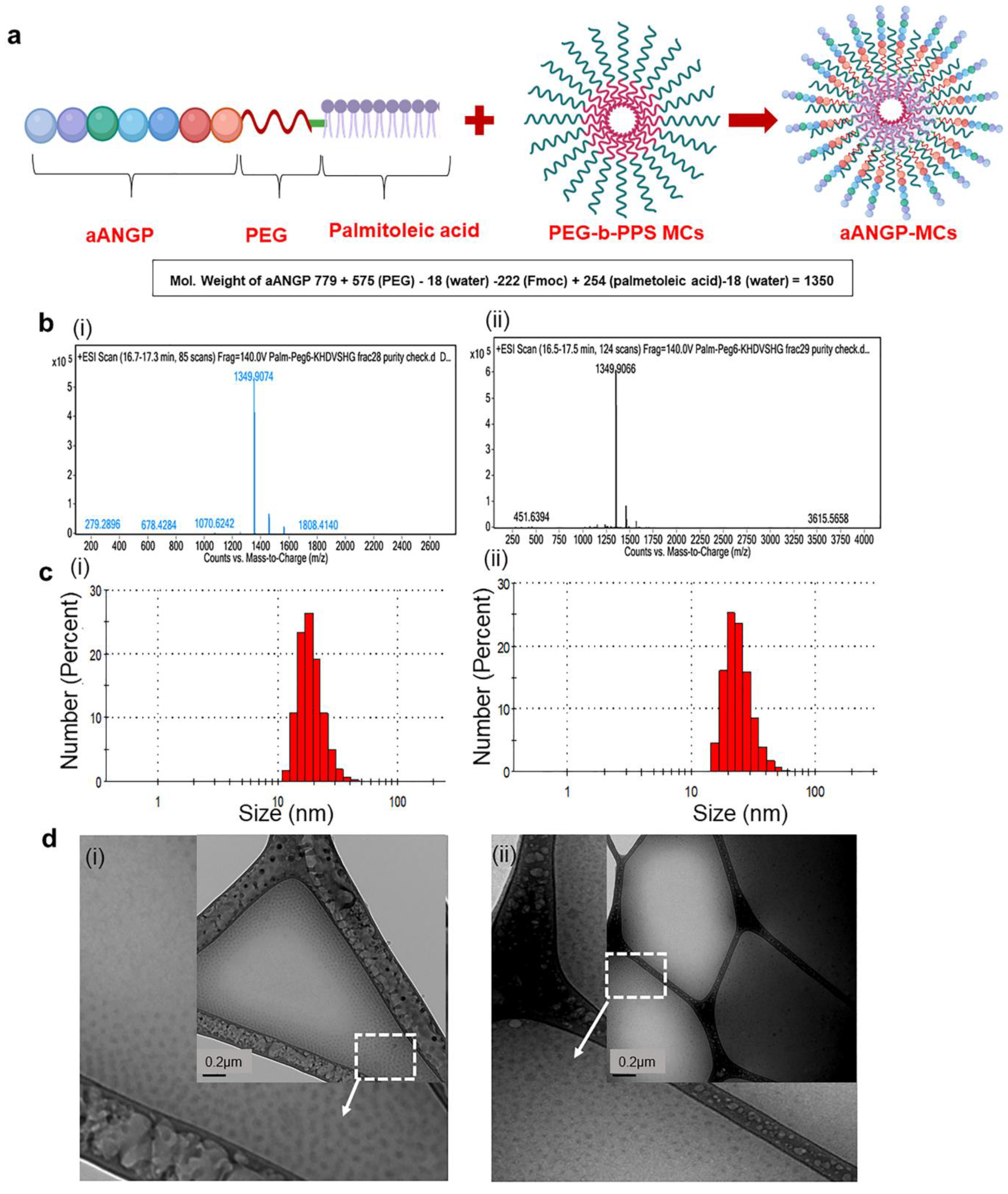
2.4. Formulation of aANGP-MC
2.5. Characterization of Developed Blank and aANGP-MCs
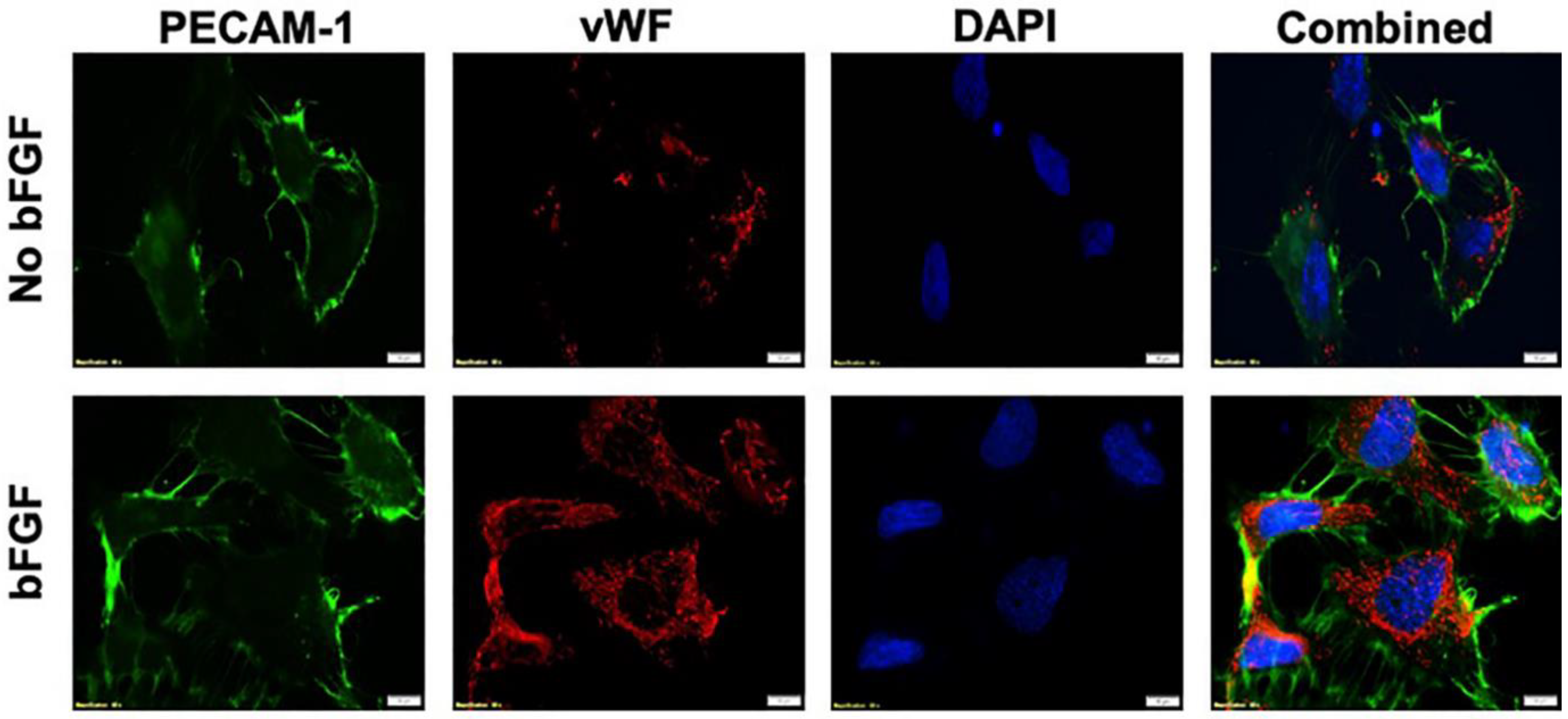
2.6. Cell Culture
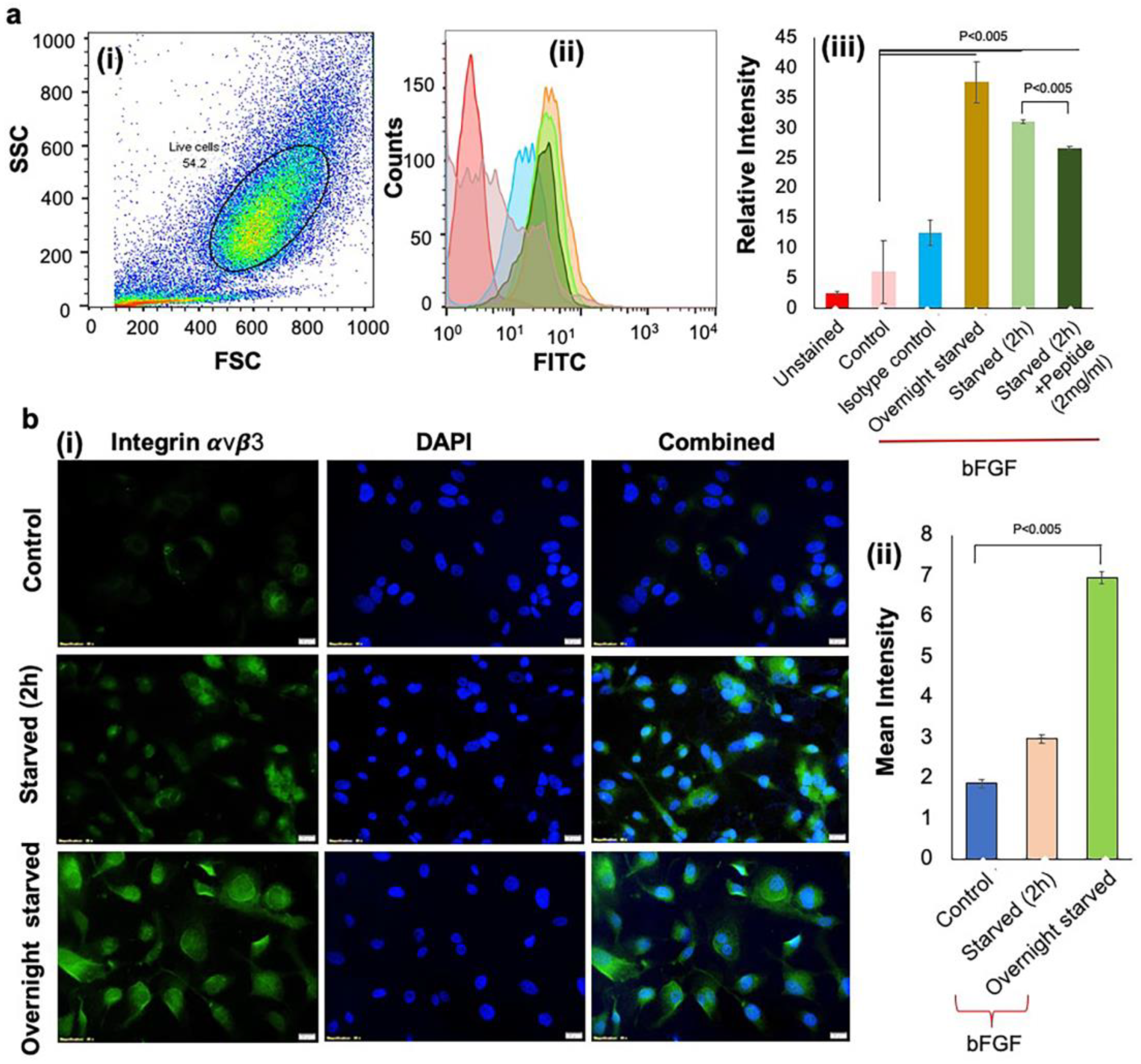
2.7. Evaluation of Integrin αvβ3 Expression
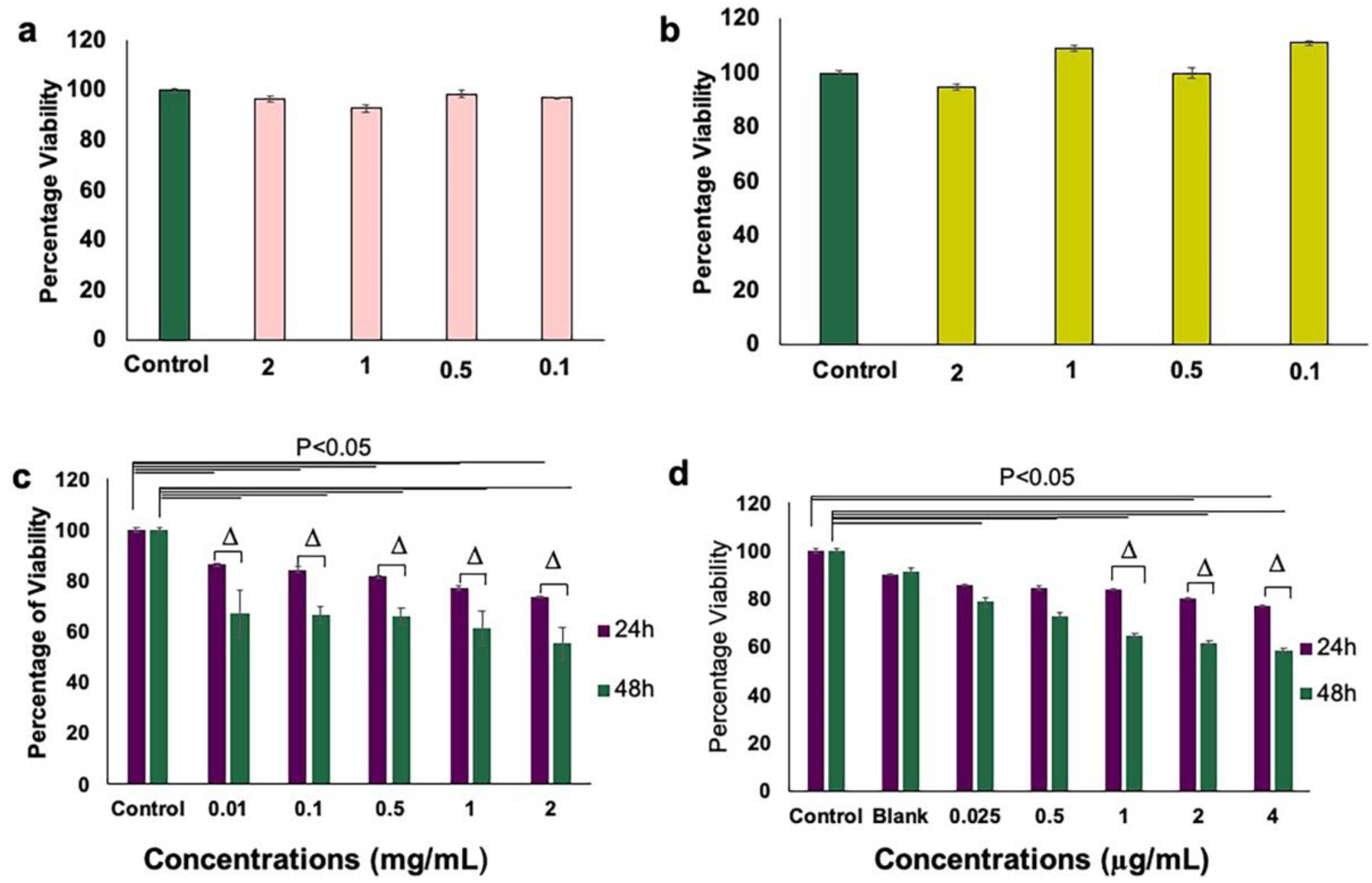
2.8. Evaluation of Endothelial Cell Growth Inhibition
2.9. Statistics
3. Results
3.1. Characterization of aANGP Micelles
3.2. Activation of Integrin αvβ3 Expressions to Mimic the Hypoxic Condition
3.3. Inhibition of Endothelial Cell Proliferation and Growth by aANGP MCs
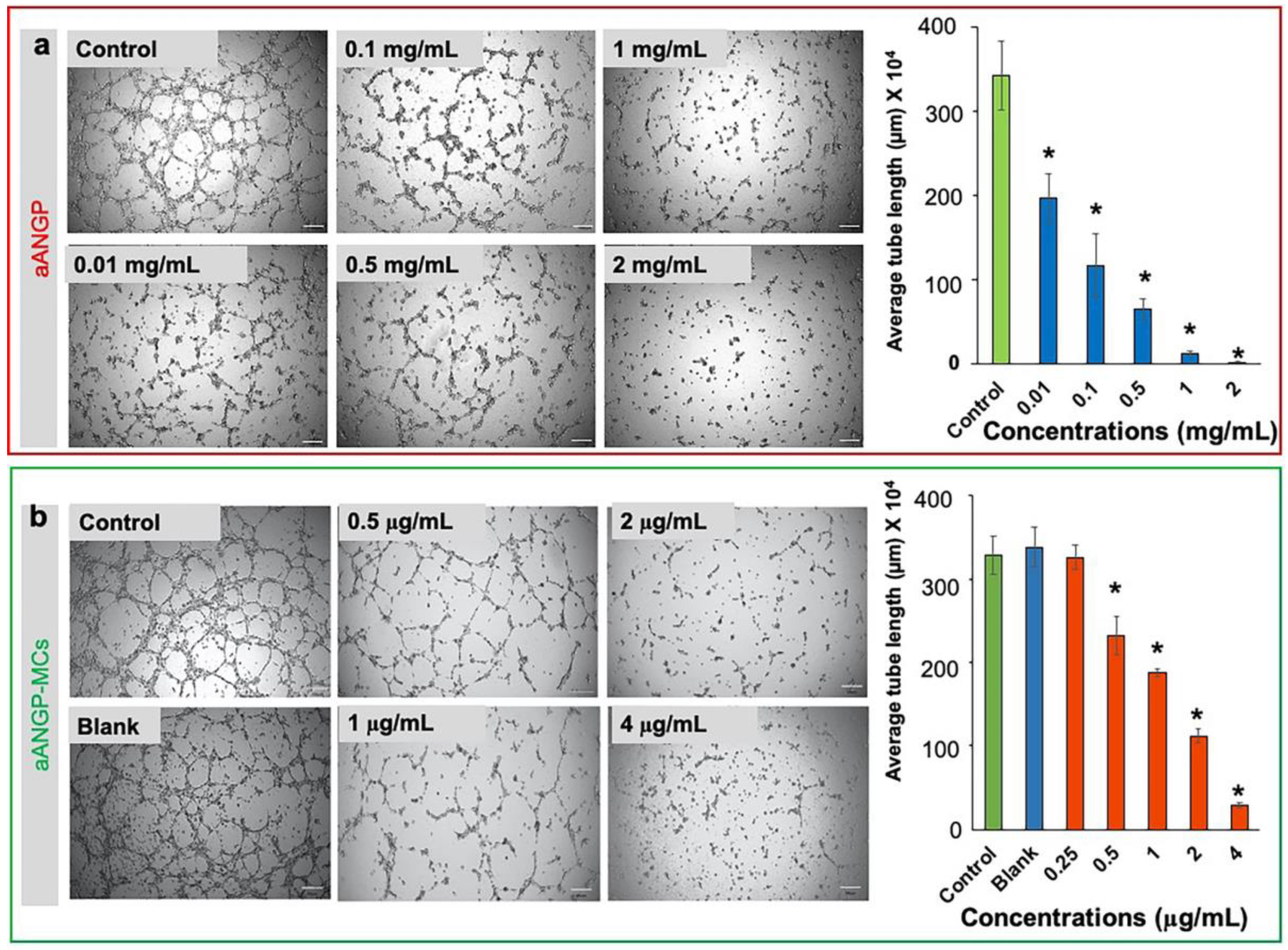
3.4. Inhibition of Endothelial Cell Migration and Tube Formation
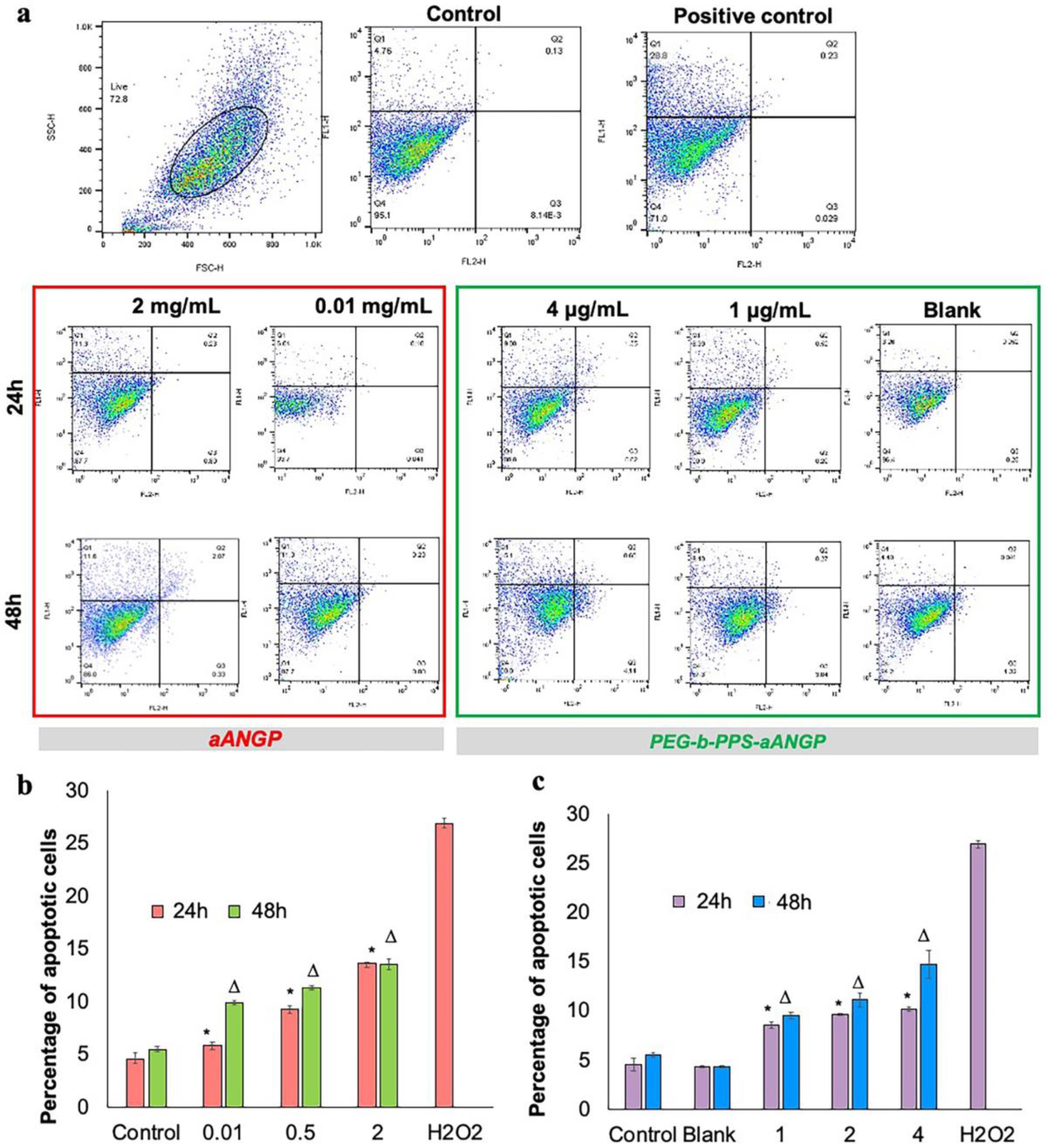
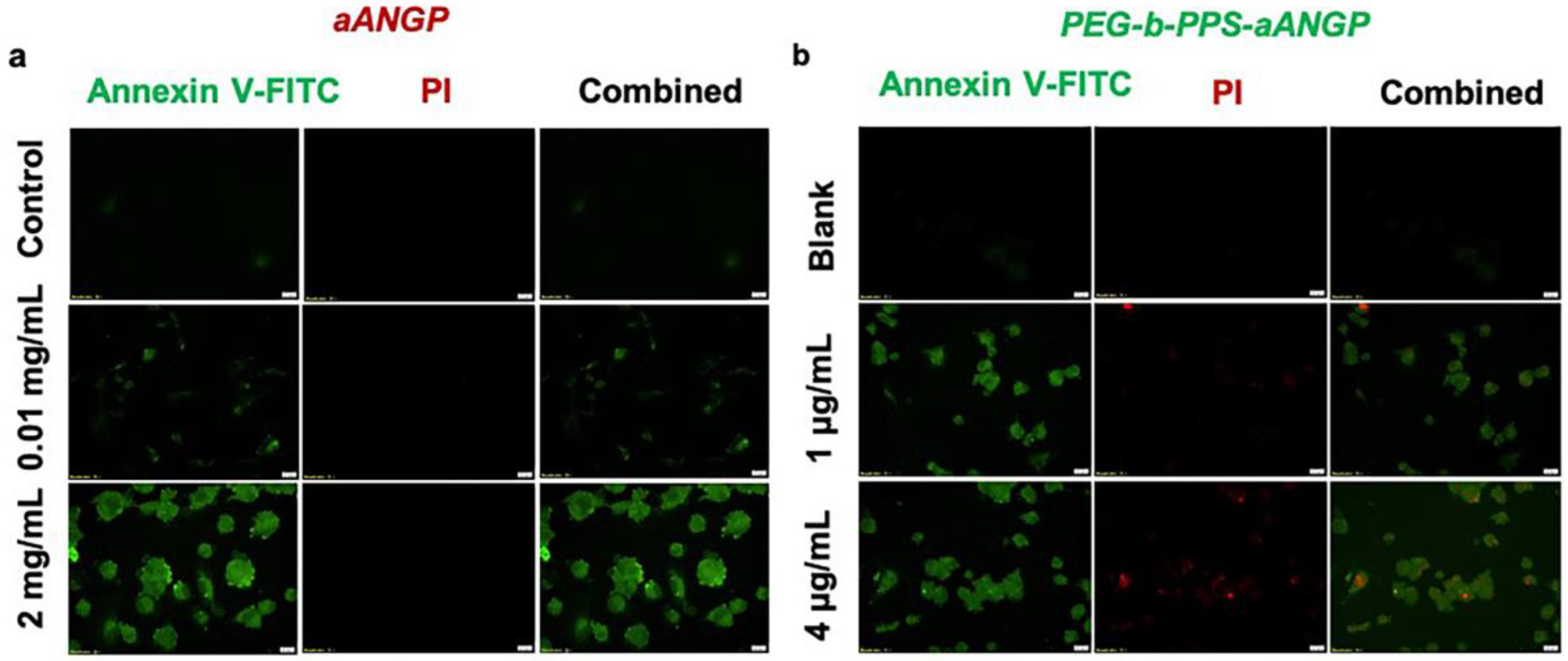
3.5. Evaluation of the Mechanism of Cell Death by Apoptosis Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tarr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of Diabetic Retinopathy. ISRN Ophthalmol. 2013, 2013, 343560. [Google Scholar] [CrossRef] [PubMed]
- Retinopathy of Prematurity|National Eye Institute. Available online: https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/retinopathy-prematurity (accessed on 20 December 2019).
- Nowak, J.Z. Age-related macular degeneration (AMD): Pathogenesis and therapy. Pharmacol. Rep. PR 2006, 58, 353–363. [Google Scholar] [PubMed]
- Brey, E.M.; McIntire, L.V. 59—Vascular Assembly in Engineered and Natural Tissues. In Principles of Regenerative Medicine; Atala, A., Lanza, R., Thomson, J.A., Nerem, R.M., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 1020–1037. ISBN 978-0-12-369410-2. [Google Scholar]
- Hennig, R.; Goepferich, A. Nanoparticles for the treatment of ocular neovascularizations. Eur. J. Pharm. Biopharm. 2015, 95, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, S.; Fukushima, Y.; Fukuhara, S.; Jakt, L.M.; Okada, M.; Shimizu, Y.; Hata, M.; Nishida, K.; Negi, A.; Hirashima, M.; et al. Arhgef15 promotes retinal angiogenesis by mediating VEGF-induced Cdc42 activation and potentiating RhoJ inactivation in endothelial cells. PLoS ONE 2012, 7, e45858. [Google Scholar] [CrossRef] [PubMed]
- Rattner, A.; Williams, J.; Nathans, J. Roles of HIFs and VEGF in angiogenesis in the retina and brain. J. Clin. Investig. 2019, 129, 3807–3820. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, H.; Robinson, R.; Sharma, A.; Sharma, S. Proteomic Biomarkers of Retinal Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2019, 20, 4755. [Google Scholar] [CrossRef]
- Li, Y.; Busoy, J.M.; Zaman, B.A.A.; Tan, Q.S.W.; Tan, G.S.W.; Barathi, V.A.; Cheung, N.; Wei, J.J.-Y.; Hunziker, W.; Hong, W.; et al. A novel model of persistent retinal neovascularization for the development of sustained anti-VEGF therapies. Exp. Eye Res. 2018, 174, 98–106. [Google Scholar] [CrossRef]
- Sun, M.; Wadehra, M.; Casero, D.; Lin, M.-C.; Aguirre, B.; Parikh, S.; Matynia, A.; Gordon, L.; Chu, A. Epithelial Membrane Protein 2 (EMP2) Promotes VEGF-Induced Pathological Neovascularization in Murine Oxygen-Induced Retinopathy. Invest. Ophthalmol. Vis. Sci. 2020, 61, 3. [Google Scholar] [CrossRef]
- Kamenskih, T.G. A differentiated approach to analysis of retinal fluid and assessment of its effect on anti-VEGF therapy of neovascular age-related macular degeneration. Vestn. Oftalmol. 2019, 135, 134–140. [Google Scholar] [CrossRef]
- Hachana, S.; Fontaine, O.; Sapieha, P.; Lesk, M.; Couture, R.; Vaucher, E. The effects of anti—VEGF and kinin B1 receptor blockade on retinal inflammation in laser—Induced choroidal neovascularization. Br. J. Pharmacol. 2020, in press. [Google Scholar] [CrossRef]
- Stepanov, A.; Středová, M.; Dusová, J.; Jirásková, N.; Studnička, J. Ranibizumab for the treatment of choroidal neovascularization due to cause other than age related macular degeneration. Ceska Slov. Oftalmol. Cas. Ceske Oftalmol. Spolecnosti Slov. Oftalmol. Spolecnosti 2019, 75, 138–144. [Google Scholar]
- Hanna, R.M.; Abdelnour, L.; Hasnain, H.; Selamet, U.; Kurtz, I. Intravitreal bevacizumab-induced exacerbation of proteinuria in diabetic nephropathy, and amelioration by switching to ranibizumab. SAGE Open Med. Case Rep. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castellanos, M.A.; González-H León, A.; Romo-Aguas, J.C.; Gonzalez-Gonzalez, L.A. A proposal of an algorithm for the diagnosis and treatment of recurrence or treatment failure of retinopathy of prematurity after anti-VEGF therapy based on a large case series. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2020, 258, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.W. Treatment of diabetic retinopathy: Recent advances and unresolved challenges. World J. Diabetes 2016, 7, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Wataru, K.; Sugiyama, A.; Yoneyama, S.; Matsubara, M.; Fukuda, Y.; Parikh, R.; Sakurada, Y. Five-year outcomes of photodynamic therapy combined with intravitreal injection of ranibizumab or aflibercept for polypoidal choroidal vasculopathy. PLoS ONE 2020, 15, e0229231. [Google Scholar] [CrossRef]
- Călugăru, D.; Călugăru, M. Intravitreal aflibercept for macular edema secondary to central retinal vein occlusion: 18-month results of the phase 3 GALILEO study. Am. J. Ophthalmol. 2015, 159, 607–608. [Google Scholar] [CrossRef]
- Qureshi, N.A.; Mansoor, H.; Ahmad, S.; Zafar, S.; Asif, M. Reducing intraocular-pressure spike after intravitreal-bevacizumab injection with ocular decompression using a sterile cotton swab soaked in proparacaine 0.5%: A quasi-experimental study. Taiwan J. Ophthalmol. 2016, 6, 75–78. [Google Scholar] [CrossRef]
- Ghasemi Falavarjani, K.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef]
- Sampat, K.M.; Garg, S.J. Complications of intravitreal injections. Curr. Opin. Ophthalmol. 2010, 21, 178–183. [Google Scholar] [CrossRef]
- Haigh, J.J. Role of VEGF in organogenesis. Organogenesis 2008, 4, 247–256. [Google Scholar] [CrossRef]
- David Salz, P.J.P.; New Brunswick, N.J.; Cunningham, E.T., Jr. Local Complications of IV Anti-VEGF Therapy. Available online: https://www.reviewofophthalmology.com/article/local-complications-of-iv-anti-vegf-therapy (accessed on 12 January 2020).
- Shikari, H.; Silva, P.S.; Sun, J.K. Complications of intravitreal injections in patients with diabetes. Semin. Ophthalmol. 2014, 29, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Park, S.W.; Cho, C.S.; Powner, M.B.; Kim, J.H.; Fruttiger, M.; Kim, J.H. Intravitreally Injected Anti-VEGF Antibody Reduces Brown Fat in Neonatal Mice. PLoS ONE 2015, 10, e0134308. [Google Scholar] [CrossRef] [PubMed]
- The Role of Anti-vascular Endothelial Growth Factor Agents in the Management of Retinopathy of Prematurity. Available online: https://www.touchophthalmology.com/2017/02/06/the-role-of-anti-vascular-endothelial-growth-factor-agents-in-the-management-of-retinopathy-of-prematurity/ (accessed on 15 January 2020).
- Huang, Q.; Zhao, P. Anti-vascular endothelial growth factor treatment for retinopathy of prematurity. Ann. Eye Sci. 2017, 2, 47. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. αv Integrins in Angiogenesis and Cancer. Cold Spring Harb. Perspect. Med. 2011, 1, a006478. [Google Scholar] [CrossRef] [PubMed]
- Rehn, M.; Veikkola, T.; Kukk-Valdre, E.; Nakamura, H.; Ilmonen, M.; Lombardo, C.R.; Pihlajaniemi, T.; Alitalo, K.; Vuori, K. Interaction of endostatin with integrins implicated in angiogenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Carman, C.V.; Kim, M.; Salas, A.; Shimaoka, M.; Springer, T.A. A small molecule agonist of an integrin, alphaLbeta2. J. Biol. Chem. 2006, 281, 37904–37912. [Google Scholar] [CrossRef]
- Brooks, P.C.; Clark, R.A.; Cheresh, D.A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994, 264, 569–571. [Google Scholar] [CrossRef]
- Friedlander, M.; Theesfeld, C.L.; Sugita, M.; Fruttiger, M.; Thomas, M.A.; Chang, S.; Cheresh, D.A. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc. Natl. Acad. Sci. USA 1996, 93, 9764–9769. [Google Scholar] [CrossRef]
- Pasqualini, R.; Koivunen, E.; Ruoslahti, E. Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat. Biotechnol. 1997, 15, 542–546. [Google Scholar] [CrossRef]
- Shlamkovich, T.; Aharon, L.; Koslawsky, D.; Einav, Y.; Papo, N. Targeting the Tie2–αvβ3 integrin axis with bi-specific reagents for the inhibition of angiogenesis. BMC Biol. 2018, 16, 92. [Google Scholar] [CrossRef]
- Abdollahi, A.; Griggs, D.W.; Zieher, H.; Roth, A.; Lipson, K.E.; Saffrich, R.; Gröne, H.-J.; Hallahan, D.E.; Reisfeld, R.A.; Debus, J.; et al. Inhibition of αvβ3 Integrin Survival Signaling Enhances Antiangiogenic and Antitumor Effects of Radiotherapy. Clin. Cancer Res. 2005, 11, 6270–6279. [Google Scholar] [CrossRef] [PubMed]
- Gomez Perdiguero, E.; Liabotis-Fontugne, A.; Durand, M.; Faye, C.; Ricard-Blum, S.; Simonutti, M.; Augustin, S.; Robb, B.M.; Paques, M.; Valenzuela, D.M.; et al. ANGPTL4-αvβ3 interaction counteracts hypoxia-induced vascular permeability by modulating Src signalling downstream of vascular endothelial growth factor receptor 2. J. Pathol. 2016, 240, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Li, X.-H.; Wang, L.-F.; Kuang, X.; Hang, Z.-X.; Deng, Y.; Du, J.-R. Therapeutic efficacy of a novel non-peptide αvβ3 integrin antagonist for pathological retinal angiogenesis in mice. Exp. Eye Res. 2014, 129, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Gong, J.; Xu, Z.; Wei, Y.; Duh, E.J. Inhibition of pathological retinal angiogenesis by the integrin αvβ3 antagonist tetraiodothyroacetic acid (tetrac). Exp. Eye Res. 2012, 94, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Suo, X.; Chu, Y.; Xu, Z.; Bao, Y.; Miao, C.; Deng, W.; Mao, K.; Gao, J.; Xu, Z.; et al. Peptides derived from the integrin β cytoplasmic tails inhibit angiogenesis. Cell Commun. Signal. CCS 2018, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Basilicata, M.F.; De Simone, M.; Del Giudice, C.; Anastasio, A.; Sorriento, D.; Saviano, M.; Del Gatto, A.; Trimarco, B.; Pedone, C.; et al. Evaluation of the anti-angiogenic properties of the new selective αVβ3 integrin antagonist RGDechiHCit. J. Transl. Med. 2011, 9, 7. [Google Scholar] [CrossRef]
- Allen, S.; Osorio, O.; Liu, Y.-G.; Scott, E. Facile assembly and loading of theranostic polymersomes via multi-impingement flash nanoprecipitation. J. Control. Release Off. J. Control. Release Soc. 2017, 262, 91–103. [Google Scholar] [CrossRef]
- Allen, S.D.; Bobbala, S.; Karabin, N.B.; Modak, M.; Scott, E.A. Benchmarking Bicontinuous Nanospheres against Polymersomes for in Vivo Biodistribution and Dual Intracellular Delivery of Lipophilic and Water-Soluble Payloads. ACS Appl. Mater. Interfaces 2018, 10, 33857–33866. [Google Scholar] [CrossRef]
- Shang, S.; Kats, D.; Cao, L.; Morgun, E.; Velluto, D.; He, Y.; Xu, Q.; Wang, C.-R.; Scott, E.A. Induction of Mycobacterium Tuberculosis Lipid-Specific T Cell Responses by Pulmonary Delivery of Mycolic Acid-Loaded Polymeric Micellar Nanocarriers. Front. Immunol. 2018, 9, 2709. [Google Scholar] [CrossRef]
- Karabin, N.B.; Allen, S.; Kwon, H.-K.; Bobbala, S.; Firlar, E.; Shokuhfar, T.; Shull, K.R.; Scott, E.A. Sustained micellar delivery via inducible transitions in nanostructure morphology. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Stack, T.; Vahabikashi, A.; Johnson, M.; Scott, E. Modulation of Schlemm’s canal endothelial cell stiffness via latrunculin loaded block copolymer micelles. J. Biomed. Mater. Res. A 2018, 106, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Zhang, X.; Sangji, M.H.; Liu, Y.; Allen, S.D.; Xiao, B.; Bobbala, S.; Braverman, C.L.; Cai, L.; Hecker, P.I.; et al. Surface Engineered Polymersomes for Enhanced Modulation of Dendritic Cells During Cardiovascular Immunotherapy. Adv. Funct. Mater. 2019, 1, 1904399. [Google Scholar] [CrossRef]
- Cerritelli, S.; O’Neil, C.P.; Velluto, D.; Fontana, A.; Adrian, M.; Dubochet, J.; Hubbell, J.A. Aggregation Behavior of Poly(ethylene glycol-bl-propylene sulfide) Di- and Triblock Copolymers in Aqueous Solution. Langmuir 2009, 25, 11328–11335. [Google Scholar] [CrossRef] [PubMed]
- DeCicco-Skinner, K.L.; Henry, G.H.; Cataisson, C.; Tabib, T.; Gwilliam, J.C.; Watson, N.J.; Bullwinkle, E.M.; Falkenburg, L.; O’Neill, R.C.; Morin, A.; et al. Endothelial Cell Tube Formation Assay for the In Vitro Study of Angiogenesis. J. Vis. Exp. JoVE 2014, 91, e51312. [Google Scholar] [CrossRef]
- Palmer, G.; Tiran, Z.; Zhou, Z.; Capozzi, M.; Park, W.; Coletta, C.; Pyriochou, A.; Kliger, Y.; Levy, O.; Borukhov, I.; et al. A novel angiopoietin-derived peptide displays anti-angiogenic activity and inhibits tumour-induced and retinal neovascularization. Br. J. Pharmacol. 2012, 165, 1891–1903. [Google Scholar] [CrossRef]
- Oba, M.; Fukushima, S.; Kanayama, N.; Aoyagi, K.; Nishiyama, N.; Koyama, H.; Kataoka, K. Cyclic RGD peptide-conjugated polyplex micelles as a targetable gene delivery system directed to cells possessing alphavbeta3 and alphavbeta5 integrins. Bioconjug. Chem. 2007, 18, 1415–1423. [Google Scholar] [CrossRef]
- Lee, K.W.; Tey, B.T.; Ho, K.L.; Tejo, B.A.; Tan, W.S. Nanoglue: An alternative way to display cell-internalizing peptide at the spikes of hepatitis B virus core nanoparticles for cell-targeting delivery. Mol. Pharm. 2012, 9, 2415–2423. [Google Scholar] [CrossRef]
- Wu, C.; Lo, S.L.; Boulaire, J.; Hong, M.L.W.; Beh, H.M.; Leung, D.S.Y.; Wang, S. A peptide-based carrier for intracellular delivery of proteins into malignant glial cells in vitro. J. Control. Release Off. J. Control. Release Soc. 2008, 130, 140–145. [Google Scholar] [CrossRef]
- Matsuo, A.L.; Juliano, M.A.; Figueiredo, C.R.; Batista, W.L.; Tanaka, A.S.; Travassos, L.R. A new phage-display tumor-homing peptide fused to antiangiogenic peptide generates a novel bioactive molecule with antimelanoma activity. Mol. Cancer Res. MCR 2011, 9, 1471–1478. [Google Scholar] [CrossRef]
- Xu, S.; Olenyuk, B.Z.; Okamoto, C.T.; Hamm-Alvarez, S.F. Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances. Adv. Drug Deliv. Rev. 2013, 65, 121–138. [Google Scholar] [CrossRef]
- Gan, B.K.; Yong, C.Y.; Ho, K.L.; Omar, A.R.; Alitheen, N.B.; Tan, W.S. Targeted Delivery of Cell Penetrating Peptide Virus-like Nanoparticles to Skin Cancer Cells. Sci. Rep. 2018, 8, 8499. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.R.; Poloukhtine, A.; Popik, V.; Tsourkas, A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Thorek, D.L.J.; Elias, D.R.; Tsourkas, A. Comparative Analysis of Nanoparticle-Antibody Conjugations: Carbodiimide Versus Click Chemistry. Mol. Imaging 2009, 8, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Vogel, K.; Wang, S.; Lee, R.J.; Chmielewski, J.; Low, P.S. Peptide-Mediated Release of Folate-Targeted Liposome Contents from Endosomal Compartments1. J. Am. Chem. Soc. 1996, 118, 1581–1586. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.-K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef]
- Guo, C.; Yu, X.; Refaely-Abramson, S.; Sepunaru, L.; Bendikov, T.; Pecht, I.; Kronik, L.; Vilan, A.; Sheves, M.; Cahen, D. Tuning electronic transport via hepta-alanine peptides junction by tryptophan doping. Proc. Natl. Acad. Sci. USA 2016, 113, 10785–10790. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Adair, T.H.; Montani, J.P. Angiogenesis. In Colloquium Series on Integrated Systems Physiology: From Molecule to Function; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010; Volume 2, pp. 1–84. [Google Scholar]
- Norgall, S.; Papoutsi, M.; Rössler, J.; Schweigerer, L.; Wilting, J.; Weich, H.A. Elevated expression of VEGFR-3 in lymphatic endothelial cells from lymphangiomas. BMC Cancer 2007, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.W.; Liau, L.L.; Chua, K.H.; Ahmad, R.; Akbar, S.A.; Pingguan-Murphy, B. Enhanced in vitro angiogenic behaviour of human umbilical vein endothelial cells on thermally oxidized TiO2 nanofibrous surfaces. Sci. Rep. 2016, 6, 21828. [Google Scholar] [CrossRef]
- Bang, J.-Y.; Kim, E.-Y.; Kang, D.-K.; Chang, S.-I.; Han, M.-H.; Baek, K.-H.; Kang, I.-C. Pharmacoproteomic analysis of a novel cell-permeable peptide inhibitor of tumor-induced angiogenesis. Mol. Cell. Proteom. MCP 2011, 10, M110.005264. [Google Scholar] [CrossRef]
- Odrljin, T.M.; Haidaris, C.G.; Lerner, N.B.; Simpson-Haidaris, P.J. Integrin alphavbeta3-mediated endocytosis of immobilized fibrinogen by A549 lung alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001, 24, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Chang, C.H.; Hsu, C.C.; Lin, K.T.; Peng, H.C.; Huang, T.F. Aggretin Venom Polypeptide as a Novel Anti-angiogenesis Agent by Targeting Integrin alpha2beta1. Sci. Rep. 2017, 7, 43612. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dong, X.; Xiu, P.; Zhong, J.; Wei, H.; Xu, Z.; Li, T.; Liu, F.; Sun, X.; Li, J. T7 peptide inhibits angiogenesis via downregulation of angiopoietin-2 and autophagy. Oncol. Rep. 2015, 33, 675–684. [Google Scholar] [CrossRef][Green Version]
- Jianjun, Z.; Li, Y.; Wang, J.; Qi, S.; Song, X.; Tao, C.; Le, Y.; Wen, N.; Chen, J. Dual redox-responsive PEG–PPS–cRGD self-crosslinked nanocapsules for targeted chemotherapy of squamous cell carcinoma. RSC Adv. 2017, 7, 53552–53562. [Google Scholar]
- Otvos, L.; Wade, J.D. Current challenges in peptide-based drug discovery. Front. Chem. 2014, 2, 62. [Google Scholar] [CrossRef] [PubMed]
- Haggag, Y.A. Peptides as Drug Candidates: Limitations and Recent Development Perspectives. Biomed. J. Sci. Tech. Res. 2018, 8. [Google Scholar] [CrossRef]
- Decuzzi, P.; Ferrari, M. The role of specific and non-specific interactions in receptor-mediated endocytosis of nanoparticles. Biomaterials 2007, 28, 2915–2922. [Google Scholar] [CrossRef]
- Gao, H.; Shi, W.; Freund, L.B. Mechanics of receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 2005, 102, 9469–9474. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagaraj, R.; Stack, T.; Yi, S.; Mathew, B.; Shull, K.R.; Scott, E.A.; Mathew, M.T.; Bijukumar, D.R. High Density Display of an Anti-Angiogenic Peptide on Micelle Surfaces Enhances Their Inhibition of αvβ3 Integrin-Mediated Neovascularization In Vitro. Nanomaterials 2020, 10, 581. https://doi.org/10.3390/nano10030581
Nagaraj R, Stack T, Yi S, Mathew B, Shull KR, Scott EA, Mathew MT, Bijukumar DR. High Density Display of an Anti-Angiogenic Peptide on Micelle Surfaces Enhances Their Inhibition of αvβ3 Integrin-Mediated Neovascularization In Vitro. Nanomaterials. 2020; 10(3):581. https://doi.org/10.3390/nano10030581
Chicago/Turabian StyleNagaraj, Rajini, Trevor Stack, Sijia Yi, Benjamin Mathew, Kenneth R Shull, Evan A Scott, Mathew T Mathew, and Divya Rani Bijukumar. 2020. "High Density Display of an Anti-Angiogenic Peptide on Micelle Surfaces Enhances Their Inhibition of αvβ3 Integrin-Mediated Neovascularization In Vitro" Nanomaterials 10, no. 3: 581. https://doi.org/10.3390/nano10030581
APA StyleNagaraj, R., Stack, T., Yi, S., Mathew, B., Shull, K. R., Scott, E. A., Mathew, M. T., & Bijukumar, D. R. (2020). High Density Display of an Anti-Angiogenic Peptide on Micelle Surfaces Enhances Their Inhibition of αvβ3 Integrin-Mediated Neovascularization In Vitro. Nanomaterials, 10(3), 581. https://doi.org/10.3390/nano10030581



